Abstract
The effect of the re-heating process on inclusion evolution in Ti-Zr deoxidized low carbon steel was investigated using high-temperature resistance furnace, high-temperature confocal laser scanning microscope, scanning electron microscope with energy-dispersive spectrometer, and electron probe microanalysis with wavelength-dispersive spectrometer. The results indicated that compared with as-cast sample, the entire inclusion characteristics in heated samples were affected by the couple effect of heating temperature and holding time. As temperature and holding time increased, the shape of the Ti-Zr oxide changed from an irregular, curved surface to a smooth, spherical surface owing to the outermost layer being enveloped by liquid inclusion of Si-Mn-Al oxide. Additionally, the Ti-Zr oxide composition distribution changed from homogeneous to heterogeneous during heating in the range of 1100 °C to 1400 °C for 2 hours. Finally, different cooling modes had a significant influence on the inclusion characteristics. Compared with cooling by direct water quenching, the process of initial cooling to 1000 °C with furnace cooling and followed by water quenching could increase the number density of nitrides and sulfides, as well as change the unstable oxide to an effective oxide by precipitation of TiN on the surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generally, inclusions have often been regarded as harmful second-phase particles. As such, the control of inclusions has been of great significance for obtaining high-performance steel.[1,2] However, as inclusions are refined and dispersed, there occurs a pinning effect on austenite grain[3,4] and induction of intragranular acicular ferrite (AF) formation,[5,6,7,8,9] gradually making them beneficial second-phase particles.[10] Therefore, controlling the beneficial effects of inclusions in steel is one of the goals pursued by metallurgical workers.[11,12,13,14,15] Moreover, the control of inclusions should not be limited to the refining and solidification stages; the evolution of inclusions in solid steel should also be considered. It is very important to study this in solid steel during the re-heating and hot working processes,[16] because the inclusion characteristics at this time are closer to the those in real steel products.[17]
At present, most of the studies on inclusion evolution in solid steel have concentrated on MnS inclusions,[18,19,20,21,22] TiN inclusions,[23,24,25] silico-manganate inclusions,[26,27,28,29,30] Ti-Al oxides,[31,32,33,34] and so on.[35,36] Most studies on MnS evolution in solid steel have focused on free-cutting steel[20,22] and non-quenched and tempered steel.[21] The changes in the morphology and size of MnS inclusions were studied during heat treatment. It was found that large-sized MnS would shrink in the radial direction and split in the axial direction, resulting in a decrease in length and a shape approaching that of a spindle.[20] The main factors affecting MnS evolution were determined to be heating rate, heating temperature, isothermal holding time, and cooling rate. In addition, Zou et al.[37] found that re-heating at 1200 °C promoted the precipitation of single MnS on oxides in shipbuilding steel with Zr addition; the same phenomenon was also found in shipbuilding steel with Mg addition.[38] However, unlike MnS inclusions that could be analyzed separately, the study of TiN evolution in solid steel was more complex, because it was often accompanied by other oxides or sulfides. Choi et al.[24] studied the effect of nitrogen content on the formation of inclusions during heating at 1200 ºC in Fe-Al-Ti-N system. It was found that the proportion of oxide inclusions with TiN in all samples increased after re-heating at 1200 ºC. From this system, they also found that promoting the formation of TiN instead of TiS was more conducive to grain refinement in the Fe-Al-Ti-O-N-S system during heating at 1200 °C.[25]
In addition to the research on the evolution of non-oxide inclusions, oxide evolution in solid steel has been paid increasing attention. Li et al. [31,34] studied the evolution of Ti-bearing oxides heated at 1300 °C. It was found that for the homogeneous Al-Ti oxide, not only does the element distribution change from a homogeneous to a heterogeneous oxide consisting of an Al-rich part and a Ti-rich part due to the diffusion of elements in the oxide, but in addition, the morphology changed from spherical to irregular. Further, the evolution of silico-manganate inclusions in stainless steel was also studied. Ren et al.[27] found that the inclusion type would transform from a silico-manganate inclusion to a manganese chromite inclusion by the reaction of Cr in the matrix with silico-manganate inclusion during heat treatment. The primary factors affecting the reaction were heating temperature and inclusion size. The experimental phenomena were similar to those reported by Shibata et al.[28] From the above results, the mechanisms of oxide evolution determined during the re-heating process were as follows: (a) the diffusion of elements inside oxides owing to the crystallization of glassy oxide; and (b) the interfacial reaction between oxides and the alloy elements in the matrix.
