Abstract
The change in the composition of oxide layers and the possibility of the formation of bonding between the two layers of a double-oxide film defect when held in an Al-0.05 wt pct Sr melt was investigated. The defect was modeled experimentally by maintaining two aluminum oxide layers in contact with one another in the liquid metal at 1023 K (750 °C) for times ranging from 5 seconds to 50 hours. Any changes in the composition and morphology of these layers were studied by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX). The results showed that the A12O3 layers started to transform to SrO gradually from the moment that they submerged into the melt. The transformation caused the two layers to bond with each other gradually. The results illustrated that the composition of the oxide layers of a double oxide film defect submerged in Sr-treated melt is different from that of pure Al, and this might affect the mechanical properties and the behavior of the defect in the melt significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Double-oxide films (bifilms) are considered as one of the most harmful defects in aluminum castings. This defect, which consists of two oxide layers and a volume of gas (presumably predominantly air) trapped between them, can be produced when the surface of the metal folds upon itself and becomes submerged in the bulk liquid.[1] The defect, therefore, necessarily resembles and acts as a crack in the liquid metal. More information about double-oxide film defects can be found in the literature.[1–7]
Campbell[1] suggested that these defects could act as initiation sites for the formation of hydrogen porosity in Al castings. He calculated the pressure required for the homogeneous and heterogeneous nucleation of a hydrogen bubble in liquid Al melt to be too high to explain the abundance of gas porosity observed in Al castings. He suggested that the existence of crack-like double oxide film defects in the liquid Al would eliminate the need for the nucleation stage to occur during the formation of a hydrogen bubble and, hence, facilitate the formation greatly. This hypothesis was later supported by the work done by Raiszadeh and Griffiths[2] and Dispinar and Campbell.[8]
To study the behavior of a double-oxide film defect in liquid Al, Raiszadeh and Griffiths[2] held a bubble of air in different liquid aluminum melts and recorded the change in the volume of the air bubble with time as its contents reacted with the surrounding melt, using real-time X-ray radiography. Their results showed that first, the oxygen and then the nitrogen of the air bubble reacted with the surrounding liquid metal to form Al2O3 and AlN, respectively. This procedure took about 5 to 8 hours for commercial purity Al. They also observed that if the initial hydrogen content of the melt was high, the air bubble expanded initially and then started to shrink. They suggested that the cracks that formed on the oxide layers of the bubble during its movement in the liquid metal provided the necessary paths for the contact of the internal atmosphere of the bubble and the surrounding melt, and it caused a penetration path for the diffusion of H atoms into (and from) the trapped atmosphere. Raiszadeh and Griffiths[2] also realized that the consumption time of the air bubble increased from 5 to 8 hours to about 20 to 30 hours when 0.05 wt pct Sr was added to the commercial purity Al melt.
Sr, which is usually used as a modifying agent for Al-Si alloys,[9] is known to cause an increased tendency to porosity formation in the alloy.[10] A tremendous amount of work has been carried out to study this effect, with no agreement achieved among the researchers. A brief but useful review of these work can be found in Shabestari et al.’s[10] report.
Iwahori et al.[11] were one of the first researchers who mentioned a possible connection between the Sr-related porosity and the oxides present in the melt. They reported that the hydrogen level in Sr-modified melts is not lowered by vacuum degassing and attributed this to the fact that hydrogen absorbed into the oxide in the melt is more strongly fixed in the oxide by the addition of Sr to the melt (the oxides being considered as inclusions in the melt).
The suggestion of Campbell[1] about double-oxide film defects acting as initiating sites for porosity growth and the curious finding of Raiszadeh and Griffiths[2] about the effect of Sr on the lifetime of an air bubble held in an Al melt suggested a comprehensive study of the behavior of double-oxide film defects in Al-0.05 wt pct Sr to be essential if the exact effect of this element on the formation of porosity in castings is to be found. Such a study was the main objective of this research.
For this reason, two aluminum oxide layers in contact with each other were maintained in the liquid alloy for varying lengths of time. Therefore, any changes in the composition and morphology of these layers were studied by scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDX) after the two layers were pulled apart after solidification.
