Abstract
Tissue browning is a major problem in tissue culturing of woody plants, especially for Ficus religiosa which occurs by the accumulation and oxidation of phenolic compounds. This study aimed to determine the effect of different concentrations of sodium nitroprusside on the appearance of callus browning from leaf explants. The results indicate that callus browning was significantly reduced by supplementation of sodium nitroprusside to the MS medium and supplemented with 2.26 μM of 2,4-dichlorophenoxyacetic acid and 0.22 μM of 6-benzyl amino purine. The accumulation of hydrogen peroxide and phenolic compounds in the callus tissues decreased at the 50 μM concentration of sodium nitroprusside. Although catalase and peroxidase activities decreased at the 50 μM concentration, the activity of superoxide dismutase and polyphenol oxidases, as well as proline content, increased exponentially. Sodium nitroprusside could be useful for the formation of non-embryogenic callus with high levels of metabolic activity for the production and isolation of secondary metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ficus religiosa L. provides a traditional remedy for various diseases because of its broad range of secondary metabolites such as lupin 3-one, bergenin, lanosterol, methyl oleanolate, stigmasterol, amides, caffeic acid, flavonoids, and β-sitosterol (Singh et al. 2011; Salmi and Hesami 2016). One of the most useful techniques for the production of secondary metabolites of Ficus religiosa is tissue culturing (Hesami and Daneshvar 2018a; Hesami et al. 2018c). The primary step during the production of secondary metabolites is callus culture that is divided into two specific types: embryogenic or non-embryogenic (Hesami et al. 2017b, 2018b; Niazian et al. 2017). The most appropriate is a non-embryogenic callus culture consisting of the homogeneous bulk of dedifferentiated cells (Jedinák et al. 2004). Extreme callus browning, an interfering phenomenon in in vitro cultures, is usually observed during culturing of F. religiosa (Hesami and Daneshvar 2018b). Callus browning is one of the most significant problems in the production and isolation of secondary metabolites for this valuable medicinal plant.
Callus browning can inhibit plant growth and reduce cell proliferation (Ling et al. 2007; Chugh et al. 2009;Mondal et al. 2014). Yoruk and Marshall (2003) indicated that there are many elements responsible for callus browning in the phenolic oxidation process such as phenolic compounds, the action and presence of reactive oxygen species (ROS), and oxidative enzymes. One of the common alarming signals for changing the expression of genes and metabolism against stress is the accumulation of hydrogen peroxide which may limit plant growth and lead to cell death (Foyer et al. 1994). Generally, ROS can be produced during the normal processes of plant metabolism such as respiration and photosynthesis. The ROS level can also be increased exponentially under various abiotic stresses such as drought, salinity, UV light, and cold (Sharma et al. 2012; Tripathy and Oelmüller 2012). Furthermore, some mechanical damages during culturing, especially in the explant preparation step such as wounding, are important factors for increased ROS that lead to callus browning (Wang et al. 2011). There are various cellular structures and organelles responsible for producing and scavenging ROS under stress, such as plasmalemma, chloroplasts, the endoplasmic reticulum (ER), mitochondria, and cell walls (Gill and Tuteja 2010). Oxidative damage in many cellular components, for example, plasmalemma disintegration, breakage of the nuclear envelope, and chloroplast deformation, results in the accumulation of ROS that leads to peroxidation of lipids, hydroxylation of nucleic acids, and oxidation of proteins (Mittler 2002; Gill and Tuteja 2010). Excessive accumulation of ROS accompanied with the disorganization of organelles, results in limiting plant growth and finally cell death (Kratsch and Wise 2000; Wi et al. 2005; Wahid et al. 2007; Sharma et al. 2012) that can be demonstrated by the browning of callus.
