Abstract
There has been a growing interest in resting-state brain alterations in people with social anxiety disorder. However, the evidence has been mixed and contested and further understanding of the neurobiology of this disorder may aid in informing methods to increase diagnostic accuracy and treatment targets. With this systematic review, we aimed to synthesize the findings of the neuroimaging literature on resting-state functional activity and connectivity in social anxiety disorder, and to summarize associations between brain and social anxiety symptoms to further characterize the neurobiology of the disorder. We systematically searched seven databases for empirical research studies. Thirty-five studies met the inclusion criteria, with a total of 1611 participants (795 people with social anxiety disorder and 816 controls). Studies involving resting-state seed-based functional connectivity analyses were the most common. Individuals with social anxiety disorder (vs. controls) displayed both higher and lower connectivity between frontal–amygdala and frontal–parietal regions. Frontal regions were the most consistently implicated across other analysis methods, and most associated with social anxiety symptoms. Small sample sizes and variation in the types of analyses used across studies may have contributed to the inconsistencies in the findings of this review. This review provides novel insights into established neurobiological models of social anxiety disorder and provides an update on what is known about the neurobiology of this disorder in the absence of any overt tasks (i.e., resting state). The knowledge gained from this body of research enabled us to also provide recommendations for a more standardized imaging pre-processing approach to examine resting-state brain activity and connectivity that could help advance knowledge in this field. We believe this is warranted to take the next step toward clinical translation in social anxiety disorder that may lead to better treatment outcomes by informing the identification of neurobiological targets for treatment.
Similar content being viewed by others
Introduction
Social anxiety disorder (SAD) is characterized by fear, anxiety, and/or avoidance of social situations [1]. Data from the World Mental Health Surveys suggest that SAD exists globally, with a lifetime prevalence rate of 5% in high-income regions (e.g., Australia, the USA, and the UK) [2]. Living with SAD can be debilitating, with the diagnosis being associated with significant impairments in multiple domains of functioning, most substantially impacting relationships, and social experiences. Despite its widespread prevalence in the community and the distressing nature of SAD, the diagnostic accuracy and treatment response rates remain poor in individuals with this disorder [3, 4]. Advances in neuroimaging techniques have only begun to provide important insights into the neurobiology of SAD. The most recent models of SAD [5, 6] propose that individuals with SAD (compared to controls) are characterized by dysfunctional fronto-limbic (fear) circuitry, with hyperactivity in limbic areas (i.e., amygdala, hippocampus, and parahippocampus) and hypoactivity in cognitive control areas (i.e., anterior cingulate cortex, ventral medial prefrontal cortex (PFC), dorsolateral PFC). Brühl et al. [5] added the role of medial parietal and occipital regions that are increasingly activated in those with SAD. The aforementioned reviews, however, primarily focused on interpreting the activation of different brain regions in response to overt tasks.
A recent trend in the clinical neuroimaging community is to study resting-state functional activity/connectivity paradigms. Compared to task-dependent methods, resting-state paradigms are not susceptible to the potential confounding effects of task performance and may be particularly useful in aiding our understanding of the intrinsic brain mechanisms implicated in the clinical presentation of SAD. Resting-state brain connectivity as a potential biomarker of psychiatric symptoms has been found useful in other disorders, including autism spectrum disorder [7] and major depression [8]. In SAD, there has been an increase in resting-state brain imaging studies in recent times. To our knowledge, Brühl et al. [5] was the most recent review that systematically examined resting-state studies (n = 11). However, the results were reported in combination with task-based functional connectivity findings, making it challenging to interpret a pattern of results specifically attributed to resting-state brain imaging studies. Other narrative reviews examining resting-state neuroimaging in SAD have been published since [9, 10] and show discrepancies within the findings of resting-state studies. Peterson et al. [10] examined resting-state connectivity across anxiety disorders and found that whilst there were overlap in the brain networks underlying the full range of anxiety disorders, there were variations in connectivity between limbic and cortical regions unique to each anxiety disorder, thereby warranting a thorough review of resting-state brain activity/connectivity of SAD on its own.
Due to the growing number of studies investigating resting-state neuroimaging in SAD, the current review aimed to use a systematic approach to obtain a critical appraisal and comprehensive understanding of the brain regions and networks implicated in SAD (compared to controls) at rest. A secondary aim of this review involved summarizing any known brain-behavior associations (i.e., links between brain function and symptoms) associated with SAD. Although the majority of the literature involves functional magnetic resonance imaging (fMRI) studies, we included a broad range of imaging modalities (including single-photon emission computed tomography (SPECT) and positron emission tomography (PET)) and various acquisition and analysis approaches relating to both activation and connectivity studies to be as comprehensive as possible. With this review, we hope to further our understanding of the neurobiology of this disorder that could aid in informing methods to increase diagnostic accuracy and treatment targets.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, which outlines a set of items to improve reporting of systematic reviews [11]. Full details regarding the search strategy, eligibility criteria, data extraction process, data synthesis, and quality assessment can be found in the Supplementary Information. Seven databases were searched on the 29th November 2020 and studies were included if they were published in English and measured brain function/activation at rest in a sample of participants that had a diagnosis of SAD and in a control group. Data extracted included demographic information, details regarding the method of neuroimaging acquisition and analysis, and the results. All included studies were quality checked using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, published by the National Heart, Lung and Blood Institute, to evaluate the internal validity of each included study [12]. The primary results of interest for the qualitative synthesis of findings were group differences in resting-state neural activity and connectivity.