Whether one oxide could become a beneficial inclusion that induces AF formation is known to depend not only on the oxide size, but also on the composition distribution of the oxide.[9,39,40] The above results confirmed that the re-heating process would cause the oxide composition distribution to change. Ti-Zr oxide is the product of the Ti-Zr complex deoxidation. As-cast Ti-Zr oxide contains the central Ti-Zr-Al-O with a homogeneous composition distribution and surface MnS.[41] Additionally, there is a Mn-depletion zone (MDZ) in the vicinity of the as-cast Ti–Zr oxide, which promotes AF formation. However, during the re-heating process, the Ti-Zr-Al-O could undergo element enrichment, MnS on the surface could decompose and lead to the disappearance of MDZ, and other inclusions, such as TiN, could precipitate on the surface of Ti-Zr-Al-O, each of these could determine whether the Ti-Zr oxide can be used as an effective inclusion to induce AF formation. Moreover, the evolution of non-oxide effective inclusions in the Ti-Zr deoxidized steel, such as TiN, also has a certain effect on AF formation. Based on this, the purpose of this study was to choose Ti-Zr deoxidized low carbon steel as the research object, exploring the effects of different heating temperatures, isothermal holding times, and cooling modes on the evolution of inclusions.
Experimental Procedure
Material
The Ti-Zr deoxidized low carbon steel was melted in a 30 kg vacuum induction furnace in a magnesia crucible. The raw materials were high-purity Fe (99.95 wt pct), Cr (99.98 wt pct), Ni (99.95 wt pct), Mo (99.86 wt pct), Mn (99.92 wt pct), Si (99.47 wt pct), C (99.95 wt pct), Ti (99.93 wt pct), and Zr (99.95 wt pct). Among of these, Fe, Cr, Ni, and Mo were first placed in the crucible, and then the power was turned on. After melting the alloys under low pressure (under 10 Pa), argon gas was introduced and the furnace pressure was increased to approximately 0.04 MPa. Then Si, Mn, C, Ti, and Zr were sequentially added at 3 minutes intervals. After refining for 20 minutes, the molten steel was cast under argon protection at 1560 °C. The detailed smelting process was the same as the one mentioned in the previous study.[42] The chemical composition of the Ti-Zr deoxidized low carbon steel is presented in Table I, and the melting point of this steel was approximately 1510 °C. It should be noted that Al was not added in the smelting process; the source of Al might have been the raw materials.
High-Temperature Resistance Furnace Experiments
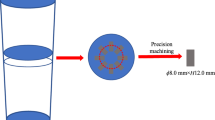
The high-temperature experiments with different heating temperatures, isothermal holding times, and cooling modes were carried out by resistance furnace with high pure Ar (purity > 99.999 pct) as protective atmosphere. First, the samples 8.0×8.0×4.0 mm in size were sectioned from the half-radius of the ingots to ensure the consistency of inclusions in all samples, as shown in Figure 1. In addition, prior to the high-temperature experiments, all sample surfaces were coated with antioxidant coating to prevent sample surface oxidation. Then, the samples were heated to the target temperatures (1100 ºC, 1200 ºC, 1300 ºC, and 1400 ºC) and held for 0.5, 1.0, or 2.0 hours. Finally, after isothermal holding, the samples were water quenched directly to retain the inclusion characteristics at the target temperatures; this cooling mode was named “cooling mode 1”. Moreover, simultaneously, another batch of identical samples that was sectioned from the same position in the same ingot was first cooled to 1000 °C with furnace cooling and then water quenched, to retain the new inclusion characteristics; this cooling mode was named “cooling mode 2”. The applied isothermal holding and cooling processes are schematically illustrated in Figure 2.
After cooling to ambient temperature, the samples were treated with standard grinding and polishing; the inclusion characteristics were then analyzed using automatic/manual scanning electron microscopy (SEM, EVO18) equipped with energy-dispersive spectroscopy (EDS). In addition, for some specific inclusions, electron probe microanalysis (EPMA, JXA-8530A) with wavelength-dispersive spectroscopy (WDS) was used for line analysis.