In the study by Liu and Chen (2010), it became clear that during callus formation, the production of various types of ROS such as superoxide anions (O2·), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH) increased significantly. Oxidative stress, which is shown by excess production of ROS, has a negative influence on plant growth and development (de Pinto and De Gara 2004; Pitzschke et al. 2009). However, there are many types of oxidative enzymes involved in enzymatic browning such as polyphenol oxidase (PPO), peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) (Xu et al. 2005; Yingsanga et al. 2008).
Sodium nitroprusside (SNP), by releasing nitric oxide (NO), a ubiquitous bioactive molecule, is a highly reactive gas that serves as an important molecule in signal transduction in plants under stress (Arasimowicz and Floryszak-Wieczorek 2007). The effect of nitric oxide on various types of cells reveals that this molecule plays a significant role as an antioxidant (Qiao and Fan 2008). Sodium nitroprusside, pH-dependent, releases nitric oxide which reduces senescence and induces plant growth and development (Kolbert et al. 2008). This valuable molecule is a new member of plant growth regulators (Jimenez-Quesada et al. 2017) that might scavenge reactive oxygen species (Laspina et al. 2005). Furthermore, the synthesis of nitric oxide was promoted by cytokinins in cell culturing of parsley, rockcress, and tobacco (Tun et al. 2001). Recently, nitric oxide has been used for developing plant tissue culture protocols (Rico-Lemus and Rodríguez-Garay 2014). Kalra and Babbar (2010) reported that nitric oxide could enhance the regeneration response by increasing the number of meristems, and suggested that it could regulate gene expression related to the differentiation of meristems. Sarropoulou and Maloupa (2017) found that nitric oxide could exert a powerful impact on cell division and could be involved in shoot organogenesis and proliferation. Han et al. (2009) and Sarropoulou et al. (2014) showed that in vitro shoot proliferation as well as root formation of plantlets were significantly promoted by applying SNP to the Murashige and Skoog (MS) medium for Chinese crabapple and cherry rootstocks. Xu et al. (2009) reported that nitric oxide exerted a strong impact on callus induction and browning of Chinese yam.
Nitric oxide promotes plant tolerance against stress by reducing the transportation of Na+ from root to shoots and the neutralization of the negative effects of oxidative damage in membranes (Guo et al. 2009; Corpas and Barroso 2017). Based on Sarropoulou et al. (2016), the positive effects of sodium nitroprusside on growth of cherry was determined under in vitro conditions. The resistance of Pinus eldarica Ten. to oxidative damage and the improvement of its growth traits increased by using sodium nitroprusside and salicylic acid (Zamani et al. 2014). In another study, Uchida et al. (2002) demonstrated that H2O2 and nitric oxide serve as significant signaling molecules for enhancing tolerance against abiotic stress in rice. Nitric oxide can improve tolerance against oxidative stress in Lycopersicon esculentum Mill. by increasing the activity of antioxidant enzymes (Hayat et al. 2012). Nitric oxide might serve as an antioxidant for reducing toxicity promoted by H2O2 (Laspina et al. 2005). As a result, this led us to study the effect of sodium nitroprusside (SNP) in controlling the browning of callus.
The aim of this study was to establish and develop appropriate and optimal protocols for the callus induction of F. religiosa. No previous study reports on the effect of sodium nitroprusside on callus browning of F. religiosa. This study focused on the effect of different concentrations of sodium nitroprusside on callus browning from the leaf explants of F. religiosa.
Materials and methods
Plant materials and sterilization
The fruits were collected from 45 to 50-year-old F. religiosa trees on the campus of the Ramin Agriculture and Natural Resources University, Khuzestan, Iran, washed with tap water for 30 min and washed again after cleaning with a liquid soap solution. Further surface sterilization was applied in a laminar air flow cabinet. The seeds were surface sterilized with 70% aqueous ethanol for 10 s, dipped in 10% (v/v) sodium hypochlorite (NaClO) solution for 5 min, and then washed three times in sterilized distilled water. The seeds were inoculated on one-tenth strength MS medium, germinated for 4 weeks, and the leaf explants used as a source of explants.
