Abstract
To increase the utilization of V-Ti-Magnetite ore in the burden of a blast furnace, we investigated replacing CaO with MgO. The high fraction of TiO2-bearing raw materials leads to a super-high TiO2 content in the blast furnace slag, typically above 30 wt pct, which affects normal blast furnace operation. The present study focuses on the sulfide capacity of slag containing a super-high TiO2 content. The effects of TiO2 content and the ratio of MgO to CaO (M/C) on the sulfide capacity of slag were examined using a gas-slag equilibrium method. In the CaO-SiO2-TiO2-MgO-Al2O3 slag system, as the content of TiO2 in the slag increased from 20 to 34 wt pct, the sulfide capacity decreased from 6.25 × 10−5 to 2.86 × 10−5. Above 30 wt pct TiO2, the influence of TiO2 content on the sulfide capacity became negligible. Increasing the (M/C) ratio induced a non-monotonic trend in the sulfide capacity, which first increased and then decreased. Thermodynamic and structural analyses were employed to characterize the experimental results. Thermodynamically, decreasing the CaO activity reduced the free oxygen in the slag with increasing TiO2 content. Furthermore, an increase of titanium suboxide slowed the reduction of the sulfide capacity. Mass substitution mainly influenced the availability of free oxygen in the slag, and then the sulfide stability and oxygen concentration worked together, resulting in a non-monotonic sulfide capacity when MgO was substituted for CaO. In addition, the sulfide capacity was dependent on the effect of both the quaternary molar basicity and the binary molar basicity. Finally, the structural analysis revealed that for a super-high TiO2 content in the slag, both the structure of Ti and the depolymerization reaction of Ti had an extremely important effect on desulfurization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The comprehensive utilization of vanadium-titanium magnetite ore (VTM) has always been a hot topic in metallurgy research. In the blast furnace ironmaking process, significantly increasing the content of VTM can effectively improve the recovery and utilization of vanadium-titanium resources.[1,2,3] Unfortunately, serious slagging difficulties, such as slag foaming and poor fluidity of the slag may occur with an increase in the TiO2 content in blast furnace slag (BFS).[4,5] In our previous studies,[3,6] an innovative slag system, in which MgO was used as a substitute for CaO, has been proposed, and some high-temperature physicochemical properties of the slag have been evaluated. Desulfurization ability is an important chemical property of BFS, and plays an essential role in determining the quality of hot metal. Sulfide capacity is the main parameter used for characterizing the desulfurization ability of slag. The sulfide capacity determines the selection of the slag desulfurization system and is also a particularly important indicator of the high-temperature physicochemical properties of slag; hence, it has received much attention in the ironmaking process.[7,8,9,10,11,12] Therefore, this study was focused on the sulfide capacity of slag containing a super-high TiO2 content in the blast furnace ironmaking process.
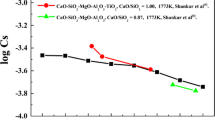
Many studies have focused on the sulfide capacity of BFS containing TiO2, as shown in Figure 1. Ghita et al.[13] measured the sulfide capacities of FeO-TiO2-SiO2 and FeO-TiO2-CaO. Brown et al.[14] investigated the sulfide capacity of CaO-(MgO)-TiO2-SiO2-Al2O3 slags. Both research groups found that the sulfide capacity of the slag increases with increasing content of basic oxides like FeO, MgO, and CaO. With a fixed basic oxide content, replacing SiO2 or Al2O3 with TiO2 may lead to increased sulfide capacity of the slag. Sommerville et al.[15] summarized and compared the desulfurization capabilities of different slag systems, and concluded that the desulfurization capability of TiO2 is lower than that of CaO, MgO, and FeO, but is higher than that of Al2O3 and SiO2. Ito[16] reported that for the MnO-TiO2-SiO2 slag system with a fixed molar ratio of MnO/SiO2, increasing the TiO2 content reduced the sulfide capacity. Che et al.[17,18,19] found that the sulfide capacity of BFS containing TiO2 is far inferior to that of ordinary BFS owing to the high TiO2 content and low CaO content of the former. Tang et al.[19] measured the sulfide capacity of CaO-SiO2-TiO2-MgO-Al2O3 by the slag-metal equilibrium method, and reported that the sulfide capacity increased with an increase in the basicity and a decrease in the content of TiO2 in the BFS. Increasing the MgO content will improve the sulfide capacity of BFS containing TiO2. However, it was reported[3,6] that MgO can affect the viscosity of BFS, and its content should not exceed 14 wt pct.
