Abstract
Summary
Vitamin D deficiency and quantitative ultrasound measurements are associated with bone fragility. We assessed these parameters and their correlates. 87.7% of the population has vitamin D inadequacy and this correlated with lifestyle factors. These results contribute to epidemiological data needed for population guidelines for bone health.
Purpose
Vitamin D deficiency and quantitative ultrasound (QUS) parameters are among the most important clinical risk factors of bone fragility. Few data are available for Greek population. The aim of the study was to evaluate the serum 25-hydroxyvitamin D [25(OH)D] level and their determinants, as well as QUS parameters in Greek population.
Methods
OSTEOS is an observational cross-sectional study conducted from June 2010 to July 2012. Nine hundred seventy adults were recruited from rural and urban areas throughout Greece and completed the appropriate questionnaire. Serum 25(OH)D measured by enzyme immunoassay, QUS parameters, broadband ultrasound attenuation (BUA), speed of sound (SOS) and stiffness index (SI), was assessed with an Achilles device. Univariate Analysis of Variance was used for the assessment of serum 25(OH)D determinants.
Results
Mean serum 25(OH)D of the total population was 20,00 ± 8,00 ng/mL. Females had lower levels than males. The negative determinants of serum 25(OH)D in the total population were the female sex and the winter-spring season of sampling while age proved negative association solely in obese subjects. Positive determinants of vitamin D status were summer sun exposure and organized physical activity as expected. Urban had lower SOS and SI than rural residents. Individuals with 25(OH)D ≥ 20 ng/mL had higher SOS than those with 25(OH)D < 20 ng/mL. BUA, SOS, and SI are positively correlated with organized physical activity and negatively with PTH.
Conclusions
This study reports that vitamin D deficiency is highly prevalent among healthy Greek men and women, demonstrates the multifactorial causation of 25(OH)D levels, and points out that further research is required to determine more factors related to vitamin D status and bone health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal multifactorial disease characterized by reduced bone mass and microarchitectural deterioration of the structure of bone tissue leading to bone fragility and increased susceptibility to fractures. Osteoporosis is a major public health concern. Women have a higher risk of osteoporotic fractures compared with men (1 in 3 women and 1 in 5 men over 50 years) [1]. Although BMD is the main predictive risk factor for an osteoporotic fracture, measurement of quantitative ultrasound (QUS) has been found to be associated with increased fracture risk [2]. The QUS measuring at the heel is an alternative, ionizing radiation-free and relatively inexpensive, portable screening technique that is able to identify women at high risk of bone fragility and fracture [3, 4] and may be used by general practitioners in primary care.
Vitamin D deficiency is also among the most important clinical risk factors of bone fragility and a subject of extensive research. The main physiological effect of vitamin D is to increase intestinal calcium absorption, as such maintaining serum calcium in order to maximize metabolic functions, signal transduction, and neuromuscular activity. The consequences of vitamin D deficiency in adults are osteomalacia, acceleration of bone loss, muscle weakness, instability, and therefore increased risk of falling [4]. In children, lack of vitamin D causes rickets and growth retardation. Vitamin D receptors are found in enterocytes, osteoblasts, the cells of the distal convoluted renal tubules, the cells of the parathyroid gland, colon, pituitary, ovaries, the cells of the immune system, etc. Therefore, vitamin D low levels were associated, by many observational studies, with major diseases, such as osteoporosis, diabetes, some forms of cancer, autoimmune diseases, infectious diseases, and hypertension but causality have not been proven [4].
Serum 25-hydroxyvitamin D (25(OH)D) is considered as the marker of vitamin D status. Guidelines about prevention of vitamin D deficiency suggest serum 25(OH)D levels above 20 ng/mL [5]. However, some experts suggest for maximal effect of vitamin D on calcium, bone, and muscle metabolism; levels of 25(OH)D should be above 30 ng/mL. Several epidemiological studies have proposed that a 25(OH)D above 30 ng/mL may have additional health benefits in reducing the risk of common cancers, autoimmune diseases, type 2 diabetes, cardiovascular disease, and infectious diseases [6].
