Abstract
Cinchona officinalis (Rubiaceae) is an endemic species of the Loja Valley in southern Ecuador with medicinal uses. Because of over-exploitation in the nineteenth century and more recent disturbances to its ecosystem, C. officinalis populations are threatened. Currently, natural regeneration of the populations is low, despite its high plant regeneration and seed formation capacity. In the present study, an efficient protocol for germination, shoot proliferation and plantlets regeneration was developed for this species. Phenolic content and germination rate of C. officinalis seeds were compared with a control species, C. pubescens. Nodal segments from seedlings of C. officinalis were cultured on Gamborg medium supplemented with different combinations of plant growth regulators. Because the phenol content is high in C. officinalis, the phenolic should be removed with hydrogen peroxide or water washes to stimulate germination. Shoots and callus developed from nodal segments within 45 days using most of the tested combinations of plant growth regulators. The best rates of shoot proliferation, callus formation and adventitious buds were obtained in medium supplemented with 5.0 mg L−1 6-benzyl-aminopurine and 3.0 mg L−1 indole-3-butyric acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cascarilla, Cinchona officinalis L. (Rubiaceae), is an endemic species from the Loja Valley of Ecuador (Andersson 1998; Garmendia 2005) that has been extremely useful for mankind (Acosta-Solís 1989). The species has enabled the development of effective treatments for the cure and prevention of malaria caused by different species of Plasmodium (Ulloa and Jorgensen 1995; McCalley 2002; Warhurst et al. 2003). Cinchona officinalis trees from Loja became quickly scarce. The species was highly exploited in the Cajanuma knot and Uritusinga, essentially for its bark to extract the active compound quinine; thus causing massive deforestation of Loja Province. The demand for the bark decreased in the 20th century when quinine was chemically synthesized, reducing the pressure on wild plant populations. However, now, human activities such as farming, ranching, and logging are causing an alarming destruction of the mountain forest, even more severe than when the bark was harvested (Madsen 2002). The small remaining populations in the cloud forest are distributed in patches, accompanied by specific vegetation (Acosta-Solís 1946). Several studies on the species denote specific conditions and limited distribution ranges, indicating a high susceptibility to deforestation and to the influence of human activities. Nevertheless, the trees of Cinchona were observed in 1946 (Acosta-Solís 1946) to have a high capacity of regrowth in natural conditions, but now only a low percentage of regeneration has been observed, suggesting low genetic diversity and reduction in reproductive success (Espinosa and Rios 2014). In contrast to the low vegetative regeneration, there is a high production of winged seeds, which are easily transport by the wind, but they are unable to germinate because of their growth and germination requirements are so specific (Acosta-Solís 1946; Garmendia 2005).
Several techniques, including culture in vitro, have been used to propagate Cinchona species and to enhance alkaloid production. Studies have mainly focused on the production of quinoline alkaloids, but a few were aimed at the propagation and recovery of natural populations of Cinchona (Hay et al. 1986; Khouri et al. 1986; Giroud et al. 1991; Hoekstra et al. 1990; Blom et al. 1992; Stevens et al. 1993; Ramos-Valdivia et al. 1997; Geerlings et al. 1999).
We carried out a preliminary study to assess seed germination. During imbibition of the seeds of C. officinalis, the soaking water turns brown caused by leaching of compounds, suggesting the presence of phenolic compounds in the dry seeds. We thus thought that the dormancy mentioned by several authors for C. josephiana (Thomas 1946) and C. ledgeriana (Barton 1947) might be caused by the presence of phenolic compounds.
In addition, Cinchona seeds were reported to need light to stimulate germination (Thomas 1946), while hydrogen peroxide can increase levels in the embryo (Verkhoturov and Frantenko 2008; Lu et al. 2013) and stimulate the oxidation process during germination (Bailly et al. 2008). Therefore, this study evaluated the influence of photoperiod and hydrogen peroxide treatments on the germination rate of C. officinalis. We also assessed the effect of various plant growth regulators (PGRs) on propagation in culture to develop techniques for massive propagation of C. officinalis as a prerequisite for its reintroduction into its native environment.