Generally, there are two main methods of measuring sulfide capacity: the slag-gas equilibrium method and the slag-metal equilibrium method.[20] This present work studies the sulfide capacity of BFS during the region of very high TiO2 content from 20 to 34 wt pct, which is yet to be done. Employing slag-gas equilibrium method can eliminate the effect of TiO2 reduction by carbon in the hot metal. Therefore, using the slag-gas equilibrium method in this study, the sulfide capacity of CaO-SiO2-TiO2-MgO-Al2O3 slag was investigated as a function of TiO2 content ranging from 20 to 34 wt pct and MgO wt pct/CaO wt pct (M/C) ratios ranging from 0.32 to 0.65.
Experimental
Materials
The synthetic slag was quenched with water after all the pure compounds were pre-melted. In the gas-slag equilibrium method, a mixture gas at a flow rate of 400 mL/min was introduced into the reaction chamber after passing the gas through the purification system. The gas mixture was composed of Ar-CO-CO2-SO2 (Ar: 99.99 pct purity, CO: 99.5 pct purity, CO2: 99.5 pct purity, SO2: 99.9 pct purity) with a volume ratio of 200:100:80:20. The flow rate of each gas was controlled by special mass flowmeters.
Experimental Procedure
The experimental setup is shown in Figure 2. The heating system consisted of electric resistance heaters, an alumina reaction tube, and a thermocouple. There were eight silicon-molybdenum heaters installed along two sides of the furnace, with a maximum operating temperature of up to 1700 °C. The constant temperature zone inside the furnace was approximately 7 cm in length. A proportional integral differential (PID) controller was used to maintain the temperature within ± 2 K. The purification system consisted of four dehydration tubes, two YJ-O100 deoxidation tubes, four special mass flowmeters, and a gas mixing chamber before entering the furnace. The dehydration tube was half-filled with color-changing silica gel and the other half was filled with molecular sieves. A YJ-O100 deoxidation tube was filled with the deoxidation agent for the palladium-catalyst oxidation system.
The time required to achieve equilibrium was established by comparing the sulfur content in the slag samples after the reaction with the mixed gas for 4, 6, and 8 hours; the results are presented in Figure 3. This indicates that the sulfur content did not vary after 6.0 hours. Hence, the optimum equilibrium time for the desulfurization reaction used for the subsequent experiment was 8 hours.
One gram of sample was weighed and placed in a platinum crucible (size: r = 0.62 cm, h = 1 cm, Pt ≥ 99.95 pct). In one experiment, three platinum crucibles were placed on the corundum boat and were positioned in the constant temperature zone of the horizontal furnace. The samples were heated in the furnace and protected by high-purity argon after purification during the heating process. The samples were held for 8 hours at 1773 K under the experimental atmosphere for equilibrium. The furnace was then shut down and the gas was simultaneously changed to purified argon. The samples were cooled in the furnace at a cooling rate of 40 K/min to solidification. The samples were crushed to particles smaller than 100 μm, and the final sulfur content in the slag was measured by chemical titration analysis.
Results and Discussion
The sulfide capacity (Cs) of slag equilibrated with the mixed gas is defined by Eq. [2], which is derived from Eq. [1]:
where (wt pct S) is the mass percentage of sulfur dissolved in the slag, while \( {\text{P}}_{{{\text{O}}_{2} }} \) and \( {\text{P}}_{{{\text{S}}_{2} }} \) are the partial pressures of oxygen and sulfur, respectively. The experimental partial pressure of the gases at 1773 K was calculated using the thermochemical program FactSage. The results are shown in Table I.