Media and culture condition
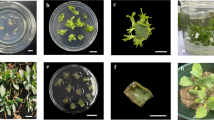
To determine the effect of sodium nitroprusside on callus browning, 5-mm leaf segments (abaxial side) from 4-week-old in vitro grown seedlings (Fig. 1a) were inoculated on MS basal medium containing 0.6% (w/v) agar (Duchefa Biochemie, Netherlands), 3% (w/v) sucrose, as well as 2.26 μM 2,4-D and 0.22 μM BAP. The pH was adjusted to 5.8 ± 0.2. The medium was autoclaved at 121 °C for 15 min at 15 psi. Subsequently, sodium nitroprusside, at different levels (10.0, 20.0, 30.0, 40.0, and 50.0 μM), was added to the basal medium via 0.2 μm filter, and distributed into 200 ml jars containing 15 ml of culture medium. All cultures were kept under 16-h light and 8-h dark photoperiod at (25 ± 2°C) with a light intensity of 45 μmol m−2 s−1 from cool white fluorescent lights. Calluses were subcultured every 3 weeks on the same composition of fresh MS medium.
Callus formation from seedling- derived leaf segments of Ficus religiosa; a 4-week-old seedling from in vitro seed germination; b brown callus on MS medium supplemented with 2.26 μM 2,4-D and 0.22 μM BAP after 8 weeks; c yellow and friable callus on MS medium containing 2.26 μM 2,4-D and 0.22 μM BAP along with 50 μM SNP after 8 weeks; Bar = 0.5 cm
Determination of H2O2 levels, activities of antioxidative enzymes, accumulations of proline, and total phenolic contents
Eight weeks after culturing, H2O2 levels, accumulations of proline in callus tissues, and the phenolic contents were evaluated based on Velikova et al. (2000), Errabii et al. (2007), and Park et al. (2006), respectively. Activities of antioxidative enzymes such as SOD (Beauchamp and Fridovich 1971), CAT (Cakmak and Horst 1991), POD (Nickel and Cunningham 1969), and PPO (Ramamoorthy et al. 2002) were measured accordingly.
Statistical analyses
The experiments were carried out in a completely randomized design of 10 replicates per treatment and each treatment repeated in three sets. The data were analyzed by Analysis of Variance (ANOVA) followed by Duncan’s multiple range test (Duncan 1955) at P < 0.05. Data analysis used SAS version 9.3.
Results and discussion
One of the most significant problems during the in vitro culturing of F. religiosa is callus browning which is associated with the overproduction of phenolic compounds and H2O2 in the tissues.
Since various phenolic compounds exist in F. religiosa which have a pharmaceutical value (Hesami et al. 2017a), the callus culture can be useful for the production and isolation of secondary metabolites (Singh et al. 2011). Thus the callus browning caused by the oxidation of phenolic compounds not only leads to plant death but also significantly reduces the production of phenolic compounds by limiting its biosynthetic capacity (Ko et al. 2009). One of the most important elements are the phenolic compounds because they serve as substrates for oxidative enzymes. Based on our results, the total phenolic content in the control callus was significantly higher than in the H2O2 treatments (Figs. 1, 2). By increasing the concentration of sodium nitroprusside, the level of browning decreased significantly; 50 μM sodium nitroprusside was the most effective concentration (Fig. 2). Furthermore, the yellowish friable callus was also obtained at this concentration. However, the over-production of phenolic compounds negatively influenced callus growth and high amounts of phenolic compounds resulted in tissue browning. This has also been reported for Cicer arietinum L. (Naz et al. 2008) as well as for Pistacia vera L. (Leng et al. 2009). According to a study by Xu et al. (2009), sodium nitroprusside was used completely for reducing or inhibiting oxidative browning in callus cultures of Dioscorea opposita Thunb. Nitric oxide also can scavenge the production of oxygen radicals where the tissue is wounded, and one of the important roles of nitric oxide in plants is to act as a protector against oxygen radicals (Hesami et al. 2018a).