This method was previously used by this research team to study the transformations that occur in the oxide layers of a double-oxide film defect when held in liquid Al alloys with different Mg contents of 0, 0.3, and 4.5 wt pct[4–6] and to find whether these transformations would cause the two oxide layers of the defect to bond with each other, in which case, the most deleterious effect of the defect (i.e., to act as a crack in the liquid metal) would be eliminated. The result of these works indicated that for the two layers of an oxide defect to bond with each other, two criteria should be met: First, the oxygen and nitrogen of the atmosphere within the defect should be consumed and the two layers should be in contact with each other, and second, a transformation that involves the rearrangement of atoms should occur at the internal surfaces of the oxide layers.
Experimental procedure
The Al-0.05 wt pct Sr alloy was prepared in a resistance-heated furnace by adding an Al-10 wt pct Sr master alloy to a commercial purity Al alloy. The composition of the resulting alloy is shown in Table I.
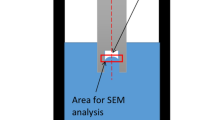
The alloy was subsequently poured into silica sand molds with 5 pct sodium silicate and CO2 gas as a binder in the shape of bars. The bars were machined to dimensions of 100 mm in length and 19 mm in diameter. Two bars were then placed in a seamless extruded steel tube (which was made specifically for gas industries) with dimensions of 210 mm in length and 20 mm in internal diameter (Figure 1). The bases of the bars that were in contact with one another in the steel tube were polished to 9 μm before the bars were inserted in the tube, so that the naturally formed oxide layers resembled the two layers of a newly formed double-oxide film defect.
The steel tube was then transferred to a cylindrical electric furnace with a sliding door at the top. The temperature of the furnace was set to 1023 K (750 °C) prior to the start of the experiment. The temperature of the Al bars increased at an average rate of 3.2 K seconds–1 (measured in a separate experiment using a K-type thermocouple inserted at the center of the steel tube), and the bars finally melted in the tube in 482 ± 1 seconds. The only possible leak path from the trapped atmosphere between the two oxide layers to the ambient atmosphere was through the gap between the oxide layer around the Al bars and the wall of the steel tube. To eliminate this leak path, the oxide layer around the top of the upper Al bar was removed with a sharp tool beneath the surface of the melt to remove the oxide separating the melt from the steel tube, ensuring direct contact between the melt and the tube. The Al bars were held in the liquid state for varying lengths of time, between 5 seconds and 50 hours, before the steel tube was raised in the furnace and held in the upper part of the heating chamber to let the liquid metal inside the tube solidify at a relatively slow rate (in approximately 40 seconds). The slow solidification of the metal was essential to prevent any thermal cracks from forming on the two oxide layers inside the melt.
After solidification, the steel tube was cut in two halves and the Al bars were removed from it. In some experiments, the bonding that formed between the two oxide layers during the experiment joined the two bars to one another. In this case, the two oxide layers were separated by pulling the two Al bars apart, using a Zwick 1484 tensile testing machine (Zwick, Kennesaw, GA) at a strain rate of 1 mm minute–1. The surfaces of these two oxide layers were then examined using optical microscopy and a Camscan scanning electron microscope (Tescan Inc., Pleasanton, CA) fitted with an Oxford Inca EDX for microanalysis (Oxford Instruments, Oxfordshire, U.K.).
Each experiment was repeated at least three times to confirm the repeatability of the results. More details of the experimental procedure can be found in another study by this research team.[4]
Results
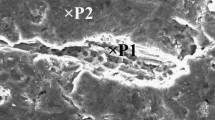
Figure 2 illustrates a photograph of the two oxide layers that were held in the liquid for 5 seconds. The two oxide layers were attached to each other at a few points. The bonds were not strong (Figure 3), and the two bars detached easily from each other after the bars were removed from the steel tube. The backscattered electron micrograph obtained from this layer and a higher magnification of this micrograph are shown in Figures 4 and 5, respectively. The EDX spectrum obtained from the white area (point P1 in Figure 5, shown in Figure 6(a) exhibited that the Al2O3 layers had already started to transform into a Sr-containing oxide at this holding time. It also appears in Figure 5 that the liquid metal has exuded through the oxide layer at point P2. The EDX spectrum from this point is shown in Figure 6(b). Such exudation has also been observed in experiments with commercial purity[6] and Al-0.3 wt pct Mg[4] alloys.
