Abstract
Rationale
In major depression, prefrontal regulation of limbic brain areas may be a key mechanism that is impaired during the processing of affective information. This prefrontal–limbic interaction has been shown to be modulated by serotonin (5-HTT) genotype, indicating a higher risk for major depressive disorder (MDD) with increasing number of 5-HTT low-expression alleles.
Objective
Functional magnetic resonance imaging was used to assess neural response to uncued unpleasant affective pictures in 21 unmedicated patients with MDD compared to 21 matched healthy controls, taking into account genetic influences of the 5-HTT (SCL6A4) high- and low-expression genotype.
Results
Healthy controls displayed greater prefrontal activation (BA10) to uncued negative pictures compared to patients with MDD. While in healthy controls prefrontal (BA10) activation and BA10–amygdala coupling increased with the number of 5-HTT low-expression risk alleles, this effect was abolished, and even reversed, in patients with MDD. In MDD, connectivity decreased with severity of depressive symptoms (HAMD total score).
Conclusion
These findings suggest that increased medial prefrontal (BA10) activation and BA10–amygdala connectivity may counteract the risk for MDD in healthy carriers of 5-HTT low-expression alleles, while this protective factor might be lost in patients who actually suffer from MDD. Prefrontal–limbic regulation in risk populations could be a target of early interventions and should be the focus of further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive control of emotions is particularly in demand when negative affective events occur unexpectedly, thus requiring cognitive appraisal (Kalisch et al. 2006; Simpson et al. 2001). Such regulation of emotional reactions involves interactions of medial and dorsal parts of the prefrontal cortex (PFC; Kalisch et al. 2006; Phan et al. 2002; Simpson et al. 2001) with emotional appraisal systems such as the amygdala (Irwin et al. 2004; Matthews et al. 2008; Ochsner and Gross 2005). The interaction of medial PFC and the amygdala during viewing of negative visual stimuli may be modulated by serotonin genotype (5-HTTLPR) carriers of short alleles of the 5-HTT promoter, who appear to display a higher risk for major depressive disorder (MDD) in response to stressful life events (Caspi et al. 2003 but see Gillespie et al. 2005) and show increased coupling between medial PFC (BA10) and the amygdala during the presentation of negative but not positive affective pictures (Heinz et al. 2005; Pezawas et al. 2005; Munafo et al. 2008). In contrast, prefrontal–amygdala coupling is decreased in patients who actually suffer from MDD (Anand et al. 2005a), indicating impaired prefrontal–limbic connectivity in this disorder (Drevets 2007; Ressler and Mayberg 2007). One explanation for these apparently contradictory findings in healthy individuals and patients with MDD could be that genetic risk for MDD may be counteracted by enhanced affect regulation through prefrontal–amygdala interaction, while the loss of this mechanism may lead to MDD.
Here, we directly compared unmedicated patients with MDD and healthy controls with respect to the modulatory effect of 5-HTT genotype on neural processing of uncued and cued negative stimuli. Bermpohl et al. (2006) observed that cues indicating the subsequent presentation of affective pictures activate the anterior cingulate cortex (ACC) and further brain areas implicated in emotion regulation and that this network is distinct from brain areas activated by the affective pictures themselves. Similarly, Ueda et al. (2003) and Onoda et al. (2008) report activation in the medial prefrontal cortex (mPFC), left ACC, and right amygdala during anticipation of negative stimuli. Furthermore, Herwig et al. (2007) describe an increased mPFC activation and decreased right amygdala activation during cognitive regulation of negative affective stimuli. Based on these findings, we hypothesized that prefrontal–amygdala connectivity would be particularly important during the presentation of aversive pictures that were not preceded by a cue and thus require the activation of compensatory centers of emotion regulation such as the ACC and medial prefrontal cortex. Therefore, negative stimuli were presented with or without prior cues to assess adaptive neural responses, comparing healthy controls and patients with MDD as well as modulatory influences of 5-HTT genotype on these neural responses. Specifically, we tested the effects of the 5-HTTLPR (Heinz et al. 2005; Pezawas et al. 2005; Praschak-Rieder et al. 2007) and a second single-nucleotide polymorphism (rs25531), which modulates 5-HTTLPR effects on serotonin transporters, resulting in reduced function of 5-HTTLPR long (L) allele carriers in the presence of a G substitution in rs25331 (LG; Hu et al. 2006; Smolka et al. 2007). We expected to find (1) increased mPFC activation and mPFC–amygdala coupling in healthy carriers of a short or LG allele of the 5-HTT gene, particularly when negative stimuli are uncued and prefrontal control of limbic activation may be required, and (2) that this potentially regulatory mechanism is dysfunctional in major depression, resulting in reduced prefrontal activation and prefrontal–amygdala coupling depending on depression severity.