Polyphenol oxidase (PPO) is an important defense enzyme against oxidative stress in various plant species and is located in the thylakoid membrane of the chloroplasts (Mayer 2006). The major role of PPO is in the enzymatic oxidation of phenolic compounds which result in necrosis and death of explants (Mayer 2006). Where PPO acts as a catalyzer in the oxidation of phenolic compounds to form quinone compounds, cell browning occurs (Dehon et al. 2002). Afterwards, quinones can polymerize to produce brown pigments called melanins. Phenols can be converted into quinones in the presence of metal ions. Nitric oxide can convert compounds by chelating metal ions to react with phenols to prevent the changing of phenols to quinones (Flora 2009). According to our study, by increasing the concentration of sodium nitroprusside, PPO activity decreased significantly in comparison with the control treatment (Fig. 3). In the medium with 50 μM sodium nitroprusside, the maximum decreased activity occurred (Fig. 3). Thus, it can be interpreted that nitric oxide has a special advantage for improving plant growth in in vitro conditions as well as for managing problems occurring by phenolic compounds. Nitric oxide can be helpful for maintaining the biosynthetic potential of the callus and for leading to the efficient production of phenolic compounds useful in medicine.
Based on our results, changes in the concentration of sodium nitroprusside can affect the browning of callus. Five days after culturing plants in the callus medium, the surfaces of explants turned brown. At the 50 μM sodium nitroprusside level, callus browning was suppressed significantly. It appears that browning is closely associated with the production of H2O2 in plant cells. The next step of this study was to evaluate the effects of sodium nitroprusside on the over accumulation of H2O2 in explants. Our results indicate that over accumulation was reduced significantly by increasing the levels of sodium nitroprusside (Fig. 4). The accumulation of H2O2 was highest in the medium without sodium nitroprusside, but significantly decreased with increase in the level of sodium nitroprusside from 10 to 50 μM (Fig. 4). Foyer et al. (1994) suggested the production of H2O2 might act as a common alarm signal leading to modification of gene expression and metabolism. Consequently, if nitric oxide reacts with H2O2, this could potentially abrogate hydrogen peroxide signaling (Neill et al. 2003). On the other hand, the overproduction of H2O2 results in inhibiting plant growth and in cell death. Nitric oxide might serve as an antioxidant for reducing toxicity promoted by H2O2 (Laspina et al. 2005). A correlation between H2O2, NO, and antioxidant levels has also been demonstrated by de Pinto et al. (2002). In tobacco BY-2 cells, neither NO nor H2O2 alone at low concentrations had any effect on programmed cell death (PCD) or on the activity of phenylalanine ammonia-lyase (PAL). However, treatment with both H2O2 and NO together induced a substantial increase in cell death with characteristics of PCD, as well as PAL activity. Moreover, this treatment also caused an increase in the activities of enzymes reducing ascorbate and glutathione (de Pinto et al. 2002), implying that both H2O2 and NO regulate cellular antioxidant levels to effect PCD, at least in some systems (Neill et al. 2003).