There have been several recent consensus statements or guidelines that have included definitions of vitamin D deficiency. It is generally agreed that the serum concentration of 25(OH)D is the best marker of an individual’s vitamin D status because it is the major circulating form and reflects the combination of dietary intake and cutaneous skin synthesis. However, different thresholds for the level of 25(OH)D that is considered to reflect efficiency are used. For example, the Institute of Medicine’s report on the dietary reference intake for vitamin D published in 2010 [6] defined a level of 50 nmol/L (< 20 ng/mL) as meeting the needs of 97.5% of the population, whereas the Endocrine Society Clinical Practice Guideline published in 2011 defined vitamin D deficiency as a level < 50 nmol/L, with levels of between 52.5 nmol/L (21 ng/mL) and 72.5 nmol/L (29 ng/mL) regarded as vitamin D insufficiency, and levels of greater than 72.5 nmol/L being regarded as optimal [7]. In the current study, we decided to use the following definitions: severe deficiency < 25 nmol/L (< 10 ng/mL), deficiency 25–50 nmol/L (10–19.9 ng/mL), insufficiency 50–75 nmol/L (20–29.9 ng/mL), sufficiency ≥ 75 nmol/L (≥ 30 ng/mL).
Although recent data indicate that prevalence of vitamin D deficiency may be common in countries previously considered as low risk (e.g., Mediterranean countries) [4], few data are available for the Greek population. Low levels occur in elderly [8], children, adolescents [7], and adults [9] mainly from urban areas of Greece. Therefore, the objective of our study was to investigate the vitamin D status in adult women and men from northern and southern regions of Greece including several islands, in relation to QUS parameters.
Methods
Subjects
OSTEOS is an observational cross-sectional study, conducted from June 2010 to July 2012. A population of 970 community-dwelling adults (133 males, 830 females) was randomly recruited at the health promotion events carried out by the Hellenic Society for the Support of Patients with Osteoporosis in rural and urban areas throughout Greece. The regions were Central Greece, including Attica, Peloponnese, Thessaly, Aegean Islands, and Macedonia, and especially in more than 10 municipalities of Attica, Ilia, Patmos island, Viotia, Fokida, Evia, Korinthos, Halkida, Amfissa, Salamina, Fthiotida, Pella, Elassona, Volos, and Thessaloniki. Subjects were informed about events by announcements at municipalities and local authorities. Demographic data and medical history of the subjects were obtained using an appropriate questionnaire.
The International Physical Activity Questionnaire (IPAQ, short version), a self-administered tool, was used for the assessment of hours spent on sedentary activities (television, personal computer) and in moderate or vigorous organized physical activity; it was completed under the surveillance of the investigator [10].
To evaluate the hours of sun exposure, the subjects were asked how many hours per week between 9:00–18:00 on average they were exposed to the sun, in the summer, and in the winter.
At around 35° north latitude and above (Greece 35°–40°), little or no vitamin D can be produced from sun exposure from November to February. 25(ΟΗ)D level reaches its nadir in late winter and it takes about 6 weeks to raise the serum levels. Therefore, we defined two seasons to classify the subjects according to the blood collection day, winter-spring (December until May) and summer-autumn (June until November) [11, 12].
Measurements
Body weight was measured with a calibrated scale to the nearest 0.1 kg wearing light clothing and no shoes. The height was measured by a mounted stadiometer to the nearest 0.5 cm. Weight and height were measured twice by the same investigator, measurements were averaged for each participant, and the body mass index (BMI) was calculated. BMI was classified according to the World Health Organization [13] into four categories: Underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obesity (≥ 30 kg/m2).