High-Temperature In Situ Observation Experiments
The in situ observation experiments at high temperature were conducted using high-temperature confocal laser scanning microscopy (HT–CLSM, VL2000DX–SVF17SP/15FTC). The samples were also sectioned from the half–radius of the ingots, as shown in Figure 1; detailed information about the sample specification, principles, and operation of the HT–CLSM experiment are provided in previous studies.[42,43] The samples were first heated to 1000 ºC with a heating rate of 10.0 ºC/s, and then to the target temperatures of 1300 and 1400 ºC with a heating rate of 2.0 ºC/s. After isothermally holding for 180 seconds at the target temperature, the samples were cooled to 1000 ºC at a cooling rate of 1.0 ºC/s to observe whether new inclusions precipitated during the cooling process. For the sake of observing inclusion evolution, images were recorded at 2 frames per second.
Results and Discussion
As-Cast Inclusion Characteristics
Figure 3 shows the inclusion characteristics in as-cast sample. It could be seen that the inclusion size distribution in as-cast samples was mainly in 1 to 3 μm. The main components of the oxides in as-cast sample were Ti, Zr, and Al, as shown in Figure 3(b), in which the content of Al in oxide was much less than that of Ti and Zr. Moreover, there were MnS precipitated during solidification process attaching on the oxide surface, as shown in Figure 4 presenting the SEM images and EDS mapping images of the typical Ti-Zr oxide. Therefore, the Ti-Zr oxides in as-cast sample were mainly compound oxides of “Ti-Zr oxide+MnS.” In this study, the compound inclusions were defined as A+B, in which A represents the main inclusion and B the inclusion attached to A.
In addition to Ti-Zr oxides, there were pure MnS, pure TiN, and “Ti-Mn-S” inclusions in as-cast sample, as shown in Figure 3(c). The “Ti-Mn-S” inclusions in the Ti-Mn-S ternary diagram actually contained two types of inclusions, namely MnS+TiN compound inclusion and TiN+MnS compound inclusion; the former main inclusion was MnS, while the latter was TiN. Since there is no N element in the ternary diagram, the pure TiN inclusions mainly located at the corner of Ti component, and the TiN+MnS compound inclusions mainly located in the vicinity of Ti component. However, the absence of detected N content in MnS+TiN compound inclusions was due to the limited accuracy of EDS analysis for the attached inclusion of TiN. For the sake of distinguishing these two types of compound inclusions, the SEM images and EDS mapping images of TiN+MnS compound inclusion and MnS+TiN compound inclusion are shown in Figure 5.
Effect of Heating Temperature and Isothermal Holding Time on Inclusion Characteristics
In order to retain the inclusion characteristics at high temperatures, the experimental samples were cooled by cooling mode 1 following high-temperature holding. Figure 6 presents the number density and size distribution of entire inclusions in the samples with different heating temperatures and holding times. Compared with the as-cast sample, the inclusions number density in the heated samples decreased except for the sample held at 1300 °C for 1 hour. When the holding time was 0.5 hours, the inclusions number density decreased with the heating temperature increase. However, as the holding time increased to 1 hour and 2 hours, respectively, in the heating temperature range of 1100 °C to 1400 °C, the inclusions number density reached the maximum value at 1300 °C and 1200 °C, which indicated both heating temperature and holding time all affected the entire inclusion number density. Similarly, the couple effect of heating temperature and holding time also affected the inclusion size distribution, due to inclusion coarsening and dissolution during re-heating process.
Figure 7 shows the ternary composition diagrams of the entire inclusions in the samples with different heating temperatures and holding times. It should be noted that there may be element enrichment areas in the oxide due to the element diffusion during re-heating process,[34] or new oxide may be formed by reaction between prior oxide with matrix alloy elements.[27] Therefore, before using automatic statistics, 5~10 oxides were detected manually to determine whether the oxide composition was uniform. Additional manual statistical analysis was used for the oxides with uneven composition. In the study of oxide composition, the automatic statistical analysis results were used for the samples heated at 1100 °C and 1200 °C, while the manual statistical analysis results were used for the samples heated at 1300 °C and 1400 °C. It could be found that the oxides in all re-heated samples were mainly Ti-Zr oxides, which was similar to that in the as-cast sample. But with the increase of heating temperature and holding time, the content of Al in oxides varied, especially when the heating temperature was 1400 °C, the content of Al in part Ti-Zr oxides was higher than that in other heating temperatures samples. Meanwhile, the Si-Mn-Al oxide was found in part Ti-Zr oxides in the sample heated at 1400 °C for 1 and 2 hours, as shown in Figure 7(k), which was not found in as-cast sample and other heating temperature samples. This will be analyzed and discussed in detail below. Moreover, compared with the as-cast Ti-Mn-S ternary diagram, there were no significant differences in composition of that in all heated samples except for the variation of number density, as shown in Figures 7(c, f, i, l).