Backscattered electron micrograph of the surface of the oxide layer shown in Fig. 2
A higher magnification of the backscattered micrograph shown in Fig. 4. The EDX spectra obtained from the points P1 and P2 are shown in Figs. 6(a) and (b), respectively. The concentration of elements in the background of this micrograph was measured to be (all in wt pct) 87 pct Al, 10 pct O, and 3 pct Sr
(a) The EDX spectrum obtained from the point P1 given in Fig. 5. The composition (all wt pct) at this point was measured to be 71 pct Al, 10 pct O, and 19 pct Sr. (b) The EDX spectrum obtained from the point P2 given in Fig. 5. The composition (all wt pct) at this point was measured to be 95 pct Al and 5 pct O
When held in the liquid metal for 12 minutes, the color of the oxide layers started to change to darker gray from the edge of the layer towards the center (Figure 7). An EDX study of the layers indicated this change in the color to be due to an increase in the concentration of Sr in the oxide layer. The two layers were bonded to each other at more points. In some samples, the ultimate tensile stress required to separate the bars was slightly higher than that of the bars that were held in the liquid metal for 5 seconds (Figure 3). However, the other samples were detached from each other with no force required. The point bonding was evident in the SEM micrograph taken from this oxide layer (Figure 8). This figure shows the oxide layer to be peeled off in some small areas. It is likely that the layers were bonded to each other in these areas, and then the bonded areas were peeled off from the oxide layer during the separation of the Al bars by the tensile machine.
SEM micrograph of the surface of the oxide layer shown in Fig. 7
A higher magnification of this micrograph is shown in Figure 9. The tip of the raised feature in this figure (denoted as P1) is flat, as if pressed against an object. The concentration of oxygen at the tip (point P1), determined by EDX (Figure 10(a)) to be 8 wt pct, was much lower than that of the hillside (point P2) (22 wt pct). These observations imply that the Al melt exuded through a discontinuity that formed on the oxide layer and contacted the opposite layer, but it could not wet and bond with it.
(a) The EDX spectrum obtained from the point P1 given in Fig. 9. The composition (all wt pct) at this point was measured to be 90 pct Al, 8 pct O, and 2 pct Sr. (b) The EDX spectrum obtained from the point P2 given in Fig. 9. The composition (all wt pct) at this point was measured to be 70 pct Al, 22 pct O, and 8 pct Sr
The backscattered micrograph presented in Figure 11 illustrates another example of the peeling of bonded oxide layers. The oxide layer at point P1 transformed completely from Al2O3 to SrO, and some part of it was peeled off the surface during the separation of the bars by the tensile machine. Then, the metal underneath (point P2) was revealed (Figures 12(a) and (b) for the EDX spectra obtained from these points).
(a) The EDX spectrum obtained from the point P1 given in Fig. 11. The composition (all wt pct) at this point was measured to be 59 pct Sr and 41 pct O. (b) The EDX spectrum obtained from the point P2 given in Fig. 11. The composition (all wt pct) at this point was measured to be 87 pct Al and 13 pct O
The transformation of Al2O3 to Sr-rich oxide continued as the oxide layers were held in the liquid metal for longer times. Figure 13 shows a photograph of the oxide layers that were held in the liquid metal for 30 minutes. As can be seen, the dark areas expanded over many parts of the layer. The backscattered micrograph obtained from this layer (presented in Figure 14) illustrates how the transformation of the Al-Sr-O layer (dark area; containing 79 wt pct Al, 15 wt pct O, and 6 wt pct Sr) to SrO (the white area consisted of 57 pct Sr, 40 pct O, and 3 pct Al) progressed in the oxide layer. Figure 15 shows another part of the oxide layer that completely transformed to SrO (white area, containing 59 pct Sr and 41 pct O). The dark area in this figure, which consisted of 88 wt pct Al and 12 wt pct O (Al2O3), was probably revealed when the SrO layer over it peeled off from the oxide layer during the separation of the Al bars by the tensile machine.
Backscattered electron micrograph of the surface of the oxide layer shown in Fig. 13. The composition (all in wt pct) of the dark area was measured to be 79 pct Al, 15 pct O, and 6 pct Sr, while the composition of the white area was measured to be 57 pct Sr, 40 pct O, and 3 pct Al
Backscattered electron micrograph obtained from another part of the oxide layer shown in Fig. 13. The composition (all in wt pct) of the dark area was measured to be 88 pct Al and 12 pct O, while the composition of the white area was measured to be 59 pct Sr and 41 pct O
Photographs of the oxide layers that were held in the liquid metal for 5 and 17 hours are shown in Figures 16 and 17, respectively. Figure 16 illustrates that the entire oxide layer turned dark gray after a holding time of 5 hours. The bonded areas, which later peeled off from one of the oxide layers, are easily visible in this figure as white points. The bonding between the two layers increased significantly for the holding time of 17 hours (Figure 17). The backscattered micrograph obtained from this layer (presented in Figure 18) showed a vast range of Al-Sr-containing oxides. The concentration of elements at points P1 to P4 on this micrograph is shown in Table II.