The chemical compositions of the samples used in the present investigation, the analyzed sulfur content in the slag, and the parameters from the sulfide capacity calculation are shown in Table II.
Effect of TiO2 on the Sulfide Capacity
The effect of the TiO2 content on the sulfide capacity is shown in Figure 4. The logarithm of the sulfide capacity decreased from − 4.20 at a 20 wt pct TiO2 content to − 4.67 at a 34 wt pct TiO2 content. The sulfide capacity declined rapidly at first with increasing TiO2 content, and then declined gradually above 30 wt pct TiO2. Because the sulfide capacity of the CaO-SiO2-MgO-Al2O3-(TiO2) slag system is dependent on the activity of CaO, which provides most of the oxygen ions available for replacement by sulfur,[21] increasing the TiO2 content rapidly decreased the sulfide capacity of the slag. However, titanium suboxides also increase with increasing TiO2 content and contribute to the stability of the slag. In other words, titanium suboxides can increase the sulfide capacity of the slag. Che confirmed this phenomenon, and pointed out that titanium suboxides are basic oxides, which favors the desulfurization reaction, based on a thermodynamic analysis.[17]
Tang et al.[19] reported the same tendency for the CaO-SiO2-TiO2-MgO-Al2O3 quinary slag system (Figure 4). However, the sulfide capacity was generally higher than that in this study because the basicity of that system was 1.2, which was higher than that in this study. On the other hand, more suboxides were formed in that system due to the use of the slag-metal method with a graphite crucible.
Effect of M/C on the Sulfide Capacity
As shown in Figure 5, the sulfide capacity of high TiO2 slag did not follow a monotonic variation but increased and then declined as the M/C ratio increased from 0.32 to 0.65. The logarithm of the sulfide capacity increased from − 4.669 to − 4.493 between an M/C ratio of 0.32 and 0.43, and then decreased to − 4.634 at an M/C ratio of 0.65. Unlike TiO2, both CaO and MgO provide free oxygen. For CaO-SiO2-Al2O3-MgO(-FeO) slag, the activity of CaO decreases with increasing M/C ratio, which can reduce the sulfide capacity, as reported by Seo[22] and Karsrud.[23,24] However, Hawkins[25] found that mass substitution of CaO with MgO (by wt pct) enhances the sulfide capacity of slag. Because in the process of equal mass substitution, due to the difference in relative atomic mass between MgO and CaO, the molar amount of MgO increases more than the reduction of CaO. On the molar fraction scale in Table III, equal mass substitution leads to an increase in the total basic oxide content while the activity of CaO decreases, resulting in a non-monotonic sulfide capacity when MgO is substituted for CaO. Combining the dissolution behavior of sulfur in the slag allows better understanding of this non-monotonicity. Kang and Park[26] reported that the sulfide capacity of ternary silicate slag is impacted by the sulfide stability and oxygen concentration. The stability of MgS is much lower than that of CaS, but at low basicity, MgO can provide additional free oxygen compared to CaO, and free oxygen plays a more important role than sulfide in slag stability. This theory can also be applied to quinary high-titanium systems, in which high-titanium plays a key role in the transition (see the detailed analysis below).
Thermodynamic Analysis
FactSage software is a thermodynamic calculator that has been widely used in the metallurgical slag field. The variation in the sulfur content of the slag with titanium suboxide (Ti2O3) under the slag-gas equilibrium condition was calculated by FactSage (Figure 6). With increasing the Ti2O3 content, the sulfur content in the slag increased, confirming that the Ti2O3 content of this slag had a positive influence on the sulfide capacity. The variation in titanium suboxide (Ti2O3) content with TiO2 in this study is shown in Figure 7. Increasing the TiO2 content increased the Ti2O3 content and improved the sulfide capacity to some extent, which slowed the sulfide capacity reduction rate. However, with an increase in the M/C ratio, the Ti2O3 content did not change, indicating that the Ti2O3 content is not the determining factor of the sulfide capacity for slag with a fixed amount of TiO2.