The antioxidative systems in plants are divided into two distinct parts; non-enzymatic antioxidants such as proline which is detoxified ROS, and enzyme antioxidants such as catalase (CAT) and superoxide dismutase (SOD) which convert ROS into water, oxygen, and hydrogen peroxide. This antioxidant system can scavenge various types of ROS and protect cells and tissues against oxidative stress (Corpas and Barroso 2017). In the present study, SOD activity increased in the 50 μM SNP concentration (Fig. 5) but CAT (Fig. 6) and POD (Fig. 7) activities decreased in this treatment. Proline is one of the major non-enzymatic antioxidants in plants. In the present study, proline content increased from 10 to 50 μM concentrations of sodium nitroprusside, i.e., the highest level was at 50 μM SNP (Fig. 8). Superoxide dismutase converts O2·− to H2O2 and O2 in the chloroplasts, mitochondria, and cytosol (Xu et al. 2009). The high activity of superoxide dismutase may protect callus tissues against oxidative stress. Xu et al. (2009) indicated that 40 μM sodium nitroprusside significantly increased the activity of superoxide dismutase in Dioscorea opposita. Catalase (CAT) is a key enzyme that converts H2O2 to H2O and O2, and peroxidase (POD) is an important component of the enzymatic antioxidant system. The activities of CAT and POD decreased at the 10–50 μM concentration of sodium nitroprusside. Our results are in line with those of Corpas and Barroso (2017) who indicated that nitric oxide might inhibit CAT and POD activities. Parani et al. (2004) reported that the gene expression of POD (At2g37130) down-regulated at 100 μM of sodium nitroprusside in Arabidopsis. In general, sodium nitroprusside decreased CAT and POD activities, while proline levels increased significantly. Proline, as the major non-enzymatic defense system, could be responsible for detoxifying ROS (reactive oxygen species) produced under biotic and abiotic stresses. Furthermore, the accumulation of proline could change the accumulation of various transcripts related to gene expression and cell division control (Maggio et al. 2002). Thus, proline accumulation along with sodium nitroprusside (SNP) induces cell proliferation and enhances the antioxidative activities of plants in tissue cultures.
Conclusion
Ficus religiosa is an important medicinal plant owing to its wide range of secondary metabolites. Therefore, callus culturing of this species would be useful for mass production of secondary metabolites. However, tissue browning is a major problem during culturing of this plant as it is with other woody plants. Our results indicate that sodium nitroprusside (SNP) has broad supplementation in culturing of F. religiosa where browning limits callus induction. SNP also could be helpful for the formation of non-embryogenic callus with high levels of metabolic activity for the production and isolation of secondary metabolites.
References
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chugh S, Guha S, Rao IU (2009) Micropropagation of orchids: a review on the potential of different explants. Sci Hortic 122:507–520
Corpas FJ, Barroso JB (2017) Lead-induced stress, which triggers the production of nitric oxide (NO) and superoxide anion (O2·−) in Arabidopsis peroxisomes, affects catalase activity. Nitric Oxide 68:103–110
de Pinto MC, De Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55:2559–2569
de Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright-Yellow 2 cells. Plant Physiol 130:698–708
Dehon L, Macheix J, Durand M (2002) Involvement of peroxidases in the formation of the brown coloration of heartwood in Juglans nigra. J Exp Bot 53:303–311
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Errabii T, Gandonou CB, Essalmani H, Abrini J, Idaomar M, Senhaji NS (2007) Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiol Plant 29:95
Flora SJ (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev 2:191–206
Foyer C, Descourvieres P, Kunert K (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Guo Y, Tian Z, Yan D, Zhang J, Qin P (2009) Effects of nitric oxide on salt stress tolerance in Kosteletzkya virginica. Life Sci J 6:67–75