Methods and material
Subjects
Twenty-one patients with unipolar major depression (mean age 40 ± 10, five women, all right-handed) were included in this study and compared to 21 healthy controls matched for age, gender, and smoking (mean age 38 ± 12, six women, all right-handed). There were no significant group effects concerning age (T = 0.734, p = 0.50), gender, and smoking (χ 2: 0.1–2.4; p > 0.1). Patients suffered from unipolar MDD according to ICD-10 and DSM-IV criteria and were included only with a Hamilton Rating Scale for Depression (HRSD-21, Hamilton 1960) score above 15 (mean ± STD: 20.19 ± 4.34; for clinical characteristics, see Table 1). They had no other psychiatric axis I disorder and no past history of drug dependence or current drug abuse (assessed with random urine drug testing and SCID interview, Wittchen et al. 1997) except for nicotine abuse.
Healthy controls had no psychiatric axis I or II disorder (SCID I and II interviews). Patients were recruited from the inpatient and outpatient center of the Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin, Campus Mitte. Healthy control participants were recruited from the local community by advertisement.
All patients were free of any medication at least 3 months before the scanning session; nine patients had never received any medication treatment (drug naïve).
Genotyping
The genotype of the promoter for the 5-HTT gene (Hu et al. 2006; Lesch et al. 1996) was assessed with polymerase chain reaction and oligonucleotide primers. Genotype effects on activation and connectivity patterns in response to negative emotional stimuli have been reported for 5-HTT low-expression alleles (Heinz et al. 2005). An A < G exchange leading to differential transcriptional activation of 5-HTT messenger RNA was established in the 5-HTT regulatory region (Hu et al. 2006). Accounting for the hypothesized functional similarity of LG and S alleles in driving 5-HTT transcription, we treated LG variants of the long 5-HTTLPR version like 5-HTT low-expression alleles (s-alleles; Hu et al. 2006; Nakamura et al. 2000; Praschak-Rieder et al. 2007; Smolka et al. 2007). 5-HTT genotype distribution did not significantly differ from Hardy–Weinberg equilibrium. The genotype distribution for patients with MDD and healthy controls with respect to A < G exchange is given in Table 2 .
fMRI data acquisition
Imaging was performed on a 1.5-T Scanner (Magnetom VISION Siemens®) with an Echo Planar Imaging (EPI) sequence (TE = 40 ms, TR = 2,300 ms, α = 90°, matrix = 64 × 64, voxel size = 4 × 4 × 3.3 mm). A 3D Magnetization Prepared Rapid Gradient Echo (TR = 9.7 ms; TE = 4 ms; flip angle 12°; matrix = 256 × 256, voxel size 1 mm x 1mm x 1mm) image data set was acquired. Head movement was minimized by using a vacuum pad.
fMRI paradigm
An event-related design with pictures of different affective valence, e.g., unpleasant (negative) and neutral pictures from the International Affective Picture System, was used for emotional stimulation (Lang et al. 1999). The 36 stimuli per category were presented for 2 s in random order and with a randomly jittered intertrial interval (1.6–3 s) in order to sample the hemodynamic response at different data points. A cue appeared 3 s before each picture (for a duration of 500 ms followed by a fixation cross). In half of the trials, the cue notified the emotional valence of the upcoming picture (e.g., “negative” or “neutral”) to the subjects (“cued” condition) and in the other half of trails the cue consisted of a meaningless letter combination that did not predict the emotional valence of the following picture (here referred to as “uncued” condition). Stimulus material was counterbalanced for the cued and uncued condition across all subjects. Participants were instructed to passively view the stimuli because even simple rating tasks can influence amygdala activation elicited by negative pictures (Taylor et al. 2003). To keep participants engaged in the task and control for a decrease in attention, subjects had to confirm every viewed picture with a button press with the right thumb.
After the MRT session, each subject rated the presented pictures on a nine-point Likert scale for valence (unpleasant vs pleasant) and arousal (high vs low).
fMRI analysis
Data preprocessing
Functional magnetic resonance imaging data were analyzed in SPM5 (www.fil.ion.ucl.ac.uk/spm). The first three volumes of each functional time series were discarded to remove non-steady-state effects caused by T1 saturation. The remaining images were corrected for differences in slice time acquisition, corrected (realigned to the mean volume), spatially normalized to the standard EPI template provided by the Montreal Neurological Institute (MNI template), and smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
Statistical analysis
First-level single-subject analysis
After preprocessing, the data were analyzed in the context of the general linear model approach as implemented in SPM5 at two levels. On the first single-subject level, the picture valence (e.g., negative or neutral) for cued and uncued pictures as well as the different cues (e.g., cue negative, cue neutral, or cue meaningless) was defined as events and were modeled separately as explanatory variables after convolution with the canonical hemodynamic response function. Realignment parameters were included as additional regressors. Individual beta images for each picture condition and the contrast for “negative − neutral pictures” (either combining cued and uncued pictures or for each condition separately) and for the contrast “[(uncued negative − uncued neutral) − (cued negative − cued neutral)]” were computed for each individual and entered into second-level random-effects models.