Materials and methods
Measurement of phenolic compounds
The amount of phenols in seeds and the germination percentage of C. officinalis were determined before and after imbibition. A parallel process was done with C. pubescens as a biological control because it does not have any problems in germinating and regenerating. Total phenols were determined by the Folin-Ciocalteu method (Jordán 1975) using tannic acid such as standard (Rosales and González 2003) and measuring absorbance at 730 nm.
In vitro germination
To evaluate germination levels of C. officinalis, we compared germination to that for C. pubescens as a biological control. The seeds of C. officinalis were obtained from the Cajanuma knot in the province of Loja, and seeds of C. pubescens were obtained from Santa Cruz Island, Galápagos. The seeds were surface-sterilized in soapy water for 5 min, then immersed in 70 % alcohol for 30 s, then in 1 % (v/v) sodium hypochlorite solution for 10 min. Three rinses in sterile water were done after each step. The seeds of both species were imbibed for in sterile distilled water 24 h and separated into two different treatment groups before being placed on Murashige–Skoog (MS) culture medium supplemented with sucrose (20 g L−1) and solidified with agar (7 g L−1), and the pH was adjusted to 5.8 prior to autoclaving. The first group of seeds was immersed in hydrogen peroxide 100 % (10 volumes) for 1 min, and the other group of seeds was sown directly onto the MS medium. Seeds were incubated at 22.0 °C with 12 h light/12 h dark or 24 h light with a photon flux density of 57 µmol m−2 s−1 provided by cool white fluorescent lamps.
Shoot proliferation
Nodal segments (approximately 1.0–1.5 cm) from 4-month-old seedlings were placed vertically on B5 medium supplemented with sucrose (20 g L−1) and solidified with agar (7 g L−1). The pH was adjusted to 5.8 before autoclaving. The explants were cultured in Gamborg medium (Gamborg 1968) or B5 without plant growth regulators (PGRs), as a control, or supplemented with 0.5, 1.0, 5.0 mg L−1 of 6-benzyl-aminopurine (BAP) or 0.2 mg L−1 of kinetin (KIN) in combination with 0.1 mg L−1 of α-naphthaleneacetic acid (NAA), 3.0 mg L−1 of indole-3-butyric acid (IBA), or 1.0, 2.0 mg L−1 of 2,4-dicholorophenoxyacetic acid (2,4-D). Cultures were maintained under 12 h light/12 h dark with a photon flux density of 57 µmol m−2 s−1 from cool white fluorescent lamps.
Histological analysis
To determine the origin of the regenerated shoots, we used several tissue samples for histological analysis. The samples were fixed in FAA (40 % formaldehyde, 50 % ethanol, 100 % glacial acetic acid) for 24 h. Samples were dehydrated in an alcohol gradient (25, 50, 70, and 95 %; 30 min each step) and 100 % alcohol for 2, 3 and 12 h. The clarification process was performed in two steps: first in ethanol–xylol (1:1) for 3 h, followed by fresh 100 % xylol for 45 min, 2 and 4 h. Samples were embedded for 24 h in xylol–paraffin (1:1) at 58.0 °C, then in 100 % paraffin and sectioned at 10 µm thickness and stained with hematoxylin and eosin (Pérez- Jiménez et al. 2012). Finally, the samples were placed on slides and were sealed with histofluid mounting medium (Marienfeld, Lauda-Königshofen, Germany) and observed with a light microscope (Labomed Lx 400).
Data collection and statistical analyses
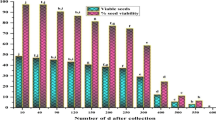
The concentration of total phenols of the complete seeds and the percentage germination (on petri dishes with wetted paper) for both species were assessed after 15 days. Overall, 300 seeds were used for each species, distributed in three replicate groups of 100 seeds. The percentage germination was evaluated after 25 days. Overall, 600 seeds of each species were used, distributed in three replicate groups of 50 seeds per treatment or combination. The percentage of shoots per explant, callus induction, adventitious shoots from callus, and the color and structure of callus were assessed after 45 days. This experiment was repeated three times with 25 explants for each combination. Mean (±SE) germination and shoot proliferation were subjected to an analysis of variance (ANOVA). Significant differences between means were assessed with Duncan’s test at P ≤ 0.05, using the program R (R Core Team 2013).