Han X, Yang H, Duan K, Zhang X, Zhao H, You S, Jiang Q (2009) Sodium nitroprusside promotes multiplication and regeneration of Malus hupehensis in vitro plantlets. Plant Cell Tiss Organ Cult 96:29–34
Hayat S, Alyemeni MN, Hasan SA (2012) Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J Biol Sci 19:325–335
Hesami M, Daneshvar MH (2018a) In vitro adventitious shoot regeneration through direct and indirect organogenesis from seedling-derived hypocotyl segments of Ficus religiosa L.: an important medicinal plant. HortScience 53:55–61
Hesami M, Daneshvar MH (2018b) Indirect organogenesis through seedling-derived leaf segments of Ficus religiosa—a multipurpose woody medicinal plant. J Crop Sci Biotechnol 21:129–136
Hesami M, Daneshvar MH, Lotfi A (2017a) In vitro shoot proliferation through cotyledonary node and shoot tip explants of Ficus religiosa L. Plant Tissue Cult Biotechnol 27:85–88
Hesami M, Naderi R, Yoosefzadeh-Najafabadi M, Rahmati M (2017b) Data-driven modeling in plant tissue culture. J Appl Environ Biol Sci 7:37–44
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M (2018a) An efficient in vitro shoot regeneration through direct organogenesis from seedling-derived petiole and leaf segments and acclimatization of Ficus religiosa. J For Res. https://doi.org/10.1007/s11676-018-0647-0
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M (2018b) Establishment of a protocol for in vitro seed germination and callus formation of Ficus religiosa L., an important medicinal plant. Jundishapur J Nat Pharm Prod 13(e62682):1–8
Hesami M, Daneshvar MH, Yoosefzadeh-Najafabadi M, Alizadeh M (2018c) Effect of plant growth regulators on indirect shoot organogenesis of Ficus religiosa through seedling derived petiole segments. J Gen Eng Biotechnol 16:175–180
Jedinák A, Faragó J, Psenakova I, Maliar T (2004) Approaches to flavonoid production in plant tissue cultures. Biologia 59:697–710
Jimenez-Quesada MJ, Carmona R, Lima-Cabello E, Traverso JÁ, Castro AJ, Claros MG, de Dios Alché J (2017) Generation of nitric oxide by olive (Olea europaea L.) pollen during in vitro germination and assessment of the S-nitroso-and nitro-proteomes by computational predictive methods. Nitric Oxide 68:23–37
Kalra C, Babbar SB (2010) Nitric oxide promotes in vitro organogenesis in Linum usitatissimum L. Plant Cell Tiss Organ Cult 103:353–359
Ko W, Su C, Chen C, Chao C (2009) Control of lethal browning of tissue culture plantlets of Cavendish banana cv. Formosana with ascorbic acid. Plant Cell Tissue Organ Cult 96:137–141
Kolbert Z, Bartha B, Erdei L (2008) Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordia. J Plant Physiol 165:967–975
Kratsch H, Wise RR (2000) The ultrastructure of chilling stress. Plant, Cell Environ 23:337–350
Laspina N, Groppa M, Tomaro M, Benavides M (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Leng P, Su S, Wei F, Yu F, Duan Y (2009) Correlation between browning, total phenolic content, polyphenol oxidase and several antioxidation enzymes during pistachio tissue culture. Acta Hortic Sin 829:127–132
Ling A, Yap C, Shaib JM, Vilasini P (2007) Induction and morphogenesis of Phalaenopsis callus. J Trop Agric Food Sci 35:147–152
Liu F, Chen L (2010) Redox dynamics during embryogenic callus induction of Phalaenopsis spp. J Wuhan Bot Res 28:737–743
Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31:699–712
Mayer AM (2006) Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry 67:2318–2331
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mondal T, Aditya S, Banerjee N (2014) In vitro axillary shoot regeneration and direct protocorm-like body induction from axenic shoot tips of Doritis pulcherrima Lindl. Plant Tissue Cult Biotechnol 23:251–261
Naz S, Ali A, Iqbal J (2008) Phenolic content in vitro cultures of chick pea (Cicer arietinum L.) during callogenesis and organogenesis. Pak J Bot 40:2525–2539
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Niazian M, Noori SAS, Galuszka P, Tohidfar M, Mortazavian SMM (2017) Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind Crops Prod 97:330–337