In addition, the equilibrium constant (K) of the slag-gas equilibrium desulfurization reaction of CaO and MgO was calculated by FactSage, and the results are shown in Eqs. [3] and [4]. It is confirmed that MgS is lower stable than CaS under the conditions studied, which are the same as those used in the study by Park et al.[26] We also focused on the change in basicity (which represents the change in the free oxygen in slag to some extent), as shown in Figure 8. Analysis of the molar basicity of the CaO-SiO2-TiO2-MgO-Al2O3 quinary slag system revealed that increasing the TiO2 content resulted in reduced binary molar basicity, while mass substitution resulted in increasing the quaternary molar basicity ((CaO+MgO)/(SiO2+TiO2)). Combined with the change in the binary basicity, we found that when the quaternary molar basicity was less than 0.875, the effect of the quaternary molar basicity dominates, and when the quaternary molar basicity was greater than 0.875, the effectiveness of the binary basicity began to dominate. In other words, titanium dioxide plays an indispensable role in the sulfide capacity transition in the substitution of MgO for CaO.
Structural Analysis
The sulfide capacity is an indicator closely associated with the slag structure.[27,28,29] In basic earth silicate melts containing alumina, when the Al2O3/MO (MO = a basicity oxide) ratio is smaller than unity, the coordination structures of aluminum oxide and silicon dioxide are the same, i.e., tetrahedral. Al3+ requires charge compensation due to the valence difference relative to that of Si4+, and charge compensation of Ca2+ takes higher priority than that of Mg2+[30,31,32,33] In titania silicate glasses and melts, Ti4+ ions can have fourfold coordination in the form of isolated clusters or substituted Si4+, and can also be coordinated to more than four oxygens.[34] Henderson,[35] Sandstorm,[36] and Greegor[37] reported that in titania silicate glasses, when the concentration of TiO2 is high, Ti4+ dominantly adopts a tetrahedral structure. Consequently, SiO, AlO, and TiO tetrahedrons exist in the CaO-SiO2-TiO2-MgO-Al2O3 slags. A previous study by Yan[38] et al. proved the applicability of the above structure in slag viscosity modeling. Therefore, we evaluated the applicability of this structure in determining the sulfide capacity.
The reaction in the slag can be reduced to a combination of basic metal ions (Mg2+and Ca2+) and three kinds of tetrahedral ([SiO4]4−, [AlO4]5−, and [TiO4]4−) species. In other words, the following three main polymerization reactions occur in the slag:
This is actually the polymerization of three oxygens reported by Finchan and Richardson[39]:
where \( {\text{O}}^{ - } \) is non-bridging oxygen, \( {\text{O}}^{2 - } \) is free oxygen, and \( {\text{O}}^{0} \) is bridging oxygen. Considering Eq. [8], as the equilibrium constant increases, the degree of melt polymerization increases.
From Eq. [1], the desulfurization of slag has an effect on the exchange of free oxygen in the slag and metal sulfur ions or gas sulfur. The basicity of slag and the temperature indirectly affect the free oxygen and sulfide capacity. Therefore, it is quite feasible and convenient to directly explore the change in free oxygen content, and thus, the sulfide capacity from the depolymerization reaction of the three oxygen species.
For the CaO-SiO2-TiO2-MgO-Al2O3 quinary slag, the depolymerization reactions and standard energy change for the specific depolymerization reactions[38] are shown in Figure 9. This indicates that the depolymerization of SiO2 and TiO2 is more sensitive to M/C, and the content of Al2O3 did not change in this study. Consequently, the structural analysis of Ti and Si was used in this study to characterize the slag, as described below.
