Abstract
Dephosphorization by using multiphase flux could considerably decrease the consumption of CaO and prevent the addition of fluorite. However, the equilibrium phase relationship within this system, which is of significant importance for understanding the formation mechanism of multiphase flux, remains unclear. Thus, it is required to provide reliable phase diagrams of the basic slag system of multiphase flux. In this research, the phase relationship of the CaO-SiO2-FeO-5 mass pct P2O5 system at 1673 K (1400 °C) with \( {P}_{{{\text{O}}_{2} }} \) of 9.24 × 10−11 atm has been studied by using the chemical equilibration method. It has been found that solid solution consists mainly of 2CaO·SiO2-3CaO·P2O5, but occasionally it contains 3CaO·SiO2. Liquidus saturated with solid solution shrinks toward the FeO corner compared with the isothermal at 1673 K (1400 °C) of the CaO-SiO2-FeO system equilibrated with metallic iron. Thermodynamically stable CaO-FeO phase is confirmed, which could promote the condensation of 3CaO·P2O5 into the solid solution and increase the phosphorus partition ratio between the solid solution and molten slag. Based on the regular solution model, the effect of T.Fe and CaO content in the liquid phase on the phosphorus partition ratio between the solid solution and molten slag is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the modern steel producing process, the dephosphorization process is often operated at the dicalcium silicate primary area for achieving a high basicity of molten flux. However, excessive CaO-CaF2 injection for maintaining a high phosphate capability often leads to environmental issues and to difficulties in phosphorus recovery from the dephosphorization slag. Because of the forbiddance of adding a melting agent such as fluorite and the pursuit of an environment friendly producing idea, such multiphase flux must be considered innovatively.
Fix et al.[1] proved that the solid solution consisting of dicalcium silicate (2CaO·SiO2) and tricalcium phosphate (3CaO·P2O5) exists over a wide concentration range at hot metal pretreatment temperature. Ito et al.[2] discovered a strategy for less CaO consumption by using dicalcium silicate as a phosphorus fixture instead of CaO is feasible, and a high phosphorus partition ratio between the 2CaO·SiO2-3CaO·P2O5 solid solution and the liquid phase is achieved with increasing the T.Fe content in the molten slag. All the preceding statements indicate that the dephosphorization process with less environment hamification and smaller slag generation could be realized by using multi phase flux.
For a more complete understanding of the multiphase flux system, Inoue and Suito[3–5] studied the transfer behavior and the partition of phosphorus between the solid solution and molten slag by adding dicalcium silicate and lime particles into a homogeneous CaO-SiO2-FetO molten slag at various temperatures. They explained the reaction mechanism of phosphorus in the CaO-SiO2-FetO system containing mesoscopic scale dicalcium silicate particles. Kitamura et al.[6] clarified the mass transfer of phosphorus between the 2CaO·SiO2-3CaO·P2O5 solid solution and the 2CaO·SiO2-3CaO·P2O5 saturated CaO-SiO2-Fe2O3-P2O5 slag at 1673 K (1400 °C). At the same temperature, the phosphorus partition was studied by Shimauchi et al.[7] for the CaO-SiO2-Fe2O3-P2O5 (6 to 18 mass pct) systems sometimes containing MgO or MnO. Also, the effects of MgO, MnO, and Al2O3 on the phosphorus partition in various slag systems with FeO or Fe2O3 as iron oxides have been discussed by Pahlevani et al.[8]
In contrast, to clarify the formation and reaction mechanism of phosphorus with the perspective of microscopic reactions in multiphase flux, first the dissolution and reaction mechanism of solid CaO with molten slag was clarified by Hamano et al.,[9] and based on that, the reaction behavior of phosphorus in both the homogeneous and heterogeneous slag systems was explained by Yang et al.[10,11]
However, because the proper phase diagram such as the CaO-SiO2-FeO-P2O5 system with various oxygen partial pressures under certain temperatures remains unclear, the equilibrium phase relationship between the solid solution and the liquid slag cannot be clarified. For this purpose, the isothermal of the CaO-SiO2-FeO-P2O5 system with low oxygen partial pressure at hot metal pretreatment temperature, which could maintain high FeO activity, has been studied experimentally in the current work.
More specifically, the P2O5 content was set to be constantly 5 mass pct before equilibration within all the experiments as considering the practical steelmaking operations, and the CO/CO2 gas was introduced for achieving low oxygen partial pressure. The equilibrium slag phases were observed and analyzed by a scanning electron microscope (SEM) and energy disperse spectroscopy (EDS). For the sake of the discussion on the thermodynamic properties of each phase, a regular solution model was adopted for calculating the activity coefficients of the components in liquid phase.