Second-level group statistical analyses
First, to determine the neural response to aversive stimuli, the individual beta images of each picture condition (combining cued and uncued pictures) were entered into a second-level random-effects model with group and picture valence (negative or neutral) as factors including subjects as a random factor. Activations for negative − neutral pictures for both groups together, for each group separately, and for differences between groups were determined using the appropriate contrasts. In a second analysis, we specifically tested for group differences between cued and uncued negative − neutral pictures and assessed the contrast “[(uncued negative − uncued neutral) − (cued negative − cued neutral)],” further referred to as “uncued compared to cued negative minus neutral pictures,” which was subjected to a two-sample t test.
In a third step, the genotype effect was estimated by including the 5-HTT low-expression allele count (0, 1, or 2) as a covariate into the above-mentioned analyses. Correlations with psychopathology measures (State Trait Anxiety Inventory (STAI) and HRSD-21 total score) were determined by entering them as covariates in separate SPM random-effects models. For the STAI, which was employed in both groups, a two-sample t test with STAI total score entered as covariate was used. For the HRSD-21, which was only administered in MDD, a one-sample t test with HRSD-21 total score as covariate was used. All tests for correlations between brain activation and behavioral data were explorative and not corrected for multiple testing; therefore, p values are exclusively given for descriptive reasons.
Statistical correction
Given our a priori hypothesis of an involvement of the amygdala and the mPFC in emotional processing, correction for multiple testing was performed using the small volume approach (Brassen et al. 2008; Heinz et al. 2005). We used the Pick Atlas (Lancaster et al. 2000; Maldjian et al. 2003; http://www.fmri.wfubmc.edu) to define regions of interest (ROI) for the amygdala (left 68 voxels, right 86 voxels). The mPFC ROI was generated by combining the BA10 ROI from the Pick Atlas with a 36-mm diameter sphere centered between the genu of the corpus callosum and the anterior pole (x/y/z = 0/52/−3, as previously used by Heinz et al. 2005) to confine the ROI to the medial part of BA10, in accord with our a priori hypothesis (BA10, left 89 voxels, right 92). We corrected for multiple comparisons using the family-wise error (FWE) procedure for the volumes of interest at a statistical threshold of p < 0.05.
Connectivity analysis: psychophysiological interaction
We used the psychophysiological interaction (PPI) approach (Gitelman et al. 2003) to assess genotype effects on coupling between the prefrontal area displaying a group effect in the whole-brain analysis (BA10) and the amygdala, following the hypothesis that genotype effects on amygdala activity (Hariri et al. 2003; Lau et al. 2008) are mediated by cortical top-down modulation (Heinz et al. 2005, Pezawas et al. 2005). To create the PPI term, the time series of the cortical seed region (e.g., the BA10) was extracted and deconvolved within a Bayesian framework to generate the neuronal signal for the seed region (Gitelman et al. 2003). The PPI was then defined as the element-by-element product of the neuronal time series of the seed region and a vector coding for the effects of emotional stimuli (uncued negative versus neutral pictures). As seed region, the peak voxel of the group effect for “uncued compared to cued negative minus neutral pictures” was chosen (x/y/z = −12/60/3). For each subject, a new first-level statistical model was set up, which included the following regressors: the individual time series of the seed region (BA10), the psychological variable (negative vs neutral uncued pictures), and the reconvolved interaction term (the psychophysiological variable). Because the seed region (BA10) showed a task-by-group interaction, it is crucial to account for variance explained by group differences of the task effect independent of connectivity differences. Therefore, all task conditions were included as regressors in the PPI single-subject analysis to control for variance explained by the task conditions (for further analysis, please see Supplementary Material).
The individual contrast images for the interaction term were taken to the second level. Group, genotype, and group-by-genotype effects were assessed with a two-sample t test with the genotype added as a covariate implicating 5-HTT low-expression allele count (0, 1, or 2).
Behavioral data analysis
Ratings for valence and arousal (on a nine-point Likert scale) were analyzed in separate repeated-measures analysis of variance with factor picture valence (negative vs neutral) as intrasubject factor and group as intersubject factor using SPSS 14.
Results
Behavioral data
As expected, negative and neutral pictures were differently rated according to their valence (F = 168.25, p < 0.001) and arousal (F = 135.54, p < 0.001) by all participants, post hoc t test revealed more aversive valence scores (T = 13.076, p < 0.001) and higher arousal scores (T = 11.68, p < 0.001) for negative pictures compared to neutral ones. There were no significant group differences in subjective ratings and no interactions (F values between 0.58 and 0.72, all p > 0.3), indicating that the stimulus material was equally appraised by the healthy controls and MDD patients.