The macroscopic appearance of the oxide layers did not change considerably for holding times longer than 17 hours. However, the bonding between the oxide layers became stronger (Figure 3) as the transformation proceeded.
Figure 19 illustrates the backscattered micrograph of an oxide layer that was held in the liquid metal for 24 hours. The EDX spectra obtained from the white areas on this micrograph (e.g., point P1, shown in Figure 20(a)) indicated that most of the oxide layer had transformed to SrO. The dark patches, the EDX spectrum from which is shown in Figure 20(b), are pure Al from underneath the oxide layer that was revealed as the SrO layer that had bonded to the other layer peeled off from the surface during the separation of the bars by the tensile machine.
Backscattered electron micrograph of the surface of an oxide layer that was held in the liquid metal for 24 h. The EDX spectra obtained from points P1 and P2 are shown in Figs. 20(a) and (b), respectively
The trend of the change in the overall composition of the oxide layers was determined by calculating the average concentrations of Al and Sr over many different points on the oxide layers for each holding time, and thereupon, the change in the ratio of Sr/Al with the holding time was calculated. The results of these calculations, along with the change in the concentration of Sr in the melt, are presented in Figure 21. The figure shows that the concentration of Sr in the oxide layer increased gradually with the holding time, whereas that of Al decreased and that the ratio of Sr/Al in the oxide layer changed with the holding time by an almost logarithmic trend (with R 2 = 0.9646). The Sr concentration meanwhile decreased in the liquid metal with an exponential decay trend, a trend typical of parameters in which the rate of decrease is proportional to their values.
Discussion
Based on the standard Gibbs free energy data,[12] the oxidation of Sr is more favorable than Al. Therefore, the chemical equilibrium path in the system Al-Sr-O is towards the complete oxidation of Sr. More specifically, in the molten Al-0.05 wt pct Sr, the oxidation process of Sr leads to the depletion of this element in the melt and, in the meantime, an increment in Sr in the oxide layer (as shown in Figure 21). As a consequence, the path to equilibrium is through the gradual oxidation of Sr, i.e., the transformation of the Al2O3 layer to a SrO one. This was observed in this research to be a fairly slow process due to (1) the low level of Sr in the melt and (2) the source of oxygen, which was limited to the oxygen in the trapped atmosphere and the oxygen provided from the aluminum oxide layer. The rate of this transformation depends on local conditions such as temperature and the concentration of Sr. The results demonstrated that the variation of such local conditions in different parts of the oxide layers was significant enough to create oxides with different compositions adjacent to each other (Figure 18 and Table II).
The results showed that the transformation of Al2O3 to a Sr-containing oxide started from (at least, or perhaps before) the very first moments after the bars were melted. Such transformation was observed over the whole of the oxide layers (Figures 4 and 5). The results obtained by Aryafar et al.[4] and Najafzadeh and Raiszadeh[5,6] have demonstrated that such transformation is necessary for the two oxide layers to bond with each other. These researchers also concluded that any bonding could take place between the two layers only if the trapped atmosphere within the defect was consumed, and therefore, the two oxide layers were in good contact with each other.
The holding time for which the oxygen and nitrogen trapped between the two oxide layers were consumed could not be determined in these experiments. However, this consumption time was estimated by Najafzadeh and Raiszadeh[5] to be about 13 hours in Al-4.5 wt pct Mg alloy. The results obtained by Raiszadeh and Griffiths[2] suggest this time to be longer for Al-0.05 wt pct Sr. Despite such long consumption times, which is probably due to the lack of considerable deformation in the oxide layers while being held in the liquid metal,[2] the two oxide layers were observed to be able to bond with each other in short holding times at some points (Figures 2 and 7). This observation suggests that despite the presence of oxygen and nitrogen in the trapped atmosphere, the two oxide layers were in contact with each other at a few points in short holding times. The oxide layers are known to have some (and sometimes, extreme) degree of roughness on a microscopic scale.[1] Hence, the contact of the two oxide surfaces, despite the presence of a gas atmosphere between them, was not unlikely.