Experimental
Sample Preparation
The CaO-SiO2-FeO-P2O5 slag was made by mixing the reagent grade of SiO2, 3CaO·P2O5, and synthesized FeO, CaO according to Table I. Both the homogeneous and heterogeneous slags after premelting are concerned as the initial conditions before equilibrium referring to the phase diagram of the CaO-SiO2-FeO ternary system equilibrated with metallic iron,[12] as shown in Figure 1. The slag with a high CaO/SiO2 ratio was paid special attention in this study. The method of making synthesized FeO and CaO are prepared as follows.
FeO: An equimolar mixture of reagent-grade Fe3O4 and Fe powder was melted at 1723 K (1450 °C) for 1 hour with argon gas by using ferrous crucible, and then it was dumped on a steel plate with argon blowing for quenching. After that, the product was ground into fine particles (≤0.15 mm), and the unreacted Fe particles were separated magnetically.
CaO: The reagent grade of calcium carbonate was calcined in an alumina crucible for 24 hours at 1273 K (1000 °C) and then kept in a desiccator until it was cooled down slowly to room temperature.
Procedure
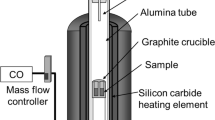
A chemical equilibration technique was adopted in this research.[13–17] Approximately 0.1 g slag mixture was put into a platinum crucible (diameter: 5 mm × height: 5 mm) and hanged quickly into the hot zone of a vertical furnace with a recrystallized alumina tube. For premelting the slag, the crucible with the slag was kept for 1 to 3 hours at 1923 K (1650 °C) with an argon gas atmosphere. After melting, the temperature was decreased slowly by 10 K/min to 1673 K (1400 °C); during this period, the atmosphere inside the tube was switched from argon to CO/CO2 gas to control the oxygen partial pressure. In this study, the oxygen partial pressure was chosen to be 9.24 × 10−11 atm for maintaining high FeO activities, and the volume ratio of the CO/CO2 gas was set to be 5:1 according to Eq. [1].[18]
All the samples were preserved for 5 to 24 hours until equilibrium based on the slag compositions. For confirming the equilibration, the repetition by using the same slag preserved for different times was done as preexperiments, and then the preservation times for equilibrium were determined. Liquid nitrogen was adopted for achieving rapid quenching. After quenching, the samples were embedded in resin and polished for an analysis. SEM and EDS were used to observe the morphology and analyze the composition of each sample.
For obtaining more accurate phase compositions, the partition of elements in each phase was examined several times in different areas of the samples by a large scale mapping analysis. The average concentration of each phase was determined through repeated analyses to conquer the roughness of EDS. Although electron probe microanalysis (EPMA) would be more accurate to measure the compositions of phases than EDS, EPMA was not applicable because of the smaller cross section of some solid phases than the EPMA spot size.
Results and Discussion
The average equilibrium compositions of each phase are shown in Table II, reordered by equilibrium status, and the typical morphology of each phase is shown in Figures 2(a) through (c). In Table II, the composition of the metallic iron of sample 12 has not been shown because the surface area of metallic iron is too small to analyze in this sample. Because the valence of ions cannot be detectable by EDS and also the Fe3+/Fe2+ ratio could not be determined appropriately in current experimental conditions,[13] only the existence of Fe2+ is assumed for presentation purpose and discussion.[15,16] Because of the low P2O5 (5 mass pct) concentration in the original slag, occasionally the P2O5 content in the liquid phase was not detected. The decrease of FeO, which forms metallic iron, occurred, but sometimes the composition of this metallic iron phase is not easy to analyze because of its tiny superficial area, just as the morphology shown in Figure 2(c).
Condensation of 2CaO·SiO2-3CaO·P2O5 Containing Solid Solution
The projection of the equilibrium concentration of the solid solution on the CaO-SiO2-P2O5 system by using all raw data is shown in Figure 3. All the points are located close to the tie line between 2CaO·SiO2 and 3CaO·P2O5 with various 3CaO·P2O5 content, but they switch a little toward the CaO corner. As neither pure 3CaO·SiO2 nor 4CaO·P2O5 was found, 3CaO·P2O5 is the only stable form for phosphorus in solid solution under the current experimental conditions.