As the structure of the slag becomes simpler, the degree of the depolymerization reaction in the slag increases, and the free oxygen content in the slag increases.[26,29,40] From our previous work,[6] Raman spectroscopy data and the deconvoluted results for the CaO-SiO2-TiO2-MgO-Al2O3 slag were obtained, and in the present work, the simple structural units of slag were used to characterize free oxygen in the slag. From the perspective of molten silicate slag,[41,42,43,44,45] structural information related to the slag can be obtained through Raman spectral analysis and other spectral experimental data, and is usually represented by Qn (where n is the bridge oxygen number). The higher the content of Q3 and Q4 in the slag, the higher the degree of slag polymerization. On the other hand, a higher content of Q0, Q1, and Q2 species indicates a higher degree of slag depolymerization. Because Raman spectroscopy only detects a fraction of the coupling \( {\text{SiO}}_{4}^{4 - } \) (i.e., Q0) and \( {\text{Ti}}_{2} {\text{O}}_{6}^{2 - } \) chains, the use of Q0 to represent the simple structure was excluded. In fact, Q0 is insignificant, as explained below. Therefore, we used the sum of Q1 and Q2 to represent the simple structural units of silicon. Due to the super-high TiO2 content, the structure of Ti4+ cannot be ignored. Compared with the \( {\text{Ti}}_{2} {\text{O}}_{6}^{2 - } \) structural unit, the \( {\text{TiO}}_{4}^{4 - } \) structural unit and the O-Ti-O deformation unit are simpler,[46,47] and are consistent; thus, \( {\text{TiO}}_{4}^{4 - } \) and O-Ti-O were used to represent the simple structural units of titanium slag. Figure 10 shows the fraction of simple structural units of (Si + Ti) and Ti as a function of the (a) TiO2 content and (b) M/C ratio. The present sulfide capacity and the fraction of simple structural units generally follow the same trend, which is consistent with the previous statement that the amount of free oxygen in the slag can greatly affect the sulfide capacity. In addition, we found that the simple structure of (Ti + Si) varies in a manner that is quite consistent with that of Ti. This could be explained by the deconvoluted Raman data, where among the structural units (Si, Al, Ti) detected by the Raman analysis of the CaO-SiO2-TiO2-MgO-Al2O3 slag, the silicon structural units accounted for only about 20 pct, which is much less than the content of titanium structural units, which was not less than 50 pct. This proves that the high-titanium slag system cannot be analyzed from the perspective of Si, but must be analyzed from the perspective of Ti, which is the main influence on the high-temperature structure of high TiO2 slag.
In titanium-based melts, the depolymerization reaction of Ti has an extremely important effect on desulfurization. The fraction of O-Ti-O deformation units in the slag as a function of the (a) TiO2 content and (b) M/C ratio, and the depolymerization reaction analysis are presented in Figure 11.
The figure indicates that the variation of the fraction of the O-Ti-O deformation units basically follows the same trend as that of logCs. The free oxygen provided by the Ti depolymerization reaction can reflect the overall free oxygen to some extent. With increasing TiO2 content, the depolymerization of Ti is promoted, which is beneficial to the generation of CaTiO3 and MgTiO3 and reduces the content of free oxygen used for replacing sulfur in the slag. That is, a 2−O decreases while f 2−S is basically unchanged, leading to a decrease in the sulfide capacity.
At 1773 K, the Gibbs energy of the depolymerization of Ti with Ca is − 94342.6 J/mol, while that of Ti with Mg is − 23693.7 J/mol, which means that the depolymerization of Ti with Ca is more sensitive than that of Ti with Mg. When MgO initially substitutes for CaO, the slag contains an excess of CaO, the depolymerization of Ti with Ca is not dominant. Eventually, the increase in the molar amount of MgO due to mass substitution outweighs the reduction of CaO, as shown in Table III, resulting in an increase in the free oxygen in the slag. That is, the increase in a 2−O is greater than the increase in f 2−S , thereby increasing the sulfide capacity. With the sequential increase in the M/C ratio, the molar amount of CaO is insufficient for the depolymerization of Ti with Ca, and the depolymerization of Ti with Ca gradually becomes the most influential reaction. Consequently, the free oxygen in the slag declines. In other words, the decrease in a 2−O and the increase in f 2−S cause the sulfide capacity to decrease.
Conclusions
The effects of the TiO2 content and M/C ratio on the sulfide capacity of super-high TiO2 slag were experimentally studied using the gas-slag phase equilibrium method, and the main conclusions are as follows:
-
1.