For the convenience of description, the inclusions in this study are divided into oxides, nitrides, and sulfides according to the types of main inclusion. For example, nitrides include not only pure TiN inclusion, but also TiN+MnS compound inclusion, because TiN is the main inclusion in the compound inclusion. Similarly, the criterion applies to sulfides and oxides.
Figure 8 presents the Ti-Zr oxides morphologies after varied heating temperatures and holding times. When the heating temperature was lower and the isothermal holding time was shorter, the morphology of Ti-Zr oxide did not noticeably change, but as both the temperature and the isothermal holding time increased, the Ti-Zr oxide shape changed from an irregular, curved surface to a smooth and spherical surface. Moreover, as the heating temperature and holding time increased, the color difference areas, corresponding to different composition, clearly began to appear in the Ti-Zr oxides.
Figure 9 shows the SEM images and EDS mapping images of Ti-Zr oxides held at 1100 ºC, 1200 ºC, 1300 ºC, and 1400 ºC for 2 hours. Some differences were observed compared with the as-cast Ti-Zr oxide. When the heating temperature was 1100 ºC, there was a Ti-rich zone, mainly TiN, in Ti-Zr oxide, while it was not found in as-cast Ti-Zr oxide. When the heating temperature was 1200 ºC, the MnS on the surface of as-cast Ti-Zr oxide decomposed and Mn diffused into the oxide,[44,45,46] or the matrix. Moreover, when the heating temperature was 1300 °C, besides a Ti-rich zone, a Ti-Al-rich zone, mainly Ti-Al oxide, was formed in Ti-Zr oxide. As the heating temperature increased to 1400 °C, the TiN dissolved; the internal elements of the Ti-Zr oxide formed not only the Ti-Al-rich region, but also a Zr-rich and a Si-Mn-Al-rich regions. Elemental Si was not found in the Ti-Zr oxides for as-cast and other heating temperatures, whereas it was found in the Ti-Zr oxide that was heated at 1400 ºC, in which it was present as a Si-Mn-Al oxide outer layer outside the inclusion. Consequently, it would be interesting to explore the Ti-Zr oxide evolution during heating at 1400 ºC. As mentioned above, as the heating temperature increased, the surface MnS decomposed. When the heating temperature reached 1400 ºC, the dissolved Mn would react with Si in the matrix and Al, O in the oxide to form a liquid inclusion of Si-Mn-Al oxide, which would wrap around the Ti-Zr oxide to form a solid inclusion in the center and a liquid inclusion on the surface. After the sample was cooled to room temperature by water quenching, the surface liquid inclusion retained its spherical shape, which might be one of the reasons for the spherical Ti-Zr oxide surface.
In order to verify liquid inclusion formed during heating process, the high-temperature in situ observation experiment was carried out. Before in situ observation experiment, the “black spots” on the sample surface under the view field were analyzed by SEM-EDS to determine whether they were Ti-Zr oxides. Figure 10 presents the inclusions SEM image, EDS mapping images of inclusion 3, and in situ observation images during heating from 200 to 1400 ºC. SEM-EDS results confirmed that the 4 inclusions showed by the color dotted circle in Figure 10(a) were all Ti-Zr oxides. The 4 inclusions observed in situ in Figure 10(c) corresponded to that in Figure 10(a). It could be seen that these 4 inclusions were coarsened to varying degrees during heating from 200.3 to 1165.2 ºC. As the heating temperature increased to 1304.9 ºC, the liquid molten pool formed at the oxide. Previous thermodynamic calculation found that solid MnS would transform as liquid MnS as the heating temperature was about 1300 ºC.[41] Therefore, the molten pool observed in situ at 1304.9 ºC may be due to the liquefaction of MnS on the Ti-Zr oxide surface. Moreover, when the heating temperature increased to 1400.2 ºC, the size of molten pool increased, which indicated that more liquid phase was formed. At this temperature, the liquid MnS would dissolve back, and Ti-Zr oxides had a high melting point and not liquefied. So there may be other liquid inclusions formed, which also confirmed the mentioned above.
