Abstract
Molten iron was equilibrated with various compositions of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO with the oxygen partial pressure of 5.22 × 10−12 atm at 1823 K (1550 °C) and 1.41 × 10−11 atm at 1873 K (1600 °C). From the concentration of P in molten iron at equilibrium, the activity of P2O5 relative to the hypothetical pure liquid P2O5 was determined. The saturating MgO phase at equilibrium was detected by X-ray diffraction for each sample, and thus, the activity of 3MgO·P2O5 was also estimated. The activity of P2O5 and 3MgO·P2O5 increased with the increase of 3CaO·P2O5 content in the solid solution. In addition, the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO was larger than that saturated with CaO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since phosphorus segregates in the grain boundary of austenite, which increases the fracture appearance transition temperature to cause the cold embrittlement of steel,[1] it should be removed from molten iron and steel during the dephosphorization process as much as possible. Usually CaO is adopted for the dephosphorization process, and in most cases, extra CaO is added to slag to achieve a high dephosphorization efficiency, while the CaO consumption and slag volume significantly increase. Meanwhile, the generated slag after the dephosphorization process is hard to be recycled since the unreacted CaO remains in slag or CaO-based compounds precipitate during the solidification of slag.
It is well known that CaO reacts with SiO2 to form 2CaO·SiO2 on the surface of CaO in the CaO-FeO-SiO2-P2O5 system during the dephosphorization process and then 2CaO·SiO2 reacts with P2O5 to form the 2CaO·SiO2-3CaO·P2O5 solid solution.[2–4] In addition, 2CaO·SiO2 and 3CaO·P2O5 form a pseudo-binary solid solution over a wide composition range at steelmaking temperature.[5] Based on the large partition ratio of P2O5 between the solid solution and liquid slag,[6–8] the multiphase flux has been considered for use in the dephosphorization process.
To understand the multiphase flux better, the reaction mechanisms between solid CaO or 2CaO·SiO2 and the FeO x -CaO-SiO2-P2O5 system have been intensively studied.[9–15] The phase relationship for the CaO-FeO-SiO2-P2O5(-Al2O3) system has been also studied.[16–18] The activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution has been measured by the chemical equilibration method at 1573 K (1300 °C) by Hasegawa et al.[19] or at 1823 and 1873 K (1550 and 1600 °C) by the current authors.[20] Since the excess CaO is being added to slag in the actual dephosphorization process, the thermodynamics of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with CaO has been also investigated by the current authors.[21] In the current study, the thermodynamic properties of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO were measured to investigate the effect of MgO on the property of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution since the dephosphorization slag contains a certain amount of MgO.
Experimental
The 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO was equilibrated with molten iron at 1823 and 1873 K (1550 and 1600 °C). The mixture of SiO2, CaHPO4·2H2O, MgO, and CaO was sintered to prepare the 2CaO·SiO2-3CaO·P2O5 solid solution powder containing MgO or both MgO and CaO. The mixture was heated in air at 1873 K (1600 °C) in a platinum crucible for 48 hours, and then the sintered material was ground into fine powder before sintering for the next 48 hours. After the second sintering, the mixture was ground into fine powder again and about 1.5 g powder was charged in steel dies to be pressed into a cylindrical specimen (diameter: 18 mm, thickness: 3 mm) at 50 MPa. The initial compositions of oxide specimens are summarized in Table I and shown on the CaO-SiO2-P2O5 ternary diagram in Figure 1.
Projection of the initial composition of oxide specimen onto the CaO-SiO2-P2O5 ternary system. The specimens marked as open circles turned into molten slag at equilibrium. The boundary and tie lines in the phase diagram were determined at 1773 K (1500 °C) by Gutt[22]
Since the phase relationship of the CaO-SiO2-P2O5 system at 1823 and 1873 K (1550 and 1600 °C) is deficient, the boundary and tie lines in Figure 1 were determined at 1773 K (1500 °C) after Gutt.[22] The formation of desired phases was confirmed by X-ray diffraction (XRD). In all oxide specimens, the MgO phase was detected and the CaO phase was also detected in oxide specimens of Nos. 5-1 and 5-2. The XRD patterns of oxide specimens Nos. 1-2 and 5-1 are shown as examples in Figures 2 and 3, respectively.