Amygdala response elicited by negative minus neutral pictures
To compare neural responses to emotional stimuli between healthy controls and patients with MDD, we assessed neural responses to negative minus neutral pictures (combining the cued and uncued conditions). As previously observed (Hariri et al. 2002; Heinz et al. 2005; Pezawas et al. 2005), there was a significant activation in both amygdalae while viewing negative minus neutral pictures across all participants (healthy controls and patients with MDD: peak left: x/y/z = −18/−6/−15, T = 3.91, p = 0.002, FWE-corrected; peak right: x/y/z = 18/−3/−15, T = 4.05, p = 0.001, FWE-corrected; healthy controls: peak left: x/y/z = −18/−6/−18, T = 3.29, p = 0.011, FWE-corrected; peak right: x/y/z = 30/3/−24, T = 3.21, p = 0.016, FWE-corrected; patients with MDD: peak left: x/y/z = −18/−6/−15, T = 2.85, p = 0.033, FWE-corrected, peak right: x/y/z = 18/−3/−15, T = 3.44, p = 0.009, FWE-corrected). There was no group difference between healthy controls and patients with MDD in the amygdala. No significant expectancy effect (uncued vs. cued) nor interactions group-by-expectancy in amygdala response to negative minus neutral pictures were found.
Correlation between amygdala response and severity of anxiety and depression
Using state anxiety as a covariate in a two-sample t test for the contrast negative minus neutral pictures (combining cued and uncued conditions), there was a significant positive correlation of right amygdala activation across both groups (T = 2.80, x/y/z = 18/3/−15, p = 0.045, FWE-corrected). This correlation was trend wise higher in patients with major depression (T = 2.38, x/y/z = 30/−9/−12, p = 0.096, FWE-corrected), the most anxious subjects showing the strongest amygdala activation to negative pictures. Severity of depression (HRSD-21 total score) did not modulate amygdala response in patients with MDD.
5-HTT genotype effect on amygdala activation
Modulation of amygdala activation by 5-HTT genotype was assessed with a two-sample t test for the contrast negative minus neutral pictures (combining cued and uncued conditions) with the number of s-alleles entered as a covariate (0, 1, or 2) for each group. There was a tendency for genotype modulation in MDD (right amygdala: T = 2.55, x/y/z = 27/−9/−12, p = 0.067, FWE-corrected), which was trend wise reversed in healthy controls (right amygdala: T = 2.43, x/y/z = 27/0/−27, p = 0.083, FWE-corrected; left amygdala: T = 2.50, x/y/z = −27/−3/−27, p = 0.066, FWE-corrected), the group-by-genotype interaction reaching significance (right amygdala: T = 2.95, x/y/z = 24/−9/−12, p = 0.030, FWE-corrected; left amygdala: T = 2.66, x/y/z = −30/−3/−27, p = 0.048, FWE-corrected): a higher number of 5-HTT low-expression alleles were associated with a significantly stronger increase in the BOLD response evoked in the amygdala in patients with MDD compared to healthy controls. With respect to previous findings (Canli et al. 2005; Heinz et al. 2007), we also assessed genotype-modulated amygdala activation for the contrast “negative minus baseline” (fixation cross) which also revealed a tendency for genotype-dependent amygdala activation across groups in the right amygdala (x/y/z = 18/3/−18, T = 2.37, p = 0.097); the group-by-genotype interaction showed a significantly stronger genotype-modulated amygdala response in MDD compared to HC (left amygdala: x/y/z = −30/0/−27, T = 3.76, p = 0.004, right amygdala: x/y/z = 24/−9/−12, T = 3.6, p = 0.006), similar to the results using the above-mentioned contrast “negative minus neutral” (see Supplementary Material Fig. 1).
Stronger mPFC activation in healthy controls but not MDD to uncued aversive pictures
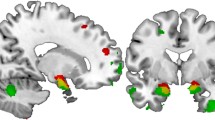
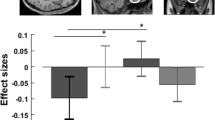
We next tested for specific effects of uncued compared to cued presentations of negative affective pictures. Healthy controls showed stronger activation in the bilateral medial PFC (BA10) to uncued compared to cued negative minus neutral pictures (left: T = 3.46, x/y/z = −6/51/6, p = 0.015, FWE-corrected; right: T = 3.43, x/y/z = 3/51/3, p = 0.017, FWE-corrected). MDD patients displayed only a trend wise effect within our predefined ROI (T = 2.82, x/y/z = −15/48/−3, p = 0.063, FWE-corrected). Directly comparing the BOLD response of healthy controls and MDD to “uncued compared to cued negative minus neutral pictures,” we found a group-by-expectancy interaction in the left medial PFC (BA10, x/y/z = −12/60/3, T = 3.10, p = 0.034, FWE-corrected). As shown in Fig. 1, this interaction was due to a stronger BA10 activation to uncued compared to cued negative minus neutral pictures in healthy controls, while in MDD this effect appeared to be reversed, with reduced neural responses to uncued compared to cued negative pictures.