Figure 11 presents images of the line scanning results of the Ti-Zr oxide heated at 1400 ºC for 2 hours to further determine the elemental distribution and the formation mechanism of each element enrichment zone. From this, no Ti concentration gradient was found in the inclusion except for at the two ends of line scanning. For Zr and Al, an evident elemental concentration gradient in the inclusion was found, which indicated that Zr and Al diffusion occurred in the inclusion; therefore, the Ti-Al-rich and Zr-rich zones could be considered to be caused by the elements diffusion. Moreover, Si was mainly in the Si-Mn-Al-rich zone, and no concentration gradient was found in other parts of the inclusion, which indicated that the Si-Mn-Al-rich zone was formed by a reaction between the inclusion and the matrix. Finally, Mn was not only in the Si-Mn-Al-rich zone, but also in the Ti-Al-rich and Zr-rich zones, which demonstrated that the dissolved Mn from the surface-decomposed MnS not only reacted with the matrix alloy element, but also simultaneously diffused into the inclusion.
Figure 12 shows the number density and equivalent circular diameter (ECD) distribution of oxides under different heating temperatures and holding times. When the heating temperature was in the range of 1100 to 1300 ºC, the oxide number density was not significantly different, but the proportion of small-sized oxides (smaller than 1.0 μm) increased with increasing holding time. However, when the heating temperature was 1400 ºC, the oxide number density increased significantly, increasing to 35~38 mm-2, which was nearly 10~12 mm-2 higher than that at other heating temperatures. The proportion of small-sized oxides initially increased and then decreased. Generally speaking, the oxide number density was not expected to change significantly during the heating process. However, in the statistical analysis, the necking phenomenon of Ti-Zr oxides was found in the samples heated at 1200 ºC and 1300 ºC, similar to the MnS evolution during heating at higher temperatures,[20] but it did not fracture after holding for 2 hours, as shown in Figure 13. Therefore, the fracture of the necking Ti-Zr oxides might be the reason for the increase in the oxide number density at 1400 ºC. However, it should be noted that the increase in oxide number density was not large, which indicated that only specific Ti-Zr oxides fractured; therefore, further investigation was required in combination with SEM-EDS and HT-CLSM.
Figure 14 shows the number density of nitrides and sulfides at different heating temperatures and holding times. It can be seen from Figure 14(a) that the change in the nitride number density was the result of the combined effect of heating temperature and holding time. When the heating temperature was 1100 ºC or 1200 ºC, there was no clear change in the nitride number density. However, when the heating temperature was higher than 1300 ºC and the holding time was longer than 1 hour, the nitride number density began to decrease, especially at 1400 ºC for 1 hour and 2 hours, for which the nitride number density decreased sharply, to approximately 5 mm-2. From Figure 14(b), it can be seen that the evolution of sulfide number density was similar to that of nitrides, which was also affected by the coupling effect of heating temperature and holding time, as confirmed in previous studies.[18,19,20,21] It is well known that the smaller the inclusion size, the larger the contact area between inclusions with the matrix, and the more easily the inclusions can dissolve into the matrix during the isothermal holding process; this might explain why small-sized inclusions preferentially dissolved in the early stage of isothermal holding. Moreover, the MnS went through necking-fracture into multiple small-sized MnS segments, which was confirmed to be the result of the combined effect of heating temperature and holding time by a mathematical model.[20] When the large-sized MnS became multiple small-sized MnS segments by surface diffusion, the number density of MnS increased; as the isothermal holding time continued to increase, these fragments dissolved and resulted in a decrease in the number density.
Effect of Cooling Mode on Inclusion Characteristics
Generally, after heating at high temperature, the as-cast low carbon steel would be cooled to a certain temperature for hot working. Figure 15 displays the in situ observation of inclusions evolution during the cooling process from 1300 to 1000 ºC. During the cooling process, coarsening of inclusion, as shown by the yellow arrow, occurred, as well as the precipitation of new inclusions, as shown by the green arrow. The in situ observations at high temperature confirmed that the inclusion characteristics changed during the cooling process. On this basis, the influence of different cooling modes on the inclusion characteristics was therefore studied at different heating temperatures using a resistance furnace.