Results
Study selection
The initial search identified 1112 possible studies. After the screening of titles, abstracts, and full-text articles and the removal of duplicates and other non-suitable studies, a final 35 studies were included in the systematic review (Fig. 1). The primary reasons for the exclusion of studies were that no participants diagnosed with SAD were involved and the data was not published in a peer-reviewed journal.
Studies were included based on the following criteria: (i) the full text was published in English, (ii) human participants were involved, (iii) brain function/activation was measured at rest, and (iv) a sample of participants with a diagnosis of SAD was compared to a control group. Reasons for exclusion are detailed in the flowchart.
Sample population
Information regarding demographic details (including age and sex), handedness, recruitment, diagnosis, and severity of participants are included in Table 1. Details regarding overlaps in samples across studies, methods of diagnosis, and medication use can be found in the Supplementary Information. For the 35 studies included, all were published between 2008 to 2020 and involved a cross-sectional design. A total of 1611 participants were included, consisting of 795 people with SAD and 816 controls. For individuals with SAD, the mean age was 26.26 (±5.48) years, and aside from one study that used a pediatric sample (results are reported separately in the Supplementary Information) [13], the remaining 34 studies used adult cohorts. The average sample size for SAD participants was 23, with a range of 7 to 53 participants.
Neuroimaging methods and analyses
Of the 35 studies included, there was a range of neuroimaging methods and analyses included. Thirty-one studies used fMRI whilst four studies used alternative imaging modalities such as SPECT and PET [14,15,16,17]. Due to difficulty integrating SPECT and PET with findings from fMRI, results from these four studies will be reported in Supplementary Table 1. For this reason, the information presented from here on will only refer to the fMRI studies (n = 31). The results from the 31 fMRI studies that used multiple neuroimaging analysis methods on their data set were considered independent of one another [18,19,20,21,22]. Information regarding the scan parameters used and the preprocessing/first-level analyses can be found in Supplementary Tables 2, 3 respectively. Of the 31 fMRI studies, the most frequently used method of analysis was seed-based/ROI-to-ROI functional connectivity analysis (n = 18), followed by the amplitude of low-frequency fluctuations (ALFF; n = 4). Other analysis methods included graph theory (n = 3), MVPA (n = 3), whole-brain analysis (a data-driven exploratory approach that seeks to identify significant correlations or activity patterns in different voxels of the brain at the size usually between 2 and 3 mm [3]; n = 2), independent component analysis (ICA; n = 2), regional homogeneity analysis (ReHo; n = 2), Granger causality analysis (GCA; n = 1), and functional connectivity density analysis (FCDA; n = 1). We summarize the findings from these studies next, however, findings involving ICA, ReHo, GCA, and FCDA can be found in the Supplementary Table 4.
Seed-based (and ROI) functional connectivity studies (n = 18)
ROI/seed-based functional connectivity analysis finds regions of the brain that are correlated with activity in a certain seed-region or the brain region of interest (ROI), with the coupling of activation (usually the Pearson’s correlation between brain regions, over the length of an fMRI scan) assumed to reflect involvement in the same underlying functional process and therefore can be interpreted as being functionally connected or correlated. Seeds are usually derived a priori, based on a hypothesis, prior results, or from statistically significant regions from other modalities such as ALFF or ReHo calculations. Four studies used seed-to-seed (ROI-to-ROI) analyses [19, 20, 23, 24], whilst the remaining 14 studies conducted seed-to-whole brain analyses. The most commonly reported seed was the amygdala (n = 11). Four studies [20, 25,26,27] reported findings of clusters of regions across brain areas and were therefore reported separately in Supplementary Table 5. Table 2 details a complete overview of the number of studies and pairings found by seed-based analyses. Overall, there were a total of 197 pairings (i.e., significant connectivity between a seed and a cluster/ROI) across 15 of these studies. The connectivity pairings most reported across studies were between frontal-amygdala regions (9 studies; 23 pairings), followed by frontal–parietal regions (7 studies; 22 pairings) and temporal-amygdala regions (6 studies; 10 pairings); see Supplementary Table 6. Of the 197 pairings, 183 pairings were positively correlated, 4 pairings from two studies were reported as negative correlations, 6 pairings across two studies reported as positively correlated in those with SAD and negatively correlated in controls, and 4 pairings across two studies were reported as negatively correlated in those with SAD and positively correlated in controls. Of the 197 pairings, 100 were found to be higher in SAD compared to controls whereas 97 showed the opposite contrast (lower in SAD compared to controls).