Nickel KS, Cunningham B (1969) Improved peroxidase assay method using leuco 2, 3′, 6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis. Anal Biochem 27:292–299
Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, Goldman SL (2004) Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2:359–366
Park S-Y, Shin KS, Paek KY (2006) Increased ethylene and decreased phenolic compounds stimulate somatic embryo regeneration in leaf thin section cultures of Doritaenopsis hybrid. J Plant Biol 49:358–363
Pitzschke A, Djamei A, Bitton F, Hirt H (2009) A major role of the MEKK1–MKK1/2–MPK4 pathway in ROS signalling. Mol Plant 2:120–137
Qiao W, Fan LM (2008) Nitric oxide signaling in plant responses to abiotic stresses. Integr Plant Biol 50:1238–1246
Ramamoorthy V, Raguchander T, Samiyappan R (2002) Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil 239:55–68
Rico-Lemus M, Rodríguez-Garay B (2014) SNP as an effective donor of nitric oxide for in vitro plant cell and tissue culture. J Plant Biochem Physiol 2:127–128
Salmi MS, Hesami M (2016) Time of collection, cutting ages, auxin types and concentrations influence rooting Ficus religiosa L. stem cuttings. J Appl Environ Biol Sci 6:124–132
Sarropoulou V, Maloupa E (2017) Effect of the NO donor “sodium nitroprusside”(SNP), the ethylene inhibitor “cobalt chloride”(CoCl2) and the antioxidant vitamin E “α-tocopherol” on in vitro shoot proliferation of Sideritis raeseri Boiss. & Heldr. subsp. raeseri. Plant Cell Tiss Organ Cult 128:619–629
Sarropoulou V, Dimassi-Theriou K, Therios I (2014) Ιn vitro plant regeneration from leaf explants of the cherry rootstocks CAB-6P, Gisela 6, and MxM 14 using sodium nitroprusside. Vitro Cell Dev Biol Plant 50:226–234
Sarropoulou V, Dimassi-Theriou K, Therios I (2016) Effect of the ethylene inhibitors silver nitrate, silver sulfate, and cobalt chloride on micropropagation and biochemical parameters in the cherryrootstocks CAB-6P and Gisela 6. Turk J Biol 40:670–683
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 217037:1–26
Singh D, Singh B, Goel RK (2011) Traditional uses, phytochemistry and pharmacology of Ficus religiosa: a review. J Ethnopharmacol 134:565–583
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7:1621–1633
Tun NN, Holk A, Scherer GF (2001) Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett 509:174–176
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223
Wang X-D, Nolan KE, Irwanto RR, Sheahan MB, Rose RJ (2011) Ontogeny of embryogenic callus in Medicago truncatula: the fate of the pluripotent and totipotent stem cells. Ann Bot 107:599–609
Wi SG, Chung BY, Kim J-H, Baek M-H, Yang DH, Lee J-W, Kim J-S (2005) Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradation. J Plant Biol 48:195–200
Xu CJ, Li L, Li H, Zhang M (2005) Preliminary studies on the elements of browning and the changes in cellular texture of leaf explant browning in Phalaenopsis. Acta Hortic Sin 32:1111–1113
Xu J, Yin H, Wang W, Mi Q, Liu X (2009) Effects of sodium nitroprusside on callus induction and shoot regeneration in micropropagated Dioscorea opposita. Plant Growth Regul 59:279–285
Yingsanga P, Srilaong V, Kanlayanarat S, Noichinda S, McGlasson W (2008) Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol Technol 50:164–168
Yoruk R, Marshall MR (2003) Physicochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem 27:361–422
Zamani M, Hakimi M, Mosleh Arany A, Kiani B, Rashtian A (2014) The effects of salicylic acid (SA) and sodium nitroprusside (SNP) on physical and growth characteristics of Pinus eldarica. Bull Environ Pharmacol Life Sci 3:31–35
Author information
Authors and Affiliations
Contributions
All authors listed have made substantial, direct and intellectual contribution to the work, and have approved it for publication.
Corresponding author
Additional information
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Rights and permissions
About this article
Cite this article
Hesami, M., Tohidfar, M., Alizadeh, M. et al. Effects of sodium nitroprusside on callus browning of Ficus religiosa: an important medicinal plant. J. For. Res. 31, 789–796 (2020). https://doi.org/10.1007/s11676-018-0860-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0860-x