Left panel: healthy controls (HC) show stronger medial prefrontal activation elicited by uncued > cued (negative minus neutral) pictures compared to patients with major depressive disorder (MDD). Right panel: parameter estimates for negative minus neutral pictures for cued and uncued condition separately are plotted for prefrontal responses (BA10) in HC and MDD
There were no significant effects within healthy controls and MDD for the reverse comparison of cued compared to uncued negative minus neutral pictures
Correlation between mPFC activation and severity of anxiety and depression
The association between mPFC activation and depression severity was tested using a one-sample t test for the contrast “uncued negative minus neutral pictures” with the HRSD-21 total score as a covariate within the MDD group. The severity of major depression correlated negatively with the bilateral mPFC (BA10; left: T = 3.75, x/y/z = −6/45/−9, p = 0.022, FWE-corrected; right: T = 3.52, x/y/z = 12/39/−6, p = 0.033, FWE-corrected), revealing decreasing mPFC activation with increasing depressive symptoms. There were no significant correlations between depression ratings and brain activation elicited by “cued negative minus neutral pictures” nor by “uncued compared to cued negative minus neutral pictures.” Anxiety (STAI total score) did not modulate mPFC activation in healthy controls or patients with MDD.
5-HTT genotype effect on mPFC activation
Modulation of mPFC activation by 5-HTT genotype was assessed with a two-sample t test for the contrast “uncued compared to cued negative minus neutral pictures” with the number of s-alleles entered as a covariate for each group. The BA10 response was modulated by 5-HTT genotype variation: a higher number of 5-HTT low-expression alleles were associated with a significantly stronger increase in the BOLD response evoked in the BA10 in healthy controls (left: T = 3.18, x/y/z = −6/42/−6, p = 0.029, FWE-corrected) but not MDD (p > 0.10), the group-by-genotype interaction approaching statistical significance (T = 2.87, x/y/z = −6/42/−9, p = 0.058, FWE-corrected).
Functional connectivity between mPFC and amygdala modulated by 5-HTT genotype
Given our previous finding (Heinz et al. 2005), we specifically tested for group-by-genotype interaction on functional connectivity between BA10 and amygdala. The peak value of the group-by-valence interaction in the BA10 (x/y/z = −12/60/3) was used as a seed region and used in a PPI analysis to assess the functional connectivity to the amygdala during uncued negative compared to neutral picture presentation.
Healthy controls showed a significant positive correlation of BA10–amygdala connectivity with the number of 5-HTT low-expression alleles (linear regression R = 0.55, p < 0.05), while this relationship was reversed in MDD (r = −0.58, p < 0.05), thus revealing a significant group-by-genotype interaction (left amygdala: T = 4.11, x/y/z = −18/0/−12, p = 0.002, FWE-corrected, right amygdala: T = 2.88, x/y/z = 18/3/−18, p = 0.042, FWE-corrected; Fig. 2a). There was no genotype modulation nor a group difference for cued negative compared to neutral pictures (p > 0.05). (For further results, see Supplementary results Fig. 1)
a Left panel: genotype-dependent medial prefrontal modulation of the left amygdala (x/y/z = −18/0/12) during uncued negative minus neutral pictures in healthy controls (HC) > patients with major depressive disorder (MDD) as determined by psychophysiological interaction analysis. Right panel: parameter estimates of connectivity plotted against 5-HTT low-expression allele number: healthy controls (HC) showed a significant increase in connectivity as a function of the number of low-expression 5-HTT risk alleles (linear regression R = 0.55, p < 0.05), while MDD showed the inverse pattern (R = −0.58, p < 0.05). b Left panel: state anxiety (STAI)-dependent medial prefrontal modulation of the right amygdala during uncued negative minus neutral pictures in healthy controls (HC) > patients with major depressive disorder (MDD; T = 5.43, x/y/z = 24/0/−21) as determined by psychophysiological interaction (PPI) analysis. Right panel: parameter estimates of connectivity plotted against STAI total score: healthy controls (HC) showed a significant increase in connectivity with increasing STAI total score (linear regression R = 0.82, p < 0.0001), while MDD show the inverse pattern (R = −0.52, p < 0.05)
Correlation between mPFC–amygdala connectivity and severity of anxiety and depression
Among MDD patients, low functional connectivity between mPFC (BA10) and right amygdala was trend-wise-associated with more severe depressive symptoms (T = 2.93, x/y/z = 24/−3/−15, p = 0.063, FWE-corrected) using the beta images of the PPI analysis in a one-sample t test with the HRSD-21 total score as a covariate within the MDD group.
Correlation between functional connectivity measures forms the PPI analysis and state anxiety was assessed within each group and compared between both groups using a two-sample t test with the STAI total score as a covariate for each group. Functional connectivity between mPFC (BA10) and amygdala displayed a positive correlation with state anxiety (STAI total score) for the bilateral amygdala in healthy controls (right: T = 4.89, x/y/z = 24/0/−21, p = 0.001, FWE-corrected; left: T = 5.53, x/y/z = −15/−6/−18, p = 0.001, FWE-corrected), whereas this effect was trend-wise-reversed in patients with MDD revealing decreasing prefrontal–amygdala connectivity in the more anxious and more depressive subjects (right: T = 2.86, x/y/z = 21/−3/−18, p = 0.051, FWE-corrected, left: T = 2.43, x/y/z = −21/0/−21, p = 0.097, FWE-corrected; Fig. 2b), the group difference reaching statistical significance (right: T = 5.43, x/y/z = 24/0/−21, p < 0.001, FWE-corrected; left: T = 5.01, x/y/z = −15/−6/−18, p < 0.001, FWE-corrected).