Positive connectivity
The majority of findings demonstrated positive connectivity between regions, with 183 pairings; see Table 2 for a full list of pairings and Fig. 2 for a visual representation of the most consistent findings. Compared to controls, those with SAD had higher connectivity between the following regions: frontal–amygdala (5 studies; 15 pairings), temporal–amygdala (4 studies; 6 pairings), and frontal–parietal (3 studies; 4 pairings). Compared to controls, those with SAD had lower connectivity between the following regions: frontal–amygdala (4 studies; 7 pairings), frontal–parietal (4 studies; 18 pairings), temporal–temporal (2 studies; 11 pairings), and frontal–temporal (2 studies; 13 pairings).
Negative connectivity
Only two studies reported higher negative connectivity in SAD compared to controls between the following regions: amygdala–lateral occipital cortex, amygdala–middle temporal gyrus, amygdala–supramarginal gyrus, and the posterior inferior temporal gyrus–inferior occipital gyrus. Both of these studies used global signal regression, a processing step that is known to induce a substantial amount of anticorrelations [28]. No studies reported lower connectivity in those with SAD compared to controls.
Mixed connectivity
Two studies reported that those with SAD (compared to controls) had higher connectivity between the amygdala–precuneus, amygdala–posterior cingulate cortex, and amygdala–left superior temporal gyrus, and lower connectivity between the amygdala–cerebellum, amygdala–anterior insula, and amygdala–supramarginal gyrus. In the aforementioned pairings, positive correlations between brain regions were reported for the SAD group and negative correlations between brain regions were reported for the control group. One study reported that those with SAD (compared to controls) had higher connectivity between the amygdala–lentiform nucleus, and lower connectivity between the amygdala–supplementary motor area, and the amygdala–right middle temporal gyrus. In all these pairings, negative correlations between brain regions were reported for the SAD group and positive correlations between brain regions were reported for the control group.
Amplitude of low-frequency fluctuations (ALFF) studies (n = 4)
The ALFF method quantifies the power of the BOLD signal is within a low-frequency range of activation (typically in the range of 0.01–0.1 Hz) that is thought to be an indirect representative of neuronal activity (e.g., cortical activity or basal ganglia activity) while containing minimal artefacts [29]. Four studies used ALFF as an analysis method [18, 30,31,32]; see Supplementary Table 7. Most consistently, three of these studies [18, 30, 32] found that individuals with SAD (vs. controls) had lower ALFF across 17 frontal regions (such as inferior, middle and superior frontal gyri, median cingulate gyrus, Rolandic operculum, precentral gyrus, prefrontal, and supplementary motor areas).
Graph theory studies (n = 3)
Graph theory is the mathematical field of network science. Graph theory quantifies the topological configuration and complexity of brain network function by delineating the local and global organization of brain networks [33]. Three resting-state brain imaging studies utilized graph theory in their methodology [19, 29, 34]; see Supplementary Table 8. Temporal (middle and inferior temporal gyrus, superior–middle temporal cortices, and hippocampus), frontal (inferior frontal cortices, middle frontal gyrus), and parietal regions (angular gyri, posterior cingulate gyrus, and supramarginal gyrus) were most implicated in the findings.
Multi-voxel pattern analysis (MVPA; n = 3)
Multi-voxel pattern analysis (MVPA) is a machine learning approach (usually based on support vector machines) that can be used to predict categories from various patterns of activation across brain voxels. Three studies used MVPA as part of their neuroimaging analysis to determine whether resting-state data distinguished between groups with and without a diagnosis of SAD [19, 35, 36]; see Supplementary Table 9. Frontal regions were most implicated in these studies in being able to distinguish between groups.
Associations between brain and dimensional measures
Of 35 studies included in the review, 17 studies reported associations between resting-state brain activity/connectivity and behavioral measures; see Supplementary Table 10. The most common behavioral outcome studied involved social anxiety symptoms across 13 studies, which all used the Liebowitz Social Anxiety Scale (LSAS; n = 13), and others additionally including the Social Phobia Scale (n = 1) or the Brief Fear of Negative Evaluation (n = 1). Of those studies assessing resting-state brain connectivity (n = 7), frontal–occipital pairings were consistently positively associated with symptoms of social anxiety (11 pairings across 1 study). Other pairings associated with symptoms of social anxiety included: amygdala–frontal (3 studies; 3 pairings positively associated, 1 pairing negatively associated), amygdala–temporal (2 studies; 3 pairings negatively associated), and frontal–temporal (1 study; 1 pairing positively associated, 1 pairing negatively associated). Other pairing combinations were found by individual studies; see Supplementary Table 10. Other studies measured general anxiety (Hamilton Anxiety Scale, n = 2; Spielberg State-Trait Anxiety Scale, n = 1), depression (Hamilton Depression Scale, n = 2; Beck Depression Inventory, n = 2), and illness duration (n = 2), and these findings are reported in the Supplementary Information.
Risk of bias
Results from the quality assessment showed consistency in the quality of studies included in this review; see Supplementary Table 11. All 35 studies stated the research question clearly, with a clearly defined study population. No studies were preregistered, and no studies provided a sample size justification or power description for the sample used. The quality check highlighted inconsistencies across studies in whether confounding variables were adjusted for statistically when examining resting-state neuroimaging between groups (SAD vs. controls). Only 14 of the 35 studies controlled for potential confounding variables in their statistical analyses, controlling for variables such as gender, age, mean framewise displacement, medication status, and education level [13, 18, 20, 22,23,24, 37,38,39,40,41].