Results and discussion
Effect of phenolic compounds on germination
In the experiments to verify preliminary findings that phenolic compounds may be present in seeds and assess any influence on the germination of two species of Cinchona, phenol concentration in C. pubescens was low in both imbibed and unimbibed seeds, and the germination percentage was similar in both cases, whereas the phenol concentration in C. officinalis was much higher in the unimbibed seeds compared with the control seeds (Table 1). This high phenol concentration corresponded to low germination. After 24 h of imbibition, seeds had a much lower concentration of phenols, and the germination percentage was approximately 9 times higher, but was still less than half the percentage germination of the control seeds.
Seed production by Cinchona is high and constant during the year in most species of this genus. However, germination and seedling survival of C. officinalis is low in natural conditions. One of the causes for this low survival is the need for specific conditions for germination and growth in the natural habitat (Acosta-Solís 1946; Garmendia 2005). In other of our results, which are not presented in this work, in the laboratory, the presence of phenols has been noted as a seed-intrinsic factor.
Phenolics act as germination inhibitors in many species of the family Rubiaceae. The presence of phenols in C. pubescens was low, and this species has no difficulty germinating in natural conditions; it is an invasive species in the Galapagos Islands. Moreover, the total phenolic content in C. officinalis higher than in C. pubescens and is directly related to the low percentage of germination.
In vitro germination
Two factors, hydrogen peroxide and the impact of photoperiod, were evaluated for promoting seed germination of C. pubescens and C. officinalis (Table 2). Hydrogen peroxide in C. pubescens caused damage to the seed coat and embryos. A 24-h photoperiod increased germination percentages in this species, and the highest percentage (90 %) was achieved without hydrogen peroxide exposure. For C. officinalis, the results were opposite. Germination rate was markedly higher after exposure to hydrogen peroxide (72.2 and 86.7 %), but exposure to the different light periods had no significant effect on seed germination.
The results showed that in the case of C. pubescens, germination was higher in the 24 h photoperiod, whereas the hydrogen peroxide treatment did not significantly influence germination. Similar observations in C. pubescens were reported by Rentería (2002), who showed that direct light exposure was required for the germination of small seeds that are characterized by a low nutrient content.
According to Ellis et al. (1985), the seeds of Cinchona are usually non-endospermic, very small and show dormancy. Some studies on seed viability showed a low percentage of germination; Díaz and Loján (2004) reported 27 % germination for C. officinalis after 34 days, whereas Koblitz et al. (1983a) obtained only 5.8 % for C. succirubra and 15 % for C. ledgeriana.
When seeds of C. officinalis had been imbibed or washed with water, the phenol content decreased and the percentage germination increased. Exposure to light did not significant affect germination (Table 2). In small seeds such as C. officinalis, phenol removal is simple with several washes or hydrogen peroxide. The effect of this substance on the elimination of phenols to facilitate germination is known in other species (Baker et al. 2005). Hydrogen peroxide can break seed dormancy or stimulate germination because it increases the activity of antioxidant enzymes in the embryo (Verkhoturov and Frantenko 2008; Lu et al. 2013) and stimulates the oxidation process during germination (Bailly et al. 2008) in several species such as Hordeum vulgare (Verkhoturov and Frantenko 2008) and Amaranthus retroflexus (Xuanyu et al. 2011).
Shoot proliferation
To increase the shoot proliferation rate, we excised nodal segments with axillary buds from seedlings and cultured them in B5 medium supplemented with various combinations of cytokinins (BAP, KIN) and auxins (NAA, IBA, 2,4-D). In all cases, the first responses shoot and callus initiations were observed after 10 days. The formation of different types of structures depended on both concentration and type of hormones (Table 3; Figs. 1, 2) on B5 medium. The role of hormones in such morphogenic responses are known in other species of Cinchona when hormones were tested for inducing the production of metabolites to maintain cell cultures (Staba and Chung 1981; Koblitz et al. 1983a, b; Hay et al. 1986; Khouri et al. 1986; Giroud et al. 1991; Hoekstra et al. 1990; Blom et al. 1992; Stevens et al. 1993; Ramos-Valdivia et al. 1997; Geerlings et al. 1999).