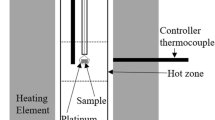
For achieving the equilibrium quickly, only 10 g of electrolytic iron with the initial concentration of P < 0.0003 mass pct was equilibrated with the prepared cylindrical oxide in a magnesia crucible (outer diameter: 25 mm, inner diameter: 20 mm, height: 60 mm) in an alumina reaction tube (outer diameter: 60 mm, inner diameter: 50 mm, length: 1000 mm). The equilibrium duration was 24 hours, which was enough to reach the equilibrium confirmed by previous research.[20,21] Temperature of the hot zone was determined by a B-type thermocouple before each experiment, and the experimental temperature was monitored by a B-type thermocouple placed at the bottom of the magnesia crucible. The oxygen partial pressure was controlled by the mixture of CO and CO2 gases with the total flow rate of 366 mL/min according to Eq. [1]. The oxygen partial pressure was 5.22 × 10−12 atm at 1823 K (1550 °C) or 1.41 × 10−11 atm at 1873 K (1600 °C) with the CO/CO2 ratio of 110/1:
After the equilibrium was established, the sample was quenched by Ar stream at the outside of the furnace. Concentrations of P in iron and P2O5 in oxide specimen were analyzed by spectrophotometry. Compositions of CaO, FeO, and MgO in oxide specimen were determined by ICP-OES and that of SiO2 was determined by gravimetry.
Results and Discussion
Oxide specimens of Nos. 2-4 and 4-4 turned into molten slag after experiments as shown by open circles in Figure 1. The concentration of P in iron and the composition of oxide specimen after reaction are summarized in Table II and shown on the CaO-SiO2-P2O5 ternary diagram in Figure 4.
Since molten iron coexisted, iron oxide in the oxide specimen was assumed to be FeO. At equilibrium, the MgO phase was detected in all oxide specimen and the CaO phase was also detected in the oxide specimen of Nos. 5-1 and 5-2 by XRD. The mole fraction of 3CaO·P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution shown in Table III was calculated from the analyzed compositions of SiO2 and P2O5 by assuming the pure pseudo-binary solid solution.
Equilibrium Concentration of P in Molten Iron and the Phosphorus Partition Ratio Between Solid Mixture and Molten Iron
The equilibrium concentration of P in molten iron and the phosphorus partition ratio between the mixture of the 2CaO·SiO2-3CaO·P2O5 solid solution and MgO and molten iron are shown in Figures 5 and 6 as a function of 3CaO·P2O5 content in the solid solution, respectively. The concentration of P in molten iron increased with the increase of the 3CaO·P2O5 content in the solid solution. By comparing at the same composition of the solid solution, the concentration of P at 1873 K (1600 °C) was larger than that at 1823 K (1550 °C). The phosphorus partition ratio did not change with the 3CaO·P2O5 content in the solid solution significantly. The effect of MgO content on the phosphorus partition ratio was also not obvious.
FeO Content and Activity Coefficient of FeO in the 2CaO·SiO2-3CaO·P2O5 Solid Solution Saturated with MgO
The FeO content in the oxide specimen at equilibrium is shown in Figure 7 as a function of P2O5 content. For most samples, the content of FeO was <1 mass pct. According to Eq. [2], the activity of FeO relative to hypothetical pure liquid FeO was calculated to be 7.75 × 10−2 at 1823 K (1550 °C) with the oxygen partial pressure of 5.22 × 10−12 atm, or 8.15 × 10−2 at 1873 K (1600 °C) with the oxygen partial pressure of 1.41 × 10−11 atm:
The activity coefficient of FeO is shown in Figure 8 and Table III as a function of the 3CaO·P2O5 content in the solid solution. The activity coefficient of FeO at 1873 K (1600 °C) was larger than that at 1823 K (1550 °C). Significant influence of CaO or MgO addition on the activity coefficient of FeO was not observed.
Activity and Activity Coefficient of P2O5 in the 2CaO·SiO2-3CaO·P2O5 Solid Solution Saturated with MgO
Due to the inevitable segregation during quenching, the concentrations of C and O in molten iron were calculated by Eqs. [3] and [4] and the reported interaction coefficients shown in Table IV instead of the analyzed values.