Discussion
This systematic review aimed to obtain a comprehensive understanding of the brain regions and networks implicated in people with SAD compared to controls, focusing on resting-state multimodal neuroimaging techniques and analysis methods. It included the examination of associations between brain and dimensional measures in people with SAD. Of the 35 studies, the most common analysis approach involved seed-based analysis. Frontal regions were most implicated across studies and analysis methods and in the relationships between brain and dimensional measures. Even when excluding findings from studies that had smaller sample sizes that were uncorrected [21, 23, 40, 41], similar findings remained. From seed-based studies, the SAD group had both higher and lower positive connectivity between the amygdala and frontal regions and between the amygdala and parietal regions, and lower positive connectivity between the amygdala and temporal regions. Findings from ALFF predominantly demonstrated lower ALFF across 17 frontal regions in those with SAD compared to controls. Likewise, across other non-seed analysis methods (e.g., graph theory, MVPA), frontal regions (i.e., superior and middle frontal gyrus) were most reported throughout. The superior frontal gyrus was most commonly implicated across all fMRI studies, being reported 63 times. It was also most consistently found to be associated with social anxiety symptoms, with results showing negative and positive correlations. The middle frontal gyrus, inferior frontal gyrus, and anterior cingulate cortex were also frequently reported across studies. Other frontal regions implicated, albeit to a lesser extent, were the dorsolateral PFC, the dorsomedial PFC, the precentral gyrus, Rolandic operculum, rectal gyrus, supplementary motor area, and the orbitofrontal gyrus.
Theoretical implications
Review findings partially deviated from the two key models of SAD [5, 6]. These models posited that those with SAD had hyperactivation of the fear circuitry (consisting of the amygdala, insula, PFC, and anterior cingulate cortex) compared to controls. The Brühl et al. [5] model additionally pointed to higher activation in the cuneus, precuneus, and the posterior cingulate cortex, which were less functionally connected to other neural regions (including the fusiform gyrus, amygdala, dorsolateral/medial PFC, and the anterior cingulate cortex) in those with SAD compared to controls. Due to the lack of studies examining neural activation in resting-state neuroimaging in SAD, we cannot comment on whether findings regarding activation differed. However, the connectivity findings from this review only partially supported the Brühl et al. [5] model of connectivity between regions; in most instances, our review demonstrated that connectivity pairings are less clear cut with findings of both hyper- and hypoconnectivity; see Supplementary Table 12. Furthermore, findings of higher connectivity between the amygdala and temporal regions (as demonstrated in 6 pairings across 4 studies of this review) were unfounded by Brühl’s model.
These discrepancies may be a result of examining only resting-state neuroimaging data in the current review as opposed to the predominantly task-based neuroimaging data that informed these neurobiological models of SAD (e.g., Brühl et al. [5]). Potentially, people with SAD have certain connectivity patterns between brain regions when encountering socially- and disorder-relevant information (e.g., emotional faces) or when they are anticipating events that would typically induce social anxiety (such as public speaking or social interactions). However, in the absence of any overt tasks (i.e., resting-state), there may be no requirement for the same connectivity patterns to arise. Therefore, the Brühl et al. [5] model of SAD may be more suitable as a neurobiological model of stimuli response, rather than as an accurate model of the normal underlying neuropathology (at rest) of the disorder. Next, we provide a discussion of the main findings, reporting on the neural pairings identified by most seed-based studies in this review.
Amygdala–frontal connectivity
The most common finding was alterations in positive connectivity between the amygdala and frontal areas, with 15 pairings across 5 studies reporting higher connectivity and 7 pairings across 4 studies reporting lower connectivity in SAD compared to controls. This suggests that alterations in this pathway are a core feature of SAD, however, we note that this effect may also be partially due to the amygdala being the most commonly used seed.
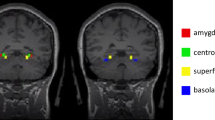
The inconsistency in findings of higher and lower connectivity between these regions in this review may be due to recent evidence in both controls and in varying clinical samples demonstrating the importance of examining subregions of the amygdala (including the centromedial, basolateral, amygdalostriatal, and superficial complex) and their connectivity patterns rather than examining the amygdala as a whole. Evidence of disturbances in the fear circuitry in only specific subregions of the amygdala has been demonstrated in clinical groups (e.g., autism spectrum disorder, post-traumatic stress disorder, major depressive disorder) and in controls [42,43,44,45]. For example, hyperconnectivity between the amygdala and the PFC was only found when looking at the centromedial complex (rather than basolateral or superficial complex) in people with autism spectrum disorder (vs. controls) [42]. In this review, one of the studies contributing to evidence of hyperconnectivity between the amygdala and PFC used amygdala subregions as seeds and found no evidence of hypoconnectivity [46]. Further research examining amygdala subregion connectivity with the PFC in those with SAD may be needed to clarify this aspect of the fear circuitry in SAD.