In vitro propagation of Cinchona officinalis in B5 medium with different combinations of plant growth regulators. a Differentiation of pre-existing axillary buds on control B5 medium. Formation of callus at the base of explants and shoots from preexisting axillary buds; b on B5 medium + 0.5 mg L−1 BAP with 0.1 mg L−1 NAA and c. B5 medium + 1.0 mg L−1 BAP with 0.1 mg L−1 NAA. d Differentiation of pre-existing axillary buds, formation of callus and compact globular structures on B5 medium + 5.0 mg L−1 BAP with 3.0 mg L−1 IBA. e Formation of callus and roots regeneration on B5 medium + 0.2 mg L−1 KIN with 1.0 mg L−1 2,4-D. f Formation of friablem reddish callus and root regeneration on B5 medium + 1.0 mg L−1 BAP with 2.0 mg L−1 2,4-D. g Formation of small, compact callus on B5 medium + 5.0 mg L−1 BAP with 1.0 mg L−1 2,4-D
Light micrographs of tissues of Cinchona officinalis regenerated in vitro after 45 days in culture (bar 10 µm). Sections were stained with hematoxylin and eosin a longitudinal section of several shoot from pre-existing axillary buds (AX) on control B5 medium. b Transversal section of formation of callus on B5 medium + 1.0 mg L−1 BAP + 2.0 mg L−1 2,4-D; parenchyma cells (PC) are present; epidermis (EP) has disintegrated. c Transversal section of differentiation of vascular bundle (VE). d Transversal section of zone active cell division and globular structures or meristemoid (ME), parenchyma cell (PC) and complete disintegration of the epidermis (EP). e Longitudinal section of stem with druses (DR). Apical bud (AP); vascular tissue (TV)
The highest proliferation rate (5.3 shoots/explant) at 45 days was obtained with BAP (5.0 mg L−1) in combination with IBA (3.0 mg L−1). These shoots originated from axillary buds. The effect of BAP for improving bud formation has been evaluated alone or in combination with auxin in Cinchona (Staba and Chung 1981; Koblitz et al. 1983a; Hoekstra et al. 1990; Giroud et al. 1991) and other medicinal plants with positive results (Siddique and Anis 2007). After 10 weeks, this combination also stimulated callus formation, and from this, the regeneration of adventitious shoots (15 shoots/callus) and also more efficient in the formation of friable callus with indirect caulogenesis (Figs. 1d, 2c, d). The same combination was used by Staba and Chung (1981) to induce the production of alkaloids in disorganized cultures and organ cultures of Cinchona. However, our study was characterized by rapid growth of shoot and roots.
Next, the callus phase was observed as small, green, globular structures (Fig. 2d) accompanied by vascular tissue (Fig. 2c) that culminated in the formation of shoots. A similar response to a combination of 2,4-D and KIN was observed by Hoekstra et al. (1990) for C. ledgeriana which formed green, compact, globular structures from a fine cell suspension culture, characterized by the presence of moderately differentiated cells. The combination of IBA with BAP in many other species induces callus formation as observed for Tylophora indica which formed a high percentage of callus and regenerated shoots on a media with BAP-IBA (Sharma et al. 2014). The response of Hydrangea macrophylla was similar but with a lower percentage of sprouting (Liu et al. 2011) and for Viburnum dentatum, in which the frequency of shoot regeneration was influenced by the presence of IBA (Dai et al. 2011).
Shoots also formed (4.3 shoots/explant) with 0.5 mg L−1 BAP and 0.1 mg L−1 NAA. In the control group (4.8 shoots/explant), sprouting could be due to breakage of apical dominance and to the presence of young tissue (Fig. 2a), whereas in the treatment group, sprouting was due to the presence of BAP, which facilitated axillary bud initiation. The combination of KIN and 2,4-D has been widely used in the culture of Cinchona to obtain secondary metabolites from callus and suspension cultures (Koblitz et al. 1983b; Schmauder et al. 1985; Wijnsma et al. 1985; Hay et al. 1986, 1987; Hoekstra et al. 1990; Blom et al. 1992; Ramos-Valdivia et al. 1997).
The first callus tissue formation in the rest of the treatments was visible at 20 days of culture, starting at the base of the explants, spreading to the rest of the tissue by 45 days. The highest rate of callus formation (100 %) was obtained with 0.2 mg L−1 KIN + 1.0 mg L−1 2,4-D. With BAP + 2,4-D, a high percentage of calluses formed. The lowest percentage of callus formation was with BAP + NAA. All calluses were translucent, whitish, and spongy; some were friable, but not all were morphogenic (Figs. 1e, f, 2b). These calluses originated in meristematic areas and subsequently formed adventitious roots, but shoots did not form.