Figure 4 shows the relationship between the 3CaO·P2O5 content and the CaO/SiO2 mole ratio (without 3CaO·P2O5) in solid solution. It should be noticed that when solid CaO occurs in the solid phase, the CaO/SiO2 mole ratio in the solid solution should be much higher than the gray solid circle in Figure 3, but the amount of this individual solid CaO phase could not be converted together with the solid solution. According to Figure 4, the content of 3CaO·P2O5 increases with the CaO/SiO2 mole ratio (without 3CaO·P2O5). Therefore, the points that are distributed between 2CaO·SiO2 and 3CaO·SiO2 prove that the solid solution responding to those parts is the mixture of 2CaO·SiO2-3CaO·P2O5 and 3CaO·SiO2. However, because the observed solid solution presents a homogeneous phase and an independent 3CaO·SiO2 phase could not be confirmed by Figure 3, the existence of 3CaO·SiO2 is ambiguous.
Concerned with the equilibrium status, the 3CaO·P2O5 content in the solid solution remains at a lower level when the liquid phase, metallic iron, and solid solution coexist; then, it shifts to a moderate level when the CaO-FeO phase occurs, and the highest value appears in the case of the solid condensed CaO. It is also considered that the existence of the solid CaO could cut down the condensation amount of 2CaO·SiO2 in the 2CaO·SiO2-3CaO·P2O5 based on the view of mass balance.
Because the T.Fe and CaO content in the liquid phase affect the partition ratio of phosphorus between the solid solution and liquid slag, somehow those two should have a certain relationship with the 3CaO·P2O5 content in the solid solution. Figures 5 and 6 show the relationship between 3CaO·P2O5 and CaO as well as the FeO content in the liquid phase. It is assumed that the liquid phase does not exist in the case of the CaO-FeO phase, solid solution, and solid CaO equilibration; therefore, the results of this equilibration have not been discussed in this article. The reason for this assumption will be discussed subsequently. The effects of the CaO and T.Fe contents in the liquid phase are not clear, but the appearance of the CaO-FeO phase promotes the condensation of 3CaO·P2O5.
Phase Relationship of the CaO-SiO2-FeO-5 mass pct P2O5 System at 1673 K (1400 °C) with \( {P}_{{{\text{O}}_{2} }} \) of 9.24 × 10−11 atm
By using the data obtained from the EDS analysis in Table II, the projections of each phase have been made by comparing with the 1673 K (1400 °C) isothermal of the CaO-SiO2-FeO system equilibrated with metallic iron as shown in Figure 7(a). The equilibrium phase sections are proposed in Figure 7(b), and a more distinct subregion of each phase can be observed in Figure 8. Even though the solid solution equilibrated with the liquid phase or other phases is marked as 2CaO·SiO2 because it has been projected on the ternary system as shown in Figure 8, this solid phase is still 3CaO·P2O5-2CaO·SiO2 containing a different P2O5 content. Thus, the 3CaO·P2O5-2CaO·SiO2 is used in the legend of Figure 8.
Projections of the various phases observed in the CaO-SiO2-FeO-5 mass pct P2O5 system on the CaO-SiO2-FeO ternary section at 1673 K (1400 °C) with \( {P}_{{{\text{O}}_{2} }} \) of 9.24 × 10−11 atm: (a) direct comparison with 1673 K (1400 °C) isothermal equilibrated with metallic iron and (b) equilibrium phase sections
In both Figures 7(a) and (b), the FeO-penetrated solid solution is located around the area between 2CaO·SiO2 and 3CaO·SiO2 because the CaO content in the solid solution is increased by the condensation of the 3CaO·P2O5-2CaO·SiO2 solid solution. An assumed liquidus with a constant FeO activity, which is equilibrated with metallic iron only, is located at a moderated SiO2 area plotted as a dotted line in Figures 7(b) and 8. In the preceding equilibrium status, the appearance of constant FeO activity is caused by the low oxygen partial pressure used in current experiments, and the reduction of FeO as mentioned is shown in Eq. [2].[18] The FeO activity of the liquid phase equilibrated with metallic iron at the current experimental conditions is calculated to be about 0.4.
The liquid surface moves toward the FeO corner compared with the isothermal at 1673 K (1400 °C) equilibrated with the metallic iron according to Figure 7(a). The formation of solid solution and the low oxygen partial pressure provide the driving force for this phenomenon.