An alternative explanation for the inconsistent findings regarding higher and lower connectivity between the amygdala and frontal regions may be due to our grouping of frontal regions as one area. The frontal lobe is thought to be structurally and functionally divided into separate regions with different connectivity patterns, and structural and functional divisions [47]. A clearer pattern of connectivity between the amygdala and frontal regions arises when frontal regions are examined as smaller subdivisions. For example, looking specifically at the dorsomedial PFC, there were consistent reports of higher connectivity between this region and the amygdala. Notably, this is shown to be associated with increased self-directed criticism and an increased tendency to exaggerate the significance of potentially self-relevant stimuli from external threat cues in those with SAD [48, 49]. Another example of the usefulness of looking at specific frontal regions is by examining the rostral medial PFC, with consistent reports of lower connectivity between this region and the amygdala in those with SAD compared to controls. Previous research suggests that a decrease in connectivity between these regions is associated with increased social interactional anxiety and decreases in emotion regulation [50]. These findings highlight the importance of considering the various smaller individual subregions of the frontal lobe when examining and interpreting findings, given the number of structurally and functionally different regions that exist. However, discrepancies in how divisions are defined and analyzed could lead to difficulty in the synthesis of findings across studies.
Parietal–frontal connectivity
Eighteen pairings across four studies found evidence of lower connectivity and four pairings across three studies found evidence of higher connectivity between parietal and frontal regions in those with SAD compared to controls. The most commonly reported pairings were lower connectivity between the bilateral posterior cingulate gyrus and the bilateral superior frontal gyrus, the bilateral precuneus and the right superior frontal gyrus, and the bilateral precuneus and the bilateral gyrus rectus.
Amygdala–temporal connectivity
Six pairings across four studies found higher connectivity between these regions in those with SAD compared to controls, and only one pairing from a separate study demonstrating evidence of lower connectivity. The most commonly reported pairings were between the left amygdala and the bilateral fusiform gyrus, and the bilateral amygdala and the bilateral parahippocampal gyrus.
Functional interpretations
The main connectivity pairings and regions identified in this review have been investigated in previous literature, with tentative interpretations being made of their function. In anxiety disorders, including SAD, it has been thought that the connection between the amygdala and PFC plays a significant role in controlling attention to salient stimuli and emotion regulation with the presence of disturbed top-down control (inability of the PFC to inhibit the amygdala response) or increased bottom-up processes (hypersensitive amygdala leading to increased activity in the PFC) in maintaining anxiety [51]. The precuneus and posterior cingulate gyrus are known to be important hubs of the default mode network and are involved in self-referential processing, the integration of present and past information, and in allowing for an observer perspective of social interactions [52, 53]. The superior frontal gyrus is thought to be involved in the initiation of novel responses and is activated during shifts of attention [54, 55]. Therefore, altered connectivity between these regions and frontal areas may be linked to impairment in socio-cognitive processes that are seen in SAD. The fusiform gyrus is implicated in facial visual processing, and hyperconnectivity between this region and the amygdala may reflect constant hypervigilance to social threats (e.g., angry faces) in people with SAD. This is consistent with Wong and Rapee [56] recent model of SAD in which they proposed that the constant alertness to social-evaluative threats in the environment serves as a maintenance factor of this disorder. Hyperactivity of the parahippocampal region has been interpreted as being indicative of disruptions to the process of assigning accurate saliency value to a stimulus [57]. Therefore, hyperconnectivity between the amygdala and this region may be contributing to dysfunction in post-event processing that also plays a role in the maintenance of this disorder [56].
Limitations and future directions
Heterogeneity in the sequence parameters and low power are likely to be significantly contributing to the inconsistencies in the findings of this review, and critically needs to be addressed in future studies. Many of the included studies involved a small sample size of participants with SAD (average n = 23; minimum n = 7; maximum n = 53). No study justified their sample size or included a power calculation. The use of small sample sizes may have contributed to the inconsistent findings [58] and/or inevitably led to an inflated risk of false positives due to the high number of variables in brain images [59]. Supplementary Fig. 1 demonstrates an increased proportion of uncorrected statistical between-group findings in the studies with smaller sample sizes compared to those with larger sample sizes. Furthermore, previous research shows that sample sizes of 20–30 subjects are likely insufficient to detect reliable relationships between brain and behavior measures that are reproducible [60]. It is therefore difficult to confidently conclude if the aforementioned resting-state neuroimaging findings are directly linked to subjective self-reported experiences of social anxiety, and replication is needed in future research. The overlap in participants across four datasets was also another limitation, as it is possible that findings from these samples were inflated due to their recurrent use. Furthermore, there is evidence that physiological confounds, such as differences in respiration and state anxiety, during the scanning process may result in changes in cerebral blood flow and ultimately influence the results [61]. For example, there is evidence of a causal role of the amygdala in respiration [62]. Whilst the potential confounding effects of state anxiety on fMRI were controlled for by most studies through the use of the State Anxiety Inventory Scale, no studies controlled for the effects of respiration on the findings. Additionally, other important confounders (e.g., medication use, psychiatric comorbidity) and the severity of the disorder (measured by the LSAS) varied widely across samples used and may also have contributed to the variation in the findings observed.