The histological analysis showed that morphological changes occurred in different tissues between weeks 6 and 10. In the control treatment, pre-existing axillary buds formed sprouts without tissue dedifferentiation; epidermis disintegration was not observed (Fig. 2a). Tissues forming callus have either low or no morphogenic capacity. These tissues did not differentiate, and had a high number of parenchyma cells and epidermal disintegration (Fig. 2b); most friable calli were characterized by the presence of cell clusters or meristemoids around these parenchymal cells (Fig. 2c). Vascular bundles were formed from these meristemoids (Fig. 2d). Finally, in few tissues, druses (a type of crystal) were occasionally present (Fig. 2e).
In our previous studies using MS medium, signs of hyperhydricity, seen as translucent tissue, were observed in C. officinalis. Many studies on Cinchona in vitro culture have reported the use of B5 medium (Koblitz et al. 1983b; Wijnsma et al. 1985; Allan and Scragg 1986; Khouri et al. 1986; Walton et al. 1987; Giroud et al. 1991; Geerlings et al. 1999) but without offering a reason for using B5, which has a lower mineral content than MS medium (Han et al. 2002). One factor that promotes hyperhydricity is the presence of a high content of salts as nitrogen in the culture medium. Several attempts to reduce or eliminate the hyperhydricity in different species. Reducing the amount of salts in medium composition and culture conditions. Hyperhydricity was reduced by modifying the nitrogen source (Ivanova and Van Staden 2009). Therefore, in this work to improve the quality of the tissue, B5 medium was used.
In conclusion, Cinchona officinalis germination was improved by applying exogenous hydrogen peroxide in the seeds. For direct shoot proliferation, nodal segments can be cultured in B5 medium with 5.0 mg L−1 BAP + 3.0 mg L−1 IBA. The same combination can also be used to regenerate shoots indirectly. Due to the ability of C. officinalis tissue to regrow, the culture of nodal segments on a hormone-free medium might be another alternative for shoot proliferation. We highly recommend the culture on a hormone-free medium to get plants with a low degree of somaclonal variation. The methods developed here improved the propagation of Cinchona, providing good potential for efficiently regenerating this species with genetically diverse germplasm starting from seed germination, to counteract the problems with reproduction and loss of genetic diversity.
References
Acosta-Solís M (1946) Cinchonas del Ecuador. Editorial Ecuador, Quito, p 271
Acosta-Solís M (1989) La Cinchona o Quina Planta Nacional del Ecuador. Rev Acad Colomb Cien 17(65):305–311
Allan EJ, Scragg AH (1986) Comparison of the growth of Cinchona ledgeriana Moens suspension cultures in shake flasks and 7 liter air-lift bioreactors. Biotechnol Lett 8(9):635–638
Andersson L (1998) A revision of the genus Cinchona (Rubiaceae-Cinchoneae). Mem N Y Bot Gard 81:1–75
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol 331:806–814
Baker K, Steadman K, Plummer J, Dixon K (2005) Seed dormancy and germination responses of nine Australian fire ephemerals. Plant Soil 277(1–2):345–358
Barton L (1947) Effect of different storage conditions on the germination of seeds of Cinchona ledgeriana Moens. Contrib Boyce Thompson Inst 15:1–10
Blom T, Kreis W, van Iren F, Libbenga K (1992) A non-invasive method for the routine-estimation of fresh weight of cells grown in batch suspension cultures. Plant Cell Rep 11(3):146–149
Dai W, Su Y, Castillo C (2011) Plant regeneration from in vitro leaf tissues of Viburnum dentatum L. Plant Cell Tiss Organ Cult 104(2):257–262
Díaz M, Loján M (2004) Fenología y propagación en vivero de especies forestales nativas del bosque protector ¨El Bosque¨. Disertación (Ingeniero Forestal). Loja, Universidad Nacional de Loja, p 119