Because Figures 7(b) and 8 are projections of the quaternary slag system on a ternary phase diagram, no liquidus could pass through the three-phase-equilibrated area. Thus, by the existence of the three phases in equilibrated zone 2 (liquid phase, metallic iron, and P2O5 containing 2CaO·SiO2 solid solution coexist) in Figure 8, the liquidus is blocked. In addition, no liquid phase is saturated with the solid solution confirmed at a moderated SiO2 content area. Similarly, zone 4 in Figure 8 becomes another blocker for the liquidus.
The CaO-FeO phase has been found thermodynamically stable with an assumed concentration range shown as a dashed line in Figures 7(b) and 8, which has been reported previously as a solid solution when solid CaO comes in contact with the molten slag.[4,19,20] However, because the existence of a low SiO2 content inside the CaO-FeO phase cannot be ignored in the current work, it is uncertain whether this CaO-FeO phase is liquid or solid judged only by the EDS analysis, although the thermodynamic calculation by FactSage 6.2 (CRCT École Polytechnique, Montreal, Canada) and the isothermal section by Riboud[21] showed it could be a liquid phase with similar equilibrium conditions. Therefore, the CaO-FeO phase has to be regarded as an individual phase different from the liquid, which agrees with the assumption proposed in the last part of Section III–A. Anyway, the appearance of this phase could promote the 3CaO·P2O5 condensation as discussed previously, and its effect on the phosphorus partition will be discussed in the next section.
Partition Ratio of Phosphorus Between Solid Solution and Liquid Slag
The phosphorus partition ratio between the solid solution and liquid slag increases with the T.Fe content in the liquid phase, as shown in Figure 9 by comparing these results with the work of Ito et al.[2] Although the linear relationship proposed by Ito et al. ends at the T.Fe content at approximately 60 mass pct, the current results fit its extension at a higher T.Fe content region. The highest value of the phosphorus partition ratio occurs when the CaO-FeO phase exists. Moreover, the CaO content in the liquid phase has the opposite effect with the T.Fe content as shown in Figure 10.
As is well known, the partition ratio of phosphorus between the solid solution and liquid phase is proportional to the activity coefficient of P2O5 in the liquid phase but inverse to that in the solid solution. Consequently, the activity coefficients of P2O5 in the liquid slag have been calculated by the regular solution model,[22] and the activity coefficient of phosphorus in the solid solution can be obtained. The calculated results are shown in Table III, in which only the results that could be calculated by the partition ratio of phosphorus have been concerned.
It is believed that the larger T.Fe content, which means a larger FeO content in this work, could increase the solubility of CaO in the liquid phase. The liquid area occurs only at a high FeO content region according to the phase diagram for the CaO-FeO x system.[12] This will affect the activity coefficient of CaO in the liquid phase as shown in Figure 11. In the case of the liquid phase equilibrated with a solid solution only, the activity coefficient of CaO seems to decrease with the T.Fe content in the liquid phase, but the trend is obscure. With the increasing T.Fe content in the liquid phase, the depressed activity coefficient of CaO in the liquid phase leads to a higher dissolved amount of CaO because the molten slag is saturated with a CaO-containing solid solution in which the CaO activity is almost constant. Then, the liquid phase of a multiphase flux could have a high CaO/SiO2 ratio, which is favorable not only for the condensation of solid solution but also for the dephosphorization reaction that occurs at the slag–metal interface.
As shown in Figures 12(a) and 13(a), the activity coefficients of P2O5 in the liquid phase decrease linearly with the increasing activity coefficient of CaO and CaO content in the liquid phase, whereas the alteration of the activity coefficient of P2O5 in the solid solution reveals a similar result in Figures 12(b) and 13(b). Coupling of the preceding two tendencies leads to the decrease of the phosphorus partition ratio between the solid solution and the liquid phase. Considering the preceding discussion, the effect of the CaO content on the phosphorus partition ratio between the solid solution and the liquid phase as mentioned in Figure 10 could be explained.
In contrast, the activity coefficient of P2O5 in the liquid phase increases with the increasing T.Fe content in the molten slag, whereas the activity coefficient of P2O5 in the solid solution decreases as shown Figure 14. This finding indicates a clear tendency that the phosphorus partition ratio between the solid solution and liquid phase increases with the T.Fe content, as discussed in Figure 9.
The coexistence of the CaO-FeO phase and the solid solution would lead to a higher FeO content in the liquid phase than the case of only the condensation of the solid solution. The precipitation of those phases would benefit the partition of phosphorus between the solid solution and the liquid, and thus, the reason for that the highest value of phosphorus partition ratio is observed when the CaO-FeO phase exists as mentioned could be explained.