The analytical concentration of P in iron was not significantly affected by the segregation since the segregated P remained in the iron sample after quenching:
The activity of P2O5 relative to the hypothetical liquid P2O5 was calculated by Eq. [5] from the concentration of P in molten iron and the reported thermodynamic data.[25] The activity and activity coefficient of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO are shown in Figures 9 and 10 comparing with the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with CaO.[21] Both the activity and activity coefficient of P2O5 increased with the increase of the 3CaO·P2O5 content in the solid solution. The activity and activity coefficient at 1873 K (1600 °C) were larger than those at 1823 K (1550 °C) comparing at the same composition of the solid solution. In addition, the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO marked by solid circles and squares was larger than that saturated with CaO marked by open circles and squares in Figures 9 and 10. Furthermore, the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with both CaO and MgO marked by solid triangles was larger than that saturated with CaO and smaller than that saturated with MgO.
The compositions of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO are projected onto the CaO-MgO-SiO2 and CaO-MgO-P2O5 systems in Figures 11 and 12. Since the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO increased with the increase of the 3CaO·P2O5 content in the solid solution, the compositions at 1823 and 1873 K (1550 and 1600 °C) cannot locate at the three-phase equilibrium region as shown in Figure 12 with the phase relationship at 1273 K (1000 °C), while experimental results were located in the region. It was because that temperature of the phase diagram in Figure 12 was lower than the present experimental temperature and oxide specimens contained a certain amount of SiO2. Moreover, not only 2CaO·SiO2 and 2MgO·SiO2 but also 3CaO·P2O5 and 3MgO·P2O5 could form a solid solution as shown in Figures 11 and 12. Since the phase relationship for the CaO-MgO-SiO2 and CaO-MgO-P2O5 systems are unavailable at 1823 and 1873 K (1550 and 1600 °C), it was assumed that the composition range of the 2CaO·SiO2-2MgO·SiO2 solid solution and the 3CaO·P2O5-3MgO·P2O5 solid solution increase with the increase of temperature similar to the 2CaO·SiO2-3CaO·P2O5 solid solution.[5]
Projection of the compositions of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO at equilibrium on the CaO-MgO-SiO2 system[30]
Projection of the compositions of the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO at equilibrium on the CaO-MgO-P2O5 system[31]
Since the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with both CaO and MgO was smaller than that saturated with MgO, it is considered that the reaction between the solid solution and MgO does not produce a pure CaO phase in the current condition. Therefore, the final composition of the mixture of 2CaO·SiO2-3CaO·P2O5 solid solution and MgO was the mixture of the 2(Ca,Mg)O·SiO2-3(Ca,Mg)O·P2O5 solid solution and MgO.
Activity of 3MgO·P2O5 in the 2CaO·SiO2-3CaO·P2O5 Solid Solution Saturated with MgO
Since the 2CaO·SiO2-3CaO·P2O5 solid solution was saturated with MgO, the activity of MgO was unity, and thus, the activity of 3MgO·P2O5 relative to pure liquid 3MgO·P2O5 was calculated from the activity of P2O5 by using Eqs. [6] and [7] as shown in Figure 13 as a function of the 3CaO·P2O5 content in the solid solution. The activity of 3MgO·P2O5 increased with the increase of the 3CaO·P2O5 content in the solid solution. The effect of temperature on the activity of 3MgO·P2O5 was not clearly observed:
Conclusions
By applying the chemical equilibration method, the equilibrium between the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO and molten iron was observed at 1823 K (1550 °C) with the oxygen partial pressure of 5.22 × 10−12 atm and at 1873 K (1600 °C) with the oxygen partial pressure of 1.41 × 10−11 atm. The activity of P2O5 relative to the hypothetical pure liquid P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO increased with the increase of the 3CaO·P2O5 content in the solid solution. The activity of P2O5 in the solid solution saturated with MgO at 1873 K (1600 °C) was larger than that at 1823 K (1550 °C). In addition, the activity of P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO was larger than that saturated with CaO. The 2CaO·SiO2-3CaO·P2O5 solid solution mixed with MgO was not saturated with CaO, but the components of the solid solution saturated with MgO were considered to be the mixture of the 2(Ca,Mg)O·SiO2-3(Ca,Mg)O·P2O5 solid solution and MgO. Moreover, the activity of 3MgO·P2O5 in the 2CaO·SiO2-3CaO·P2O5 solid solution saturated with MgO also increased with the increase of the 3CaO·P2O5 content in the solid solution.