Discussion
Our results demonstrate that neural responses to uncued negative minus neutral stimuli and the interaction of 5-HTT genotype with these neural responses are altered in unmedicated patients with MDD. Compared to healthy controls, they showed (1) reduced PFC activation in the BA10, (2) a diminished effect of 5-HTT genotype on PFC activation, and (3) a reversed influence of 5-HTT genotype and anxiety on functional connectivity between BA10 and the amygdala. In patients with major depression, reduced activation of the mPFC (BA10) and weaker connectivity between the mPFC and amygdala elicited by uncued negative minus neutral stimuli was correlated with the severity of depression and anxiety, whereas in healthy subjects, anxiety interacted with stronger prefrontal–amygdala connectivity.
Our results suggest that in healthy individuals mPFC activation may reflect a regulatory mechanism for the control of affective responses to negative events that occur without prior anticipation. In contrast, this regulatory mechanism may be deficient in MDD, in line with earlier findings (Keedwell et al. 2005). In subjects suffering from high anxiety, Bishop et al. (2004) observed a high amygdala response independent of directed attention, indicating a potential lack of (prefrontal) attentional regulation of emotional responses. Interestingly, PFC responses have previously been reported to be more pronounced in healthy 5-HTT low-expression allele carriers (Canli et al. 2005; Smolka et al. 2007), who are thought to be at higher risk for stress-induced negative mood states (Caspi et al. 2003 but see Gillespie et al. 2005) and anxiety (Gonda et al. 2008). In contrast, the effect of 5-HTT genotype was reversed in patients with MDD in our study. This may indicate that increased PFC activation in healthy carriers of 5-HTT low-expression alleles reflects a protective mechanism that breaks down in MDD. Similar to the responses to uncued negative minus neutral stimuli in BA10, connectivity between this region and the amygdala was stronger in healthy subjects carrying 5-HTT low-expression alleles, in line with previous findings (Heinz et al. 2005; Pezawas et al. 2005). Anxiety was associated with higher (and potentially compensatory) PFC–limbic connectivity in healthy subjects, whereas, in MDD, the influence of 5-HTT genotype and anxiety on prefrontal–amygdala connectivity was reversed. Simpson et al. (2001) reported regional cerebral blood flow (rCBF) increases in the mPFC (BA10/32) during anticipatory anxiety for anxious but healthy subjects, whereas the least anxious subjects demonstrated a strong decrease in regional rCBF of the mPFC. This finding supports the notion that prefrontal regulatory control of limbic responses may be a compensatory mechanism, which is even more in demand in healthy but anxious subjects, particularly if carrying the 5-HTT risk genotype and which breaks down in MDD. Our findings are also in line with the previous observation of impaired prefrontal–amygdala connectivity in patients with MDD (Johnstone et al. 2007; Matthews et al. 2008), as we observed reduced functional connectivity in MDD patients compared to controls with respect to 5-HTT genotype. Indeed, in our study, low mPFC activation and decreased connectivity with the amygdala correlated with symptom severity and anxiety in MDD patients supporting the notion that this interaction may be clinically relevant.
Such dysfunctional corticolimbic connectivity may serve as a target for therapeutic interventions (Anand et al. 2005a, b, 2007; Irwin et al. 2004; Seminowicz et al. 2004). Chen et al. (2008) reported decreased functional coupling between the amygdala and right frontal and cingulate cortex, striatum, and thalamus in unmedicated patients with MDD compared to healthy controls during the processing of aversive stimuli (sad faces). Decreased coupling was positively modified via antidepressant medication, extinguishing differences in prefrontal–amygdala coupling between healthy controls and patients with MDD. Antidepressants thus may increase corticolimbic coupling and enhance the cortical regulation of potentially abnormal limbic activation (Anand et al. 2005b; Chen et al. 2008).
In the light of the present study, we can only speculate with respect to factors in healthy control 5-HTT risk allele carriers that may protect prefrontal–amygdala connectivity. However, nonhuman primate studies revealed that rhesus monkeys carrying a comparable 5-HTT risk allele display reduced serotonin turnover rates (as measured with the serotonin metabolite 5-hydroxyindole acetic acid in the cerebrospinal fluid; Bennett et al. 2002; Lesch et al. 1997; Spinelli et al. 2007) after social separation stress compared with carriers of two long alleles. Interestingly, stimulation of the medial prefrontal cortex in cats increased serotonin release in raphe neurons projecting to cortical and subcortical brain areas (Juckel et al. 1999). Therefore, increased stress vulnerability (Brown and Harris 2008; Caspi et al. 2003; Kendler et al. 2005; Zalsman et al. 2006 but see Gillespie et al. 2005) and anxiety (Gonda et al. 2008), as clinically seen in 5-HTTLPR short allele carriers, may interfere with medial prefrontal regulation of raphe serotonin neurons that project to the amygdala and other limbic areas. In the amygdala, indices of serotonergic neurotransmission have been shown to directly affect amygdala responses to negative (Hariri et al. 2005) and fearful (Lau et al. 2008) stimuli. Multimodal imaging studies that combine the assessment of functional activation with measurement of serotonergic neurotransmission and genotyping may further explore these interactions.