Figure 16 presents the effect of the cooling mode on the entire inclusion number density. When the heating temperature was 1100 ºC or 1200 ºC, the cooling mode had no apparent influence on any inclusion number density. However, when the heating temperature was increased to 1300 ºC or 1400 ºC, more inclusions could be obtained by cooling mode 2. Moreover, it can be observed in Figures 16(b) and (c) that different cooling modes had no significant effect on the oxide number density, while the number densities of nitrides and sulfides were greatly affected by the cooling mode. Specifically, more nitrides and sulfides could be obtained by cooling mode 2 regardless of whether the heating temperature was 1300 ºC or 1400 ºC. This was mainly due to the fact that when samples were cooled from the target temperature to 1000 ºC, the slower cooling rate of cooling mode 2 allowed more time for new inclusions to precipitate. In addition, the cooling time from 1400 ºC to 1000 ºC was longer than that from 1300 ºC to 1000 ºC; hence, the former provided a longer time for the new inclusion precipitation. Therefore, compared with heating at 1300 ºC, the heating temperature of 1400 ºC could result in more nitrides and sulfides using the same cooling mode.
Although under different cooling modes, the oxide number density did not change significantly, the morphology and composition distribution of the oxides varied. Figure 17 shows SEM images and EDS mapping images of the Ti-Zr oxides cooled by cooling mode 2. It can be seen that the Ti-Zr oxide surface was covered with MnS (and TiN), which was not observed on Ti-Zr oxides cooled by cooling mode 1. For the sample heated at 1300 ºC, the effect of cooling mode 2 was only the precipitation of MnS in part of the Ti-Zr oxide surface. For the sample heated at 1400 ºC, not only did MnS precipitate on the inclusion surface, but a layer of TiN did as well. Compared with Figure 9(d), when the sample was directly water quenched after holding at 1400 ºC for 2 hours, the outermost layer of the Ti-Zr oxide was primarily Si-Mn-Al oxide. However, after being cooled to 1000 °C with furnace cooling, MnS and TiN precipitated on the outermost layer of Ti-Zr oxide.
Figure 18 shows the images of the line scanning results of the Ti-Zr oxide cooled by cooling mode 2. A concentration gradient of Ti, Zr, and Al from the MnS zone was found in the outer layer to the Zr-rich zone. This indicated that the precipitation mechanism of MnS might be another in which MnS possessed a similar crystal structure to that of the core-rich oxide,[47] which reduced the MnS nucleation barrier and promoted MnS precipitation. In addition, the concentration gradient of Ti could be clearly observed in the Si–Mn–Al oxide zone adjacent to the TiN zone; this indicated that the formation of the outermost TiN was due not only to the reaction between the Ti and N dissolved in the matrix but also to the diffusion of elemental Ti in the inclusion, thus producing a higher Ti concentration at the edge of the Si-Mn-Al oxide zone, which also promoted the precipitation of TiN.
The above results confirmed that different cooling modes not only had a significant effect on the number density of nitrides and sulfides, but also had a certain effect on the morphology and composition distribution of the Ti-Zr oxide. For nitrides, which could usually be inclusions that effectively induce AF formation,[48,49,50] the increase in number density was bound to increase the probability of AF formation. Moreover, the change in the composition distribution of the Ti-Zr oxide also determined whether it could be used as an effective inclusion to induce AF formation. According to the research results, when cooling mode 1 was used after holding completion, there would be a Ti-rich zone in the oxide with heating temperature in the range of 1100 ºC to 1300 ºC, primarily TiN. Because of the low lattice misfit between TiN and ferrite,[48] these oxides could be used as effective inclusions to induce AF formation. However, when the heating temperature was 1400 ºC, the outermost layer of the Ti-Zr oxide was enveloped in Si-Mn-Al oxide; therefore, this oxide might not be as effective at inducing AF formation. However, at the same heating temperature of 1400 ºC, when cooling mode 2 was used, a layer of TiN precipitated on the oxide surface, which might render the unstable oxide an effective oxide for inducing AF formation.
Conclusions
In the present work, the effects of different heating temperatures, isothermal holding times, and cooling modes on the evolution of inclusions in Ti-Zr deoxidized low carbon steel were investigated. The results obtained were as follows:
-
(1)
Compared with as-cast sample, the entire inclusion characteristics in heated samples were affected by the couple effect of heating temperature and holding time.
-
(2)
During the re-heating process, with the increase in heating temperature and holding time, the shape of the Ti-Zr oxide changed from an irregular, curved surface to a smooth and spherical surface due to a liquid inclusion of Si-Mn-Al oxide covering the outermost layer.