Conclusions
By using the chemical equilibration method, the phase relationship of the CaO-SiO2-FeO-5 mass pct P2O5 system at 1673 K (1400 °C) with \( {P}_{{{\text{O}}_{2} }} \) of 9.24 × 10−11 atm has been studied. The following results were obtained:
-
1.
The solid solution is confirmed to be 2CaO·SiO2-3CaO·P2O5, which occasionally contains 3CaO·SiO2.
-
2.
The liquid area shrinks toward the FeO corner compared with the isothermal at 1673 K (1400 °C) of the CaO-SiO2-FeO system equilibrated with metallic iron. The liquidus equilibrated with metallic iron is proposed, which has a constant FeO activity.
-
3.
The thermodynamically stable CaO-FeO phase is confirmed and the condensation of 3CaO·P2O5 into the solid solution is promoted; in addition, the phosphorus partition ratio between the solid solution and the liquid phase increased.
-
4.
The phosphorus partition ratio between the solid solution and the liquid phase increases with the increasing T.Fe content in the liquid phase; in contrast, the CaO content in the liquid phase shows the opposite effect.
References
W. Fix, H. Heymann, and R. Heinke: J. Am. Ceram. Soc., 1969, vol. 52, pp. 346–47.
K. Ito, M. Yanagisawa, and N. Sano: Tetsu-to-Hagané, 1982, vol. 68, pp. 342–43.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46, pp. 174–79.
H. Suito and R. Inoue: ISIJ Int., 2006, vol. 46, pp. 180–87.
R. Inoue and H. Suito: ISIJ Int., 2006, vol. 46, pp. 188–94.
S. Kitamura, S. Saito, K. Utagawa, H. Shibata, and D.G.C. Robertson: ISIJ Int., 2009, vol. 49, 1838–44.
K. Shimauchi, S. Kitamura, and H. Shibata: ISIJ Int., 2009, vol. 49, pp. 505–11.
F. Pahlevani, S. Kitamura, H. Shibata, and N. Maruoka: ISIJ Int., 2010, vol. 50, pp. 822–29.
T. Hamano, S. Fukagai, and F. Tsukihashi: ISIJ Int., 2006, vol. 46, pp. 490–95.
X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2009, vol. 49, pp. 1298–1307.
X. Yang, H. Matsuura, and F. Tsukihashi: ISIJ Int., 2010, vol. 50, pp. 702–11.
Slag Atlas: Deutscher Eisenhütten (VDEh), 1995, pp. 57,126.
H. Kimura, S. Endo, K. Yajima, and F. Tsukihashi: ISIJ Int., 2004, vol. 44, pp. 2040–45.
H. Matsuura, M. Kurashige, M. Naka, and F. Tsukihashi: ISIJ Int., 2009, vol. 46, pp. 1283–89.
H.M. Henao, F. Kongoli, and K. Itagaki: ISIJ Int., 2005, vol. 46, pp. 812–19.
H.M. Henao, C. Pizarro, J. Font, A. Moyano, P.C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2010, vol. 41B, pp. 1116–93.
Y.B. Kang and H.G. Lee: ISIJ Int., 2005, vol. 45, pp. 1552–60.
E.T. Turkdogan: Physical Chemistry of High Temperature Technology, Academic Press, New York, NY, 1980, pp. 7, 14.
R. Saito, H. Matsuura, K. Nakase, X. Yang, and F. Tsukihashi: Tetsu-to-Hagané, 2009, vol. 95, pp. 72–81.
L. Hachtel, W. Fix, and G. Trömel: Arch. Eisenhüttenwes, 1972, vol. 43, pp. 361–69.
P. Riboud: Memoires Scientifiques de la Revue de Metallurgie, 1973, vol. 70, pp. 771–82.
S. Banya: ISIJ Int., 1993, vol. 33, pp. 2–11.
Acknowledgment
This research was partly supported by Strategic International Cooperative Program, Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 24, 2011.
Rights and permissions
About this article
Cite this article
Gao, X., Matsuura, H., Sohn, I. et al. Phase Relationship of CaO-SiO2-FeO-5 mass pct P2O5 System with Low Oxygen Partial Pressure at 1673 K (1400 °C). Metall Mater Trans B 43, 694–702 (2012). https://doi.org/10.1007/s11663-012-9651-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9651-5