We did not find a significant group difference between MDD and healthy controls in amygdala response to negative minus neutral pictures in this sample. This finding is in line with previous research (Lee et al. 2008), although the majority of studies reported amygdala hyperactivation in MDD (Anand et al. 2005a; Sheline et al. 2001; Siegle et al. 2002). However, we did find a significant group difference in 5-HTT genotype modulation of amygdala activation to negative minus neutral pictures between patients with MDD compared to healthy controls. This increased limbic activation to aversive stimuli in 5-HTT genotype carriers with MDD compared to healthy controls might be the result of lacking medial prefrontal (BA10) regulation (Heinz et al. 2005; Pezawas et al. 2005). Lau et al. (2008) reported the reverse activation pattern in healthy 5-HTT risk allele carriers compared to MDD, using fearful faces to induce aversive emotional processing in adolescents. Studies on adults (for meta-analysis, see Munafo et al. 2008) and 5-HTT knockout mice (Wellman et al. 2007) match our results with opposite gene–amygdala response patterns. Dannlowski et al. (2008) also reported increased amygdala activation in 5-HTT risk allele carriers to masked (unconscious) emotional faces (angry, sad, and happy compared to neutral) in MDD and healthy controls but did not find a group-by-genotype interaction.
These conflicting results might be due to differences in stimulus material (e.g., IAPS pictures vs. faces) and paradigms (e.g., conscious vs. unconscious processing of emotional stimuli) as well as sample characteristics (Mayberg 2003; Mitterschiffthaler et al. 2006), medication status (Dannlowski et al. 2008) and sample size (which constrains the explanatory power of our genetic data and will have to be further validated in bigger samples).
Besides 5-HTT genotype effets on amygdala activation (Munafo et al. 2008) and functional connectivity between the mPFC (BA10) and amygdala, which were seen in two studies (Heinz et al. 2005; Pezawas et al. 2005), one of these studies also reported further 5-HTT genotype-driven connectivity between the amygdala and another prefrontal brain area, the ACC (Pezawas et al. 2005), which can contribute to fear extinction. However, we did not observe a significant activation in this brain area with our paradigm, neither in healthy controls nor MDD patients and therefore did not use this region as a seed in our functional connectivity analysis.
Heterogeneity in anatomical structures, connections, and directions of neural responses between prefrontal and limbic brain areas to emotional information across published reports pay tribute to the richness and complexity of anatomical connections and the heterogeneity of ongoing intrinsic compensatory processes observed on a clinical and neural basis in patients with MDD (Lee et al. 2008; Mayberg 2002).
One major limitation of our study is the relatively small sample size (see Munafo et al. 2008), which was mainly due to the exclusion criterion of antidepressive medication in MDD subjects. Indeed, we only observed trends towards 5-HTT genotype effect looking at each group separately on amygdala activation elicited by aversive pictures, although genotype effects on neural responses have been observed in comparable samples before (Dannlowski et al. 2007; Domschke et al. 2006; Lau et al. 2008; Smolka et al. 2007). Furthermore, life events were not recorded, so gene-by-environment interactions could not be assessed.
Taken together, our pilot study suggests that healthy individuals with 5-HTT low-expression risk alleles display increased prefrontal activation in connection with reduced amygdala responses to sudden information. In healthy controls, prefrontal activation is modulated by anxiety, i.e., more anxious subjects display increased mPFC activation. In contrast, MDD patients with higher levels of anxiety display reduced prefrontal activation and a diminished 5-HTT-driven prefrontal–amygdala connectivity. This reduced connectivity may contribute to the severity of depressive symptoms in MDD.