Ellis R, Hong T, Roberts E (1985) Handbook of seed technology for genebanks, vol 2. International Board for Plant Genetic Resources (IBPGR), Rome, p 715
Espinosa C, Ríos G (2014) Patrones de crecimiento de Cinchona officinalis in vitro y ex vitro; respuestas de plántulas micropropagadas y de semillas. Rev Ecuat Med Cienc Biol 35(1 y 2):73–82
Gamborg O, Miller R, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Garmendia A (2005) El Árbol de la Quina (Cinchona spp.): Distribución, caracterización de su hábitat y arquitectura. Loja, Editorial Universidad Técnica Particular de Loja, p 187
Geerlings A, Hallard D, Martinez A, Lopes I, van der Heijden R, Verpoorte R (1999) Alkaloid production by a Cinchona officinalis “Ledgeriana” hairy root culture containing constitutive expression constructs of tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus. Plant Cell Rep 19:191–196
Giroud C, van der Leer T, van der Heijden R, Verpoorte R, Heeremans C, Niessen W, van der Greef J (1991) Thermospray liquid chromatography/mass spectrometry (TSP LC/MS) analysis of the alkaloids from Cinchona in vitro cultures. Planta Med 57(2):142–148
Han Y-S, van der Heijden R, Vepoorte R (2002) Improved anthraquinone accumulation in cell cultures of Cinchona robusta by feeding of biosynthetic precursors and inhibitors. Biotechnol Lett 24(9):705–710
Hay C, Anderson L, Phillipson J, Roberts M (1986) In vitro culture of Cinchona species. Precursor feeding of C. ledgeriana root suspension cultures with L-tryptophan. Plant Cell Rep 5(1):1–4
Hay C, Anderson L, Phillipson J, Curless D, Brown T (1987) In vitro culture of Cinchona species. Part II Time-course studies on the uptake of radio-labelled alkaloid precursors and alkaloids by C. ledgeriana root suspension cultures. Plant Cell Rep 9(3):197–206
Hoekstra S, Harkes P, Verpoorte R, Libbenga K (1990) Effect of auxin on cytodifferentiation and production of quinoline alkaloids in compact globular structures of Cinchona ledgeriana. Plant Cell Rep 8(10):571–574
Ivanova M, Van Staden J (2009) Nitrogen source, concentration, and NH4+:NO3− ratio influences shoot regeneration and hyperhydricity in tissue cultured Aloe polyphylla. Plant Cell Tissue Organ Cult 99(2):167–174
Jordán M (1975) Histologische und physiologische Untersuchungen zur Kapazität der Androgenese bei in vitro kultivierten Prunus-, Pyrus-, Ribes- und Nicotiana Antheren. Inaugural-Dissertation. Gieben, Justus Liebig-Universität, p 113
Khouri H, Ibrahim R, Rideau M (1986) Effects of nutritional and hormonal factors on growth and production of anthraquinone glucosides in cell suspension cultures of Cinchona succirubra. Plant Cell Rep 5(6):423–426
Koblitz H, Koblitz D, Schmauder H, Gröger D (1983a) Studies on tissue cultures of the genus Cinchona L. Alkaloid production in cell suspension cultures. Plant Cell Rep 2(3):122–125
Koblitz H, Koblitz D, Schmauder H, Gröger D (1983b) Studies on tissue cultures of the genus Cinchona L. In vitro mass propagation through meristem-derived plants. Plant Cell Rep 2(2):95–97
Liu F, Huang L, Yang L, Reinhound P, Jongsma M, Wang C (2011) Shoot organogenesis in leaf explants of Hydrangea macrophylla ‘Hyd1’ and assessing genetic stability of regenerants using ISSR markers. Plant Cell Tiss Organ Cult 104:111–117
Lu J, Li X, Yang Y, Jia L, You J, Wang W (2013) Effect of hydrogen peroxide on seedling growth and antioxidants in two wheat cultivars. Biol Plant 57(3):487–494
Madsen J (2002) Historia cultural de la cascarilla de Loja En: Aguirre Z, Madsen J, Cottas E, Balslev H (eds) Botánica Austroecuatoriana: estudios sobre los recursos naturales en las provincias de El Oro, Loja y Zamora Chinchipe. Ediciones AbyaYala. Quito, pp 385–399
McCalley D (2002) Analysis of the Cinchona alkaloids by high-performance liquid chromatography and other separation techniques. J Chromatogr A 967(1):1–19