In the CaO-SiO2-TiO2-MgO-Al2O3 slag system, as the content of TiO2 in the slag increased from 20 to 34 wt pct, the sulfide capacity declined from 6.25 × 10−5 to 2.86 × 10−5, and when the TiO2 content exceeded 30 wt pct, the influence on the sulfide capacity became negligible. With an increasing M/C ratio, the slag sulfide capacity followed a non-monotonic trend, where it first increases and then decreased.
-
2.
Thermodynamically, the decrease in the CaO activity reduced of the free oxygen in the slag with increasing TiO2 content. The increase in titanium suboxide slowed the reduction of the sulfide capacity. Mass substitution mainly influenced the change of free oxygen in the slag, and then the sulfide stability and oxygen concentration worked together, leading to non-monotonic sulfide capacity of the slag in which MgO substitutes for CaO. Focusing on the basicity change, the sulfide capacity was dependent on the effect of both the quaternary molar basicity and binary molar basicity. When the quaternary molar basicity is less than 0.875, the effect of the quaternary molar basicity dominates, and when the quaternary molar basicity is greater than 0.875, the effective binary basicity begins to dominate.
-
3.
Considering structural changes within the CaO-SiO2-TiO2-MgO-Al2O3 slag system, the anion groups mainly existed in the form of three kinds of tetrahedral ([SiO4]4−, [AlO4]5−, and [TiO4]4−) species. For the high TiO2 slag, the structure of Ti and the depolymerization reaction of Ti both had an extremely important effect on desulfurization. The change in the fraction of the O-Ti-O deformation units follows basically the same trend as logCs. The present results can be attributed to the depolymerization reaction.
References
Z. Yuan, X. Wang, C. Xu, W. Li, and M. Kwauk: Miner. Eng., 2006, vol. 19, pp. 975-78.
F. Valighazvini, F. Rashchi, and R. Khayyam Nekouei: Ind. Eng. Chem. Res., 2013, vol. 52, pp. 1723-30.
Z. Pang, X. Lv, Y. Jiang, J. Ling, and Z. Yan: Metall. Mater. Trans. B, 2020, vol. 51, pp. 722-31.
A. Shankar, M. Görnerup, A. K. Lahiri, and S. Seetharaman: Metall. Mater. Trans. B, 2007, vol. 38, pp. 911-15.
T. Nagasaka, M. Hino, and S. Ban-Ya: Metall. Mater. Trans. B, 2000, vol. 31, pp. 945-55.
Z. Pang, X. Lv, J. Ling, Y. Jiang, Z. Yan, and J. Dang: Metall. Mater. Trans. B, 2020, vol. 51, pp. 2348-57.
A. Shankar: Ironmak. Steelmak., 2006, vol. 33, pp. 413-18.
A. Shankar, M. Görnerup, S. Seetharaman, and A. K. Lahiri: Metall. Mater. Trans. B, 2006, vol. 37, pp. 941-47.
M. K. Cho, J. Cheng, J. H. Park, and D. J. Min: ISIJ Int., 2010, vol. 50, pp. 215-21.
G.-H. Park, Y.-B. Kang, and J. H. Park: ISIJ Int., 2011, vol. 51, pp. 1375-82.