The integration of findings from studies in this review was also hindered by heterogeneity in scanning methods and in the analysis techniques used (see Supplementary Tables 2, 3). It can be problematic to integrate results across differing fMRI acquisition settings due to the potential effects that these settings have on results. For example, differences in repetition time can impact on the discrimination between activated and nonactivated brain tissue [63]. However most notably, the scan time varied between studies (ranging from 200 to 471 s) and this likely had an impact on the results. Noble et al. [64] demonstrated that resting-state scans with a duration of 300 s or less were associated with poor test-retest reliability of connectivity. It has been suggested that 10 min or more of resting-state data is needed for good intra- and intersession reliability [65], and a higher image sampling rate is unlikely to make up for shorter scan duration [66]. We support the recommendation of longer scan times to increase the amount of data per subject, which also allows researchers to investigate dynamical brain properties and state changes in functional imaging data [67]. We were unable to draw inference from non-seed analyses given the limited number of studies using these methods (e.g., graph theory, MVPA) to date. Perhaps in future reviews this may be possible and is needed if we are to fully understand the biological mechanisms underlying SAD.
We find it encouraging that the field of resting-state fMRI is moving onto a common data processing framework called fMRIprep [68]. Streamlined pre-processing frameworks lead to a methodological consistency between studies which will make it easier for scientists to replicate prior research. Differences in the management of common artefacts (such as head motion or breathing effects), the application of global signal regression, and motion thresholds, are susceptible to false-positive results and need to be critically assessed when interpreting findings. Image registration, smoothing, and filtering of resting-state fMRI data also matter in terms of how brain regions are allocated, and differences in these may also influence the interpretation of the findings [69].
Given the cross-sectional design of all studies included in this systematic review, it is currently not possible to derive any etiological theories of SAD as causation cannot be implied (i.e., do neural alterations cause SAD?). Whilst having a snapshot of the neurobiology of SAD in a pre-diagnosed sample is helpful, other brain areas and connections may play a role in the development of this disorder. Longitudinal study designs with sufficient samples from adolescence into adulthood may help increase our understanding of whether neural alterations are unique to or contribute to the development and maintenance of this disorder.
Conclusions
This review suggests that the neurobiology of SAD may differ from previously proposed models that were derived predominantly from the synthesis of task-based neuroimaging studies. In the absence of a task (i.e., resting-state), the literature shows that on average, those with SAD have aberrant connectivity between the amygdala and temporal, parietal, and frontal regions. Additionally, there appear to be differences in the activity of frontal regions in those with SAD compared to controls as shown by a range of neuroimaging analyses. Frontal regions were also found to have significant associations with social anxiety severity. Even amongst the most consistent findings demonstrated across studies, there remained great variation in the direction of activity (hypo- vs. hyperactivity) within regions and connectivity (SAD > HC vs. HC > SAD) between regions. The wide range of analysis methods and seeds used for functional connectivity analyses may have contributed to the presence of mixed findings and led to difficulty in synthesizing results across studies to form strong conclusions regarding the neurobiology of those with SAD at rest. Therefore, this review has led us to provide recommendations to improve methodology to ensure greater rigor for future studies. Further research using studies with larger sample sizes of clinical participants and more consistent analysis methods is necessary to provide further clarification of the resting-state neurobiology of SAD.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Stein DJ, Lim CCW, Roest AM, de Jonge P, Aguilar-Gaxiola S, Al-Hamzawi A, et al. The cross-national epidemiology of social anxiety disorder: data from the World Mental Health Survey Initiative. BMC Med. 2017;15:143.
Chapdelaine A, Carrier J-D, Fournier L, Duhoux A, Roberge P. Treatment adequacy for social anxiety disorder in primary care patients. PloS ONE. 2018;13:e0206357.
Vermani M, Marcus M, Katzman MA. Rates of detection of mood and anxiety disorders in primary care: a descriptive, cross-sectional study. Prim Care Companion CNS Disord. 2011;13:PCC.10m01013.
Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–80.
Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88.
Hull JV, Dokovna LB, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. Resting-state functional connectivity in autism spectrum disorders: a review. Front Psychiatry. 2017;7:205.
Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12.
Kim Y-K, Yoon H-K. Common and distinct brain networks underlying panic and social anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:115–22.
Peterson A, Thome J, Frewen P, Lanius RA. Resting-state neuroimaging studies: a new way of identifying differences and similarities among the anxiety disorders? Can J Psychiatry. 2014;59:294–300.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies [Internet]. Bethesda (MD): National Heart, Lung, and Blood Institute. 2014 [cited 2020 Dec 01]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
Dorfman J, Benson B, Farber M, Pine D, Ernst M. Altered striatal intrinsic functional connectivity in pediatric anxiety. Neuropsychologia. 2016;85:159–68.
Warwick JM, Carey P, Jordaan GP, Dupont P, Stein DJ. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1251–6.