Pérez-Jiménez M, Carrillo-Navarro A, Cos-Terrer J (2012) Regeneration of peach (Prunus persica L. Batsch) cultivars and Prunus persica × Prunus dulcis rootstocks via organogenesis. Plant Cell Tiss Organ Cult 108:55–62
R Core Team (2013) R: a language and environment for statistical computing. Vienna: the R Foundation for Statistical Computing. ISBN: 3-900051-07-0. http://www.R-project.org/
Ramos-Valdivia A, van der Heijden R, Verpoorte R (1997) Elicitor-mediated induction of anthraquinone biosynthesis and regulation of isopentenyl diphosphate isomerase and farnesyl diphosphate synthase activities in cell suspension cultures of Cinchona robusta How. Planta 203:155–161
Rentería J (2002) Ecología y Manejo de la Cascarilla (Cinchona pubescens Vahl), en Santa Cruz, Galápagos, Disertación (Ingeniería Forestal). Loja, Universidad Nacional de Loja, p 101
Rosales M, González R (2003) Comparación del contenido de compuestos fenólicos en la corteza de ocho especies de pino. Madera y Bosques 9(2):41–49
Schmauder H, Groger D, Koblitz H, Koblitz D (1985) Shikimate pathway activity in shake and fermenter cultures of Cinchona succirubra. Plant Cell Rep 4(5):233–236
Sharma M, Verma R, Singh A, Batra A (2014) Assessment of clonal fidelity of Tylophora indica (Burm. f.) Merrill “in vitro” plantlets by ISSR molecular markers. SpringerPlus 3:400
Siddique I, Anis M (2007) In vitro shoot multiplication and plantlet regeneration from nodal explants of Cassia angustifolia (Vahl.): a medicinal plant. Acta Physiol Plant 29(3):233–238
Staba J, Chung A (1981) Quinine and quinidine production by Cinchona leaf, root and unorganized cultures. Phytochemistry 20(11):2495–2498
Stevens L, Giround C, Pennings J, Verpoorte R (1993) Purification and characterization of strictosidine synthase from a suspension culture of Cinchona robusta. Phytochemistry 33(1):99–106
Thomas A (1946) Cinchona en Uganda. Empire J Exp Agric 14:75–84
Ulloa C, Jorgensen P (1995) Árboles y Arbustos de los Andes del Ecuador. Ediciones Abya-Yala, Quito, p 329
Verkhoturov V, Frantenko V (2008) Effect of hydrogen peroxide on anti- and prooxidant status of barley seeds during germination. Russ Agric Sci 34(1):11–13
Walton N, Parr A, Robins R, Rhodes M (1987) Toxicity of quinoline alkaloids to cultured Cinchona ledgeriana cells. Plant Cell Rep 6(2):118–121
Warhurst D, Craing J, Adagu I, Meyer D, Lee S (2003) The relationship of physico-chemical properties and structure to the differential antiplasmodial activity of the Cinchona alkaloids. Malar J 2:26
Wijnsma R, Go J, van Weerden I, Harkes P, Verpoorte R, BaerheimSvendsen A (1985) Anthraquinones as phytoalexins in cell and tissue cultures of Cinchona spec. Plant Cell Rep 4(5):241–244
Xuanyu L, Zhijun D, Hongyan Ch, Xinhua H, Songquan S (2011) Nitrite, sodium nitroprusside, potassium ferricyanide and hydrogen peroxide release dormancy of Amaranthus retroflexus seeds in a nitric oxide-dependent manner. Plant Growth Regul 64:155–161
Acknowledgments
This study was part of the project “PROY_IECOLOGIA_0036” financed by the third internal call for projects of the Universidad Técnica Particular de Loja (UTPL). We also appreciate the support provided by Augusta Cueva, Pablo Ramón, Chris Brinegar and Máximo Moreira.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This study was part of the project “PROY_IECOLOGIA_ 0036” financed by the third internal call for projects of the Universidad Técnica Particular de Loja (UTPL).
The online version is available at http://www.springerlink.com
Corresponding Editor: Zhu Hong
Rights and permissions
About this article
Cite this article
Armijos-González, R., Pérez-Ruiz, C. In vitro germination and shoot proliferation of the threatened species Cinchona officinalis L (Rubiaceae).. J. For. Res. 27, 1229–1236 (2016). https://doi.org/10.1007/s11676-016-0272-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-016-0272-8