-
(3)
During heating in the range of 1100 to 1400 ºC for 2 hours, the composition distribution of Ti-Zr oxide changed from homogeneous to heterogeneous. This is mainly due to the diffusion of elements in the Ti-Zr oxide, but during heating at 1400 ºC, the reaction between Ti-Zr oxide and elements in the matrix might have been another cause.
-
(4)
Compared with cooling mode 1, cooling mode 2 both caused the number density of nitrides and sulfides to increase and changed the unstable oxide to an effective one by TiN precipitating on the surface, particularly when the heating temperature was 1400 ºC.
References
L. F. Zhang and B. G. Thomas: ISIJ. Int., 2003, vol. 43, pp. 271-91.
L. F. Zhang and B. G. Thomas: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 733-61.
M. Fattahi, N. Nabhani, M. Hosseini, N. Arabian, and E. Rahimi: Micron, 2013, vol. 45, pp. 107-14.
H. Suito, H. Ohta, and S. Morioka: ISIJ Int., 2006, vol. 46, pp. 840-46.
S. S. Babu and H. K. D. H. Bhadeshia: Mater. Trans. JIM, 1991, vol. 32, pp. 679-88.
J. H. Shim, Y. W. Cho, S. H. Chung, J. D. Shim, and D. N. Lee: Acta Mater., 1999, vol. 47, pp. 2751-60.
I. Madariaga and I. Gutiérrez: Acta Mater., 1999, vol. 47, pp. 951-60.
Z. Zhang and R. A. Farrar: Mater. Sci. Technol., 1996, vol. 12, pp. 237-60.
W. Z. Mu, H. Shibata, P. Hedström, P. G. Jönsson, and K. Nakajima, Metall. Mater. Trans. B, 2016, vol. 47B, pp. 2133-47.
J. H. Shim, Y. J. Oh, J. Y. Suh, Y. W. Cho, J. D. Shim, J. S. Byun, and D. N. Lee: Acta Mater., 2001, vol. 49, pp. 2115-22.