Evans KC, Simon NM, Dougherty DD, Hoge EA, Worthington JJ, Chow C, et al. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2009;34:390–8.
Doruyter A, Dupont P, Taljaard L, Stein DJ, Lochner C, Warwick JM. Resting regional brain metabolism in social anxiety disorder and the effect of moclobemide therapy. Metab Brain Dis. 2018;33:569–81.
Doruyter A, Lochner C, Jordaan GP, Stein DJ, Dupont P, Warwick JM. Resting functional connectivity in social anxiety disorder and the effect of pharmacotherapy. Psychiatry Res Neuroimaging. 2016;251:34–44.
Yuan C, Zhu H, Ren Z, Yuan M, Gao M, Zhang Y, et al. Precuneus-related regional and network functional deficits in social anxiety disorder: a resting-state functional MRI study. Compr Psychiatry. 2018;82:22–9.
Zhu H, Qiu C, Meng Y, Yuan M, Zhang Y, Ren Z, et al. Altered topological properties of brain networks in social anxiety disorder: a resting-state functional MRI Study. Sci Rep. 2017;7:43089.
Cui Q, Vanman EJ, Long Z, Pang Y, Chen Y, Wang Y, et al. Social anxiety disorder exhibit impaired networks involved in self and theory of mind processing. Soc Cogn Affect Neurosci. 2017;12:1284–95.
Geiger MJ, Domschke K, Ipser J, Hattingh C, Baldwin DS, Lochner C, et al. Altered executive control network resting-state connectivity in social anxiety disorder. World J Biol Psychiatry. 2016;17:47–57.
Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. Plos ONE. 2010;5:e15238.
Rabany L, Diefenbach GJ, Bragdon LB, Pittman BP, Zertuche L, Tolin DF, et al. Resting-state functional connectivity in generalized anxiety disorder and social anxiety disorder: evidence for a dimensional approach. Brain Connect. 2017;7:289–98.
Yang X, Liu J, Meng Y, Xia M, Cui Z, Wu X, et al. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 2019;190:213–23.
Choi S-H, Shin J-E, Ku J, Kim J-J. Looking at the self in front of others: Neural correlates of attentional bias in social anxiety. J Psychiatr Res. 2016;75:31–40.
Ergul C, Ulasoglu-Yildiz C, Kurt E, Koyuncu A, Kicik A, Demiralp T, et al. Intrinsic functional connectivity in social anxiety disorder with and without comorbid attention deficit hyperactivity disorder. Brain Res. 2019;1722:146364.
Manning J, Reynolds G, Saygin ZM, Hofmann SG, Pollack M, Gabrieli JDE, et al. Altered resting-state functional connectivity of the frontal-striatal reward system in social anxiety disorder. Plos ONE. 2015;10:e0125286.
Murphy K, Fox MD. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage. 2017;154:169–73.
Yang H, Long X-Y, Yang Y, Yan H, Zhu C-Z, Zhou X-P, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36:144–52.
Qiu C, Feng Y, Meng Y, Liao W, Huang X, Lui S, et al. Analysis of altered baseline brain activity in drug-naive adult patients with social anxiety disorder using resting-state functional MRI. Psychiatry Investig. 2015;12:372–80.
Yuan M, Zhu H, Qiu C, Meng Y, Zhang Y, Ren Z, et al. Altered regional and integrated resting-state brain activity in general social anxiety disorder patients before and after group cognitive behavior therapy. Psychiatry Res. 2018;272:30–7.
Zhang Y, Zhu C, Chen H, Duan X, Lu F, Li M, et al. Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder. J Affect Disord. 2015;174:329–35.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Yun JY, Kim JC, Ku J, Shin JE, Kim JJ, Choi SH. The left middle temporal gyrus in the middle of an impaired social-affective communication network in social anxiety disorder. J Affect Disord. 2017;214:53–9.
Liu F, Guo WB, Fouche JP, Wang YF, Wang WQ, Ding JR, et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct Funct. 2015;220:101–15.
Zhang W, Yang X, Lui S, Meng Y, Yao L, Xiao Y, et al. Diagnostic prediction for social anxiety disorder via multivariate pattern analysis of the regional homogeneity. Biomed Res Int. 2015;2015:763965.
Liu F, Zhu C, Wang Y, Guo W, Li M, Wang W, et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol. 2015;126:1711–6.
Yuan ML, Meng YJ, Zhang Y, Nie XJ, Ren ZJ, Zhu HR, et al. Cerebellar neural circuits involving executive control network predict response to group cognitive behavior therapy in social anxiety disorder. Cerebellum. 2017;16:673–82.
Jung YH, Shin JE, Lee YI, Jang JH, Jo HJ, Choi SH. Altered amygdala resting-state functional connectivity and hemispheric asymmetry in patients with social anxiety disorder. front psychiatry. 2018;9:164.
Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage. 2011;56:881–9.
Pannekoek JN, Veer IM, van Tol M-J, van der Werff SJA, Demenescu LR, Aleman A, et al. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. Eur Neuropsychopharmacol. 2013;23:186–95.