Raiszadeh and Griffiths[2] suggested that the rate of consumption of the atmosphere within a double-oxide film defect depends on the rate of formation of cracks on the oxide layers when the defect deforms in the fluid flow. Hence, the rate of consumption of oxygen and nitrogen trapped within the atmosphere of a real double-oxide film defect moving in the melt (due to fluid flow or liquid convection) should presumably be greater than that of the atmosphere between the two relatively stagnant oxide layers in the current experiments, and therefore, it is likely that the two oxide layers would bond with each other in holding times shorter than what was observed in this study.
On the other hand, the addition of Sr to Al was found in some studies[9,13,14] to cause the hydrogen content of the melt to increase. Although not confirmed in other studies,[15,16] if true, then the diffusion of hydrogen from the melt to the atmosphere of the defect could cause the distance between the two oxide layers to increase and, therefore, prevent any bonding to form between the layers. In this case, when whole of the layer transforms to SrO, no further transformation would occur in the layers, and even if the layers come in contact with each other later, they would not be able to bond with each other anymore.
The exact mechanism of the exudation of liquid metal from some points at the short holding times (Figures 5, 8, and 9) is not clear. Such exudation is only possible if the oxide layer either cracks or spalls due to stresses generated in the oxide layer. The presence of stresses in oxide layers and their effects on cracking, spalling and decohesion of oxide layers have been recognized for some time.[17] A variety of parameters (e.g., interfacial tension, mechanical effect of vibrations, falling dust, gaseous diffusion, and mechanical properties of the oxide layer) are recognized to be able to produce discontinuities or defects in the oxide layer, particularly once it has attained a certain thickness. The metal is sometimes able to penetrate such discontinuities by a capillary effect.[18]
Figure 9, however, clearly indicates that the liquid metal that exuded from one of these discontinuities did not bond with the opposite oxide layer. This was due to the inability of the exuded metal to wet the opposite layer in the presence of oxygen in the trapped atmosphere. It has been reported in the literature,[19] using a sessile drop technique, that Al does not wet Al2O3 below 1173 K (900 °C). Further studies (for example, References 20 and 21) revealed that in sessile drop experiments, the presence of a thin surface Al2O3 layer on the Al melt accounts for the nonwetting of Al2O3 by Al, and once this oxide layer is eliminated, Al melt can wet the Al2O3. Such a feature has also been observed on the oxide layers that were held in commercial-purity Al melt for 1 hour.[6]
Comparing the results obtained in this study to those obtained by Najafzadeh and Raiszadeh[6] with commercial-purity Al melt indicates that the presence of 0.05 wt pct Sr in the liquid metal changed the behavior of the oxide defect in the melt completely. In commercial-purity Al melt, the two oxide layers did not start to bond with each other considerably before the holding time of 5 hours, and the bonding that happened after this holding time was attributed to the transformation of γ- to α-Al2O3; the transformation of Al2O3 to SrO and the bonding between the two oxide layers was gradual in the Al-0.05 wt pct Sr melt (Figure 3) and started immediately after (or perhaps even before) the melting of the Al bars was completed.
The results of Raiszadeh and Griffiths[2] showed that when 0.05 wt pct Sr was added to the commercial-purity Al melt, the duration of the air bubble held in the liquid metal increased about four times. They did not offer any explanation for this observation in their publication. Also, Iwahori et al.[11] realized that the hydrogen level in Sr-modified melts is not lowered by vacuum degassing. The current study showed that the nature of the oxide layers of the defect would change with time from the very first moment after the defect is entrained into the melt (Figure 21). The rate of transformation in the oxide layers is relatively high during the first minutes after the entrainment of the defect such that according to Figure 21, the transformation of an almost Al2O3 layer (with an Sr/Al ratio of 0.33) to an Al-Sr oxide layer with an Sr/Al ratio of 0.85 (which is about 20 pct of the transformation from an Al2O3 layer to an SrO layer) takes place during the first 12 minutes after the melting of the bars. It is possible that such rapid change in the nature of the oxide layer would cause its mechanical properties (i.e., tensile strength and ductility) to change considerably with time as the defect is submerged in the Al-0.05 wt pct Sr melt. If the strength of the oxide layer (i.e., its resistance against cracking) increases due to such changes in the composition (or perhaps thickness) of the oxide layer, then the rate of consumption of oxygen and nitrogen from its atmosphere as well as the rate of diffusion of hydrogen into and out of the atmosphere would decrease considerably with time. This theory certainly requires more study for verification, but if confirmed, it might be able to explain the observations of Raiszadeh and Griffiths[2] and Iwahori et al.[11]
Conclusions
-
1.