W. Z. Mu, H. Shibata, P. Hedström, P. G. Jönsson, and K. Nakajima, Steel Res. Int., 2016, vol. 87, pp. 10-14.
Y. Liu, G. Q. Li, X. L. Wan, X. G. Zhang, Y. Shen, and K. M. Wu: Ironmak. Steelmak., 2019, vol. 46, pp. 113-23.
J. Pu, S. F. Yu, and Y. Y. Li: J. Alloys Compd., 2017, vol. 692, pp. 351-58.
H. Nako, Y. Okazaki, and J. G. Speer: ISIJ Int., 2015, vol. 55, pp. 250-56.
C. K. Lin, Y. C. Pan, Y. H. F. Su, G. R. Lin, W. S. Hwang, and J. C. Kuo: Mater. Charact., 2018, vol. 141, pp. 318-27.
A. I. Zaitsev, A. V. Koldaev, A. V. Amezhnov, and N. G. Shaposhnikov: Metallurgist, 2016, vol. 60, pp. 721-29.
S. Y. Kitamura: ISIJ Int., 2011, vol. 51, pp. 1943.
D. Gnanamuthu, T. Z. Kattamis, M. C. Flemings, and R. Mehrabian: Metall. Trans., 1974, vol. 5, pp. 2557-67.
Y. V. Murty, T. Z. Kattamis, R. Mehrabian, and M. C. Flemings: Metall. Mater. Trans. A, 1977, vol. 8A, pp. 1275-82.
X. J. Shao, X. H. Wang, M. Jiang, W. J. Wang, and F. X. Huang: ISIJ Int., 2011, vol. 51, pp. 1995-01.
X. J. Shao, X. H. Wang, C. X. Ji, H. B. Li, Y. Cui, and G. S. Zhu: Int. J. Min. Met. Mater., 2015, vol. 22, pp. 483-91.
22. X. J. Shao, X. H. Wang, M. Jiang, W. J. Wang, F. X. Huang, Y. Q. Ji (2011) Acta Metall. Sin. 47:1210-15
S. Matsuda, and N. Okumura: ISIJ. Int., 1978, vol. 18, pp. 198-05.
W. Choi, H. Matsuura, and F. Tsukihashi: ISIJ. Int., 2013, vol. 53, pp. 2007-12.
W. Choi, H. Matsuura, and F. Tsukihashi: Metall. Mater. Trans. B, 2016, vol. 47B, pp. 1851-57.
J. Guo, X. R. Chen, S. W. Han, Y. Yan, and H. J. Guo: Int. J. Min. Met. Mater., 2020, vol. 27, pp. 328-39.
Y. Ren, L. F. Zhang, P. C. Pistorius (2007) Metall. Mater. Trans. B, 48B:2281-92
H. Shibata, T. Tanaka, K. Kimura, and S. Y. Kitamura: Ironmak. Steelmak., 2010, vol. 37, pp. 522-28.
X. L. Zhang, S. F. Yang, C. S. Liu, J. S. Li, and W. X. Hao: JOM, 2018, vol. 70, pp. 958-62.
C. S. Liu, K. H. Kim, S. J. Kim, J. S. Li, S. Ueda, X. Gao, H. Shibata, and S. Y. Kitamura: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 1875-84.
M. G. Li, H. Matsuura, and F. Tsukihashi: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 1915-23.
W. Choi, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2011, vol. 51, 1951-56.
M. G. Li, H. Matsuura, and F. Tsukihashi: Mater. Charact., 2018, vol. 136, pp. 358-66.
M. G. Li, H. Matsuura, and F. Tsukihashi: Metall. Mater. Trans. A, 2019, vol. 50A, pp, 863-73.
G. Cheng, W. F. Li, X. G. Zhang, and L. F. Zhang: Metals, 2019, vol. 9, p. 642.
Y. P. Chu, W. F. Li, Y. Ren, and L. F. Zhang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2047-62.
X. D. Zou, H. Matsuura, and C. Wang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 1134-38.
Q. Y. Wang, X. D. Zou, H. Matsuura, and C. Wang: JOM, 2018, vol. 70, pp. 521-26.
D. S. Sarma, A. V. Karasev, and P. G. Jönsson: ISIJ Int., 2009, vol. 49, pp. 1063-74.
W. Z. Mu, P. G. Jönsson, and K. Nakajima: High Temp. Mat. Proc., 2017, vol. 36, pp. 309-25.
Y. K. Yang, D. P. Zhan, H. Lei, G. X. Qiu, Z. H. Jiang, and H. S. Zhang: ISIJ Int., 2019, vol. 59, pp. 1545-51.
Y. K. Yang, D. P. Zhan, H. Lei, Y. L. Li, G. X. Qiu, R. J. Wang, Z. H. Jiang, and H. S. Zhang: Metall. Mater. Trans. B, 2020, vol. 51B, pp. 480-91.
Y. K. Yang, D. P. Zhan, H. Lei, G. X. Qiu, Y. L. Li, Z. H. Jiang, and H. S. Zhang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2536-46.
J. S. Byun, J. H. Shim, Y. W. Cho, and D. N. Lee: Acta Mater., 2003, vol. 51, pp. 1593-06.
K. Yamamoto, T. Hasegawa, and J. I. Takamura: ISIJ Int., 1996, vol. 36, pp. 80-86.
B. W. Zhou, G. Q. Li, X. L. Wan, Y. Li, and K. M. Wu: Met. Mater. Int., 2016, vol. 22, pp. 267-75.
Y. Li, X. L. Wan, W. Y. Lu, A. A. Shirzadi, O. Isayev, O. Hress, and K. M. Wu: Mater. Sci. Eng. A, 2016, vol. 659, pp. 179-87.
J. M. Gregg and H. K. D. H. Bhadeshia: Acta Mater., 1997, vol. 45, pp. 739-48.
J. M. Dowling, J. M. Corbett, and H. W. Kerr: Metall. Trans. A, 1986, vol. 17A, pp.1611-23.
W. Z. Mu, P. G. Jönsson, and K. Nakajima: J. Mater. Sci., 2016, vol. 51, pp. 2168-80.
Acknowledgments
The authors are grateful for the support from the National Natural Science Foundation of China (No. 51874081, 51574063) and Fundamental Research Funds for the Central Universities (N150204012, N180725021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted on August 11, 2021; accepted March 8, 2021.
Rights and permissions
About this article
Cite this article
Yang, Y., Zhan, D., Lei, H. et al. Research on Inclusion Evolution during Re-Heating Process in Ti-Zr Deoxidized Low Carbon Steel. Metall Mater Trans B 52, 1839–1853 (2021). https://doi.org/10.1007/s11663-021-02150-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02150-5