J. H. Park, and G.-H. Park: ISIJ Int., 2012, vol. 52, pp. 764-69.
J. Zhang, X. Lv, Z. Yan, Y. Qin, and C. Bai: Ironmak. Steelmak., 2016, vol. 43, pp. 378-84.
I. Ghita, and H. Bell: Ironmak. Steelmak., 1982, vol. 9, pp. 239-43.
S. Brown, R. Roxburgh, I. Ghita, and H. Bell: Ironmak. Steelmak., 1982, vol. 9, pp. 163-67.
I. D. Sommerville, and H. B. Bell: Can. Metall. Quart., 1982, vol. 21, pp. 145-55.
M. Ito, K. Morita, and N. Sano: ISIJ Int., 1997, vol. 37, pp. 839-43.
C. Che, and W. Wang: Iron Steel V.Ti., 1984, vol. 3, pp. 004.
J. Liu, and D. Li: Eng. Chem.Metall., 1987, vol. 1, pp. 008.
X. Tang, and C. S. Xu: Isij Int., 1995, vol. 35, pp. 367-71.
H. Bell: Can. Metall. Quart., 1981, vol. 20, pp. 169-79.
Z. Yan, X. Lv, Z. Pang, W. He, D. Liang, and C. Bai: Metall. Mater. Trans. B, 2017, vol. 48, pp. 2607-14.
J. D. Seo, and S. H. Kim: Steel Res, 1999, vol. 70, pp. 203-08.
K. Karsrud: Scand. J. Metall., 1984, vol. 13, pp. 265-68.
K. Karsrud: Scand. J. Metall., 1984, vol. 13, pp. 144-50.
R. J. Hawkins, G. S. Meherali, and M. W. Davies: Ironmak. Steelmak., 1971, 209, 646-57.
Y.-B. Kang, and J. H. Park: Metall. Mater. Trans. B, 2011, vol. 42, pp. 1211-17.
L. Wang, Y. Wang, K.-c. Chou, and S. Seetharaman: Metall. Mater. Trans. B, 2016, vol. 47, pp. 2558-63.
L.-j. Wang, M. Hayashi, K.-c. Chou, and S. Seetharaman: Metall. Mater. Trans. B, 2012, vol. 43, pp. 1338-43.
S. Lee, and D. J. Min: J Am Ceram Soc, 2018, vol. 101, pp. 634-43.
J. H. Park, D. J. Min, and H. S. Song: Metall. Mater. Trans. B, 2004, vol. 35, pp. 269-75.
K. C. Mills: ISIJ Int., 1993, vol. 33, pp. 148-55.
Q. Shu: Steel. Res. Int., 2009, vol. 80, pp. 107-13.
G. Zhang, K. Chou, and K. Mills: Metall. Mater. Trans. B, 2014, vol. 45, pp. 698-706.
Mysen BO, and Richet P: Silicate Glasses and Melts: Properties and Structure, 1st ed., Elsevier, San Diego, 2005, pp. 322–26.
G. S. Henderson, X. Liu, and M. E. Fleet: Phys Chem Miner, 2002, vol. 29, pp. 32-42.
D. R. Sandstrom, F. W. Lytle, P. S. P. Wei, R. B. Greegor, J. Wong, and P. Schultz: J Non-Cryst Solids, 1980, vol. 41, pp. 201-07.
R. B. Greegor, F. W. Lytle, D. R. Sandstrom, J. Wong, and P. Schultz: J Non-Cryst Solids, 1983, vol. 55, pp. 27-43.
Z. Yan, R.G. Reddy, X. Lv, Z. Pang, and W. He: Ironmak. Steelmak., 2018, pp. 1-7.
C. J. B. Fincham, and F. D. Richardson: Proc. R. Soc. Lond. Ser. A, 1954, vol. 223, pp. 40-62.
Z. Ren, X. Hu, and K. Chou: J Iron Steel Res Int, 2013, vol. 20, pp. 21-25.
B.O. Mysen, P. Richet, Silicate Glasses and Melts, Elsevier, Amsterdam, 2005.
B.O. Mysen, Eur. J. Mineral. 15 (2003) 781.
B.O. Mysen, Phys. Earth Planet. Inter. 107 (1998) 23.
J.H. Park, J. Non-Cryst. Solids, 358 (2012) 3096-3102.
J.H. Park, Met. Mater. Int., 19 (2013) 577-584.
K. Zheng, Z. Zhang, L. Liu, and X. Wang: Metall. Mater. Trans. B, 2014, vol. 45, pp. 1389-97.
J. Ryerson: Am. Miner., 1980, vol. 65, pp. 1150–65.
Acknowledgments
This study was supported by National Key R&D Program of China (Grant No. 2018YFC1900500).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted October 21, 2020; accpeted April 15, 2021.
Rights and permissions
About this article
Cite this article
Ling, J., Pang, Z., Jiang, Y. et al. Blast Furnace Ironmaking Process with Super-High TiO2 in the Slag: Sulfide Capacity. Metall Mater Trans B 52, 2786–2795 (2021). https://doi.org/10.1007/s11663-021-02192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02192-9