References
R. Viswanathan: Metall. Trans., 1971, vol. 2, pp. 809-15.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46, pp. 174-9.
H. Suito and R. Inoue: ISIJ Int., 2006, vol. 46, pp. 180-7.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46, pp. 188-94.
W. Fix, H. Heymann, and R.S. Heinke: J. Am. Ceram. Soc., 1969, vol. 52, pp. 346-7.
F. Pahlevani, S. Kitamura, H. Shibata, and N. Maruoka: ISIJ Int., 2010, vol. 50, pp. 822-9.
K. Shimauchi, S. Kitamura, and H. Shibata: ISIJ Int., 2009, vol. 49, pp. 505-511.
K. Ito, M. Yanagisawa, and N. Sano: Tetsu-to-Hagané, 1982, vol. 68, pp. 342-4.
S. Kitamura, S. Saito, K. Utagawa, H. Shibata, and D.G.C. Robertson: ISIJ Int., 2009, vol. 49, pp. 1838-44.
T. Hamano, S. Fukagai, and F. Tsukihashi: ISIJ Int., 2006, vol. 46, pp. 490-5.
S. Fukagai, T. Hamano, and F. Tsukihashi: ISIJ Int., 2007, vol. 47, pp. 187-9.
X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2009, vol. 49, pp. 1298-307.
X. Yang, H. Matsuura, and F. Tsukihashi: Tetsu-to-Hagané, 2009, vol. 95, pp. 268-74.
X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2010, vol. 50, pp. 702-11.
X. Yang, H. Matsuura, and F. Tsukihashi: Mater. Trans., 2010, vol. 51, pp. 1094-101.
X. Gao, H. Matsuura, I. Sohn, W. Wang, D.J. Min, and F. Tsukihashi: Metall. Mater. Trans. B, 2012, vol. 43B, pp. 694-702.
X. Gao, H. Matsuura, I. Sohn, W. Wang, D.J. Min, and F. Tsukihashi: Mater. Trans., 2013, vol. 54, pp. 544-52.
X. Gao, H. Matsuura, M. Miyata, and F. Tsukihashi: ISIJ Int., 2013, vol. 53, pp. 1381-85.
M. Hasegawa, Y. Kashiwaya, and M. Iwase: High Temp. Mater. Proc., 2012, vol. 31, pp. 421-30.
M. Zhong, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2015, vol. 55, pp. 2283-8.
M. Zhong, H. Matsuura, and F. Tsukihashi: Mater. Trans., 2015, vol. 56, pp. 1192-8.
W. Gutt: Nature, 1963, vol. 197, pp. 142-3.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, p. 7.
S. Ban-ya and S. Matoba: Tetsu-to-Hagané, 1962, 48, pp. 925-32.
E.T. Turkdogan: ISIJ Int., 2000, vol. 40, pp. 964-70.
K. Yamada and E. Kato: Tetsu-to-Hagané, 1979, vol. 65, pp. 264-72.
H.G. Hadrys, M.G. Frohberg, and J.F. Elliott: Metall. Trans., 1970, vol. 1, pp. 1867-74.
D. Dutilloy and J. Chipman: Trans. Metall. Soc. AIME, 1960, vol. 218, pp. 428-30.
H. Sakao and K. Sano: J. Jpn. Inst. Met., 1959, vol. 23, pp. 671-4.
Verein Deutscher Eisenhüttenleute (VDEh) ed.: SLAG ATLAS, 2nd edn. Verlag Stahleisen GmbH, Düsseldorf, 1995, p. 134.
Verein Deutscher Eisenhüttenleute (VDEh) ed.: SLAG ATLAS, 2nd edn. Verlag Stahleisen GmbH, Düsseldorf, 1995, p. 133.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, p. 14.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 13, 2015.
Rights and permissions
About this article
Cite this article
Zhong, M., Matsuura, H. & Tsukihashi, F. Thermodynamic Properties of Phosphorus Oxide in the 2CaO·SiO2-3CaO·P2O5 Solid Solution Saturated with MgO. Metall Mater Trans B 47, 1745–1752 (2016). https://doi.org/10.1007/s11663-016-0639-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0639-4