References
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ (2005a) Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 57:1079–1088
Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ (2005b) Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology 30:1334–1344
Anand A, Li Y, Wang Y, Gardner K, Lowe MJ (2007) Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci 19:274–282
Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD (2002) Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 7:118–122
Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G (2006) Attentional modulation of emotional stimulus processing: an fMRI study using emotional expectancy. Hum Brain Mapp 27:662–77
Bishop S, Duncan J, Brett M, Lawrence AD (2004) Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7:184–188
Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buchel C (2008) Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biol Psychiatry 64:349–355
Brown GW, Harris TO (2008) Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene–environment interaction. J Affect Disord 111:1–12
Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP (2005) Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A 102:12224–12229
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389
Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore E (2008) Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33:1909–1918
Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T (2007) Serotonergic genes modulate amygdala activity in major depression. Genes Brain Behav 6:672–676
Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, Arolt V, Heindel W, Kersting A, Baune BT, Suslow T (2008) 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology 33:418–424
Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, Bauer J, Hohoff C, Kersting A, Engelien A, Arolt V, Heindel W, Deckert J (2006) Association of the functional −1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. Int J Neuropsychopharmacol 9:349–355
Drevets WC (2007) Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci 1121:499–527
Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG (2005) The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med 35:101–111
Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003) Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage 19:200–207
Gonda X, Fountoulakis KN, Juhasz G, Rihmer Z, Lazary J, Laszik A, Akiskal HS, Bagdy G (2008) Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. Eur Arch Psychiatry Clin Neurosci 259:106–113
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR (2002) The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage 17:317–323
Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR (2005) A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry 62:146–152
Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C (2005) Amygdala–prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8:20–21
Heinz A, Smolka MN, Braus DF, Wrase J, Beck A, Flor H, Mann K, Schumann G, Büchel C, Hariri AR, Weinberger DR (2007) Serotonin transporter genotype (5- HTTLPR): effects of neutral and undefined conditions on amygdala activation. Biol Psychiatry 61:1011–1014
Herwig U, Baumgartner T, Kaffenberger T, Brühl A, Kottlow M, Schreiter- Gasser U, Abler B, Jäncke L, Rufer M (2007) Modulation of anticipatory emotion and perception processing by cognitive control. Neuroimage 37:652–662
Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D (2006) Serotonin transporter promoter gain-of-function genotypes are linked to obsessive–compulsive disorder. Am J Hum Genet 78:815–826
Irwin W, Anderle MJ, Abercrombie HC, Schaefer SM, Kalin NH, Davidson RJ (2004) Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. NeuroImage 21:674–686
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007) Failure to regulate: counterproductive recruitment of top-down prefrontal–subcortical circuitry in major depression. J Neurosci 27:8877–8884
Juckel G, Hegerl U, Molnar M, Csepe V, Karmos G (1999) Auditory evoked potentials reflect serotonergic neuronal activity—a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology 21:710–716
Kalisch R, Wiech K, Critchley HD, Dolan RJ (2006) Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. NeuroImage 30:1458–1466
Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML (2005) A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 58:495–503
Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B (2005) The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry 62:529–535
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131
Lang PJ, Bradley MM, Cuthbert BN (1999) International Affective Picture System (IAPS) Instruction Manual and Affective Ratings. Gainesville, University of Florida
Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen PH, Pine DS, Ernst M (2008) Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry 65:349–355
Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU, Chae JH, Choi IG, Ham BJ (2008) Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 32:778–785
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A (1997) The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm 104:1259–1266
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19:1233–1239
Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP (2008) Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord 111:13–20
Mayberg HS (2002) Modulating limbic–cortical circuits in depression: targets of antidepressant treatments. Semin Clin Neuropsychiatry 7:255–268
Mayberg HS (2003) Modulating dysfunctional limbic–cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207
Mitterschiffthaler MT, Ettinger U, Mehta MA, Mataix-Cols D, Williams SC (2006) Applications of functional magnetic resonance imaging in psychiatry. J Magn Reson Imaging 23:851–861
Munafo MR, Brown SM, Hariri AR (2008) Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry 63:852–857
Nakamura M, Ueno S, Sano A, Tanabe H (2000) The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry 5:32–38
Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci 9:242–249
Onoda K, Okamoto Y, Toki S, Ueda K, Shishida K, Kinoshita A, Yoshimura S, Yamashita H, Yamawaki S (2008) Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia 46:102–110
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005) 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16:331–348
Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH (2007) Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [(11) C] DASB positron emission tomography study. Biol Psychiatry 62:327–331
Ressler KJ, Mayberg HS (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10:1116–1124
Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S (2004) Limbic–frontal circuitry in major depression: a path modeling metanalysis. NeuroImage 22:409–418
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001) Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50:651–658
Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS (2002) Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 51:693–707
Simpson JR Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME (2001) Emotion-induced changes in human medial prefrontal cortex: II during the anticipatory anxiety. Proc Natl Acad Sci U S A 98:688–693
Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, Zimmer A, Wrase J, Flor H, Mann K, Braus DF, Goldman D, Heinz A (2007) Gene–gene effects on central processing of aversive stimuli. Mol Psychiatry 12:307–317
Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ, Higley JD, Goldman D, Barr CS (2007) Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol 19:977–987
Taylor SF, Phan KL, Decker LR, Liberzon I (2003) Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18:650–659
Ueda K, Okamoto Y, Okada G, Yamashita H, Hori T, Yamawaki S (2003) Brain activity during expectancy of emotional stimuli: an fMRI study. NeuroReport 14:51–55
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A (2007) Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27:684–691
Wittchen HU, Zaudig M, Fydrich T (1997) SKID. Strukturiertes klinisches Interview für DSM-IV. Achse I und II. User manual. Göttingen: Hogrefe
Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, Ellis SP, Goldman D, Mann JJ (2006) Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry 163:1588–1593
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) DFG He 2597/4-3 and 7-3 and STE 1430/2-1.
Competing interest statement
The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eva Friedel, Florian Schlagenhauf, and Philipp Sterzer contributed equally to this work.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 121 kb)
Rights and permissions
About this article
Cite this article
Friedel, E., Schlagenhauf, F., Sterzer, P. et al. 5-HTT genotype effect on prefrontal–amygdala coupling differs between major depression and controls. Psychopharmacology 205, 261–271 (2009). https://doi.org/10.1007/s00213-009-1536-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1536-1