The Al2O3 layers started to transform to SrO gradually as they were submerged in the Al-0.05 wt pct Sr melt.
-
2.
This transformation caused the two oxide layers to bond with each other in short holding times at a few points. The bonding between the two oxide layers increased gradually, as the transformation in the oxide layer progressed.
-
3.
The addition of 0.05 wt pct Sr to the commercial-purity Al melt changed the behavior of oxide layers in the melt completely. Previous studies have shown that in commercial-purity Al melt, the two oxide layers do not start to bond with each other before the holding time of 5 hours, while the bonding between the two oxide layers held in Al-0.05 wt pct Sr melt (in this study) started immediately after the entrainment of the defect into the melt.
-
4.
The results obtained in this study showed that Sr can change the composition of the oxide layers of double-oxide film defects submerged into the melt. Therefore, the composition of double-oxide film defects in the Sr-modified cast aluminum alloys would be different from that of pure aluminum alloys, and this might affect the mechanical properties and the behavior of the defect in the melt significantly.
References
J. Campbell: Complete Casting Handbook, Butterworth-Heinemann, London, U.K., 2011.
R. Raiszadeh and W.D. Griffiths: Metall. Mater. Trans. B, 2006, vol. 37B, pp. 865-71.
R. Raiszadeh and W.D. Griffiths: Metall. Mater. Trans. B, 2008, vol. 39B, pp. 298-303.
M. Aryafar, R. Raiszadeh, and A. Shalbafzadeh: J. Mater. Sci., 2010, vol. 45, pp. 3041-51.
F. Najafzadeh-Bakhtiarani and R. Raiszadeh: J. Mater. Sci., 2010, vol. 46, no. 5, pp. 1305-15.
F. Najafzadeh-Bakhtiarani and R. Raiszadeh: Metall. Mater. Trans. B, 2011, vol. 42B, pp. 331-40.
A. Ardekhani and R. Raiszadeh: J. Mater. Eng. Perform., 2012, vol. 21, no. 7, pp. 1352-62.
D. Dispinar and J. Campbell: Int. J. Cast Metals Res., 2004, vol. 17, pp. 280-6.
X. Bian, Z. Zhang, and X. Lin: Mater. Sci. Forum, 2000, vols. 331-7, pp. 361-6.
S.G. Shabestari, S.M. Miresmaeili, and S.M.A. Boutorabi: J. Mater. Sci., 2003, vol. 38, pp. 1901-7.
H. Iwahori, K. Yonekura, Y. Yamamoto, and M. Nakamura: AFS Trans., 1990, vol. 98, pp. 167-73.
S.M. Miresmaeili: Oxid. Met., 2009, vol. 71, pp. 107-23.
J.R. Denton and J.A. Spittle: Mater. Sci. Technol., 1985, vol. 1, p. 305.
H. Guthy, S. Shankar, and M. Makhlouf: 6 th International AFS Conference, Molten Aluminum Processing, Orlando, FL, 2001.
M.H. Mulazimoglu, N. Handiak, and J.E. Gruzleski: AFS Trans., 1989, vol. 17, pp. 225-32.
B. Kotte: Modern Cast., 1985, vol. 76, p. 33.
O. Kubaschewski and B.E. Hopkins: Oxidation of Metals and Alloys. Butterworths, London, U.K., 1967.
M. Drouzy and C. Mascre: Metall. Rev., 1969, vol. 14, pp. 25-46.
H. Kambayashi and H. Miyake: AFS Trans., 2005, vol. 5-016, no. 5, pp. 1-19.
H. John and H. Hausner: J. Mater. Sci. Lett., 1986, vol. 5, pp. 549-51.
S.A. Impey, D.J. Stephenson, and J.R. Nicholls: Mater. Sci. Technol., 1988, vol. 4, pp. 1126-32.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted November 12, 2011.
Rights and permissions
About this article
Cite this article
Nateghian, M., Raiszadeh, R. & Doostmohammadi, H. Behavior of Double-Oxide Film Defects in Al-0.05 wt pct Sr Alloy. Metall Mater Trans B 43, 1540–1549 (2012). https://doi.org/10.1007/s11663-012-9708-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-012-9708-5