Kleinhans NM, Reiter MA, Neuhaus E, Pauley G, Martin N, Dager S, et al. Subregional differences in intrinsic amygdala hyperconnectivity and hypoconnectivity in autism spectrum disorder. Autism Res. 2016;9:760–72.
Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. NeuroImage. 2009;45:614–26.
Tang S, Li H, Lu L, Wang Y, Zhang L, Hu X, et al. Anomalous functional connectivity of amygdala subregional networks in major depressive disorder. Depression Anxiety. 2019;36:712–22.
Yuan M, Pantazatos SP, Zhu H, Li Y, Miller JM, Rubin-Falcone H, et al. Altered amygdala subregion-related circuits in treatment-naïve post-traumatic stress disorder comorbid with major depressive disorder. Eur Neuropsychopharmacol. 2019;29:1092–101.
Yoon H-J, Kim JS, Shin Y-B, Choi S-H, Lee S-K, Kim J-J. Neural activity during self-referential working memory and the underlying role of the amygdala in social anxiety disorder. Neurosci Lett. 2016;627:139–47.
Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Ann NY Acad Sci. 1995;769:191–212.
Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: Evidence for separate disorders. Am J Psychiatry. 2008;165:1193–202.
Yuan ML, Zhu HR, Qiu CJ, Meng YJ, Zhang Y, Shang J, et al. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. Bmc Psychiatry. 2016;16:198.
Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, et al. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39:2061–9.
Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16.
Petrini K, Piwek L, Crabbe F, Pollick FE, Garrod S. Look at those two!: the precuneus role in unattended third-person perspective of social interactions. Hum Brain Mapp. 2014;35:5190–203.
Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage. 2011;55:225–32.
Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage. 1999;10:193–9.
Peraud A, Meschede M, Eisner W, Ilmberger J, Reulen H-J. Surgical resection of grade II astrocytomas in the superior frontal gyrus. Neurosurgery. 2002;50:966–77.
Wong QJJ, Rapee RM. The aetiology and maintenance of social anxiety disorder: a synthesis of complimentary theoretical models and formulation of a new integrated model. J Affect Disord. 2016;203:84–100.
Binelli C, Subirà S, Batalla A, Muñiz A, Sugranyés G, Crippa JA, et al. Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: a systematic review and voxel-based meta-analysis of functional resonance imaging studies. Neuropsychologia. 2014;64:205–17.
Blackford JU. Leveraging statistical methods to improve validity and reproducibility of research findings. JAMA Psychiatry. 2017;74:119–20.
Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113:7900–5.
Grady CL, Rieck JR, Nichol D, Rodrigue KM, Kennedy KM. Influence of sample size and analytic approach on stability and interpretation of brain-behavior correlations in task-related fMRI data. Human Brain Mapping. 2021;42:204–19.
Giardino ND, Friedman SD, Dager SR. Anxiety, respiration, and cerebral blood flow: implications for functional brain imaging. Compr Psychiatry. 2007;48:103–12.
Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, et al. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol. 2018;83:460–71.
Constable RT, Spencer DD. Repetition time in echo planar functional MRI. Magn Reson Med. 2001;46:748–55.
Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb Cortex. 2017;27:5415–29.
Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–8.
Airan RD, Vogelstein JT, Pillai JJ, Caffo B, Pekar JJ, Sair HI. Factors affecting characterization and localization of interindividual differences in functional connectivity using MRI. Hum Brain Mapp. 2016;37:1986–97.
Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–78.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Weinberger DR, Radulescu E. Finding the elusive psychiatric “Lesion” with 21st-century neuroanatomy: a note of caution. Am J Psychiatry. 2016;173:27–33.
Anteraper SA, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4:81–90.
Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, et al. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging. 2011;29:701–11.
Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage. 2010;52:1549.
Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JEC, et al. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–77.
Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala–frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depression Anxiety. 2013;30:234–41.
Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, et al. Regional homogeneity changes in social anxiety disorder: a resting-state fMRI study. Psychiatry Res Neuroimaging. 2011;194:47–53.
Acknowledgements
This work was supported by the Australian Government Research Training Program Scholarship (SM).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mizzi, S., Pedersen, M., Lorenzetti, V. et al. Resting-state neuroimaging in social anxiety disorder: a systematic review. Mol Psychiatry 27, 164–179 (2022). https://doi.org/10.1038/s41380-021-01154-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01154-6
- Springer Nature Limited
This article is cited by
-
Resting-state amygdala subregion and precuneus connectivity provide evidence for a dimensional approach to studying social anxiety disorder
Translational Psychiatry (2024)
-
Dissecting task-based fMRI activity using normative modelling: an application to the Emotional Face Matching Task
Communications Biology (2024)
-
A brain model of altered self-appraisal in social anxiety disorder
Translational Psychiatry (2023)
-
Identification of spatial patterns with maximum association between power of resting state neural oscillations and trait anxiety
Neural Computing and Applications (2023)
-
Structural and functional deficits and couplings in the cortico-striato-thalamo-cerebellar circuitry in social anxiety disorder
Translational Psychiatry (2022)