Following a 12-h fast, all subjects had a sample of venous blood withdrawn for serum isolation between 08:00–09:00 am. Total calcium (Ca), phosphorus (P), parathyroid hormone (PTH), and 25(OH)D were measured. Intact parathyroid hormone (iPTH) measurements were performed on a Roche/Modular Analytics analyzer, which employs electrochemiluminescence immunoassay technology (ECLIA). The intra- and inter-assay coefficients of variation (CVs) of the iPTH assay were less than 7% and the analytical sensitivity was 1.2 pg/mL. Serum 25(OH)D levels were determined by enzyme immunoassay [Immunodiagnostic Systems, 25(OH)D; Boldon, UK]. All the assays were performed in a single batch and in the same laboratory. The sensitivity of this assay is 5 nmol/L and intra- and inter-assay coefficients of variation of 5.3% and 4.6%, respectively. Standardization of the different vitamin D assays is the key to achieving comparable results across different methods and manufacturers. Furthermore, assay standardization is of critical importance for the establishment of common clinical cutoffs and their use in routine practice. Applying a common cutoff value on results generated with poorly standardized assays will inevitably lead to inconsistent patient classification and inappropriate therapeutic decisions. In 2010, the Vitamin D Standardization Program (VDSP) was established to improve the standardization of 25(OH)D assays. The aim of VDSP is that 25(OH)D measurements are accurate and comparable over time, location, and laboratory procedure to the values obtained using reference measurement procedures (RMPs) developed at the NIST [14] and Ghent University [15]. According to VDSP, a routine method is considered as standardized if the CV is < 10% and the bias < 5% [16]. The method we have used fulfills the criteria to be considered standardized according to CDC website http://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Vitamin_D_Procedures.pdf.
Heel bone properties were measured using the Achilles quantitative ultrasound (QUS) device, a water-bath ultrasound system into which the subject places his heel. Achilles generates a band of frequencies from 200 to 600 kHz. It measures the broadband ultrasound attenuation (BUA), expressed in dB/MHz, and measures the ultrasound attenuation with the incident frequency of wave sound. The speed of sound (SOS) is expressed in metres per second and means the necessary time for ultrasound waves to go through a determined distance inside the calcaneus bone. The third variable, stiffness index (SI), is automatically calculated by Achilles from the BUA and the SOS, using the equation SI=0.67*BUA+0.28*SOS-240 [17]. The lower the QUS parameter values, the higher the fracture risk [18, 19]. For normative data, we used reference data for the QUS measurements of the calcaneus specific for Greek population [20]. A quality-control procedure using the standard phantom was performed daily before the measurements in the present study. In vivo short-term precision calculated from four repeated measurements by the same operator, with repositioning, on 15 unselected subjects and expressed as the root mean square of the coefficients of variation (CV) was 2.28% for SI. A single ultrasonometer was used throughout the study, and all measurements were carried out by specially trained technicians.
Subjects with a known history of metabolic bone diseases were excluded from the study. People with endocrine diseases, malignancies, connective tissue diseases, malabsorption syndrome, inflammatory bowel diseases (ulcerative colitis, Crohn’s disease), liver cirrhosis, renal failure, and having taken drugs that affect bone metabolism were excluded from the study. Early menopause (< 40 years) and amenorrhea > 1 year were also exclusion criteria. Finally, subjects taking calcium and/or vitamin D supplements were excluded from the study.
Statistical analyses
Statistical analysis was conducted using SPSS 19 statistical software. Continuous variables are presented as mean ± standard deviation, while categorical variables are presented as relative frequencies. Analysis of variance (ANOVA) was used to examine differences among the groups for different continuous, while the chi-square test was used to evaluate associations between categorical variables. Independent relationships between vitamin D status and other variables were assessed by stepwise multiple regression. All tests are two-sided with significance level < 0.05.
A voluntary participation agreement was obtained from each participant. The study was approved by the Ethics Committee of Harokopio University.
Results
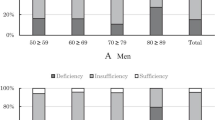
A total of 970 subjects were included in this study. The mean age of the population was 49.58 years (range, 18–86 years), while 87.2% were 18–65 years old. The mean serum 25(OH)D level of the population was 20.00 ± 8.00 ng/mL. The 57% of specimens were collected during the summer-autumn period while the rest 43% were collected during the winter-spring period. The remarkable percentage of 54% within our study population was found vitamin D deficient while only 12.3% reached the adequate level (data not shown). Figure 1 shows the percentages of men and women having severe deficiency (≤ 10 ng/mL), deficiency (10–19.9 ng/mL), insufficiency (20–29.9 ng/mL), and adequacy (≥ 30 ng/mL) of serum 25(OH)D. Descriptive characteristics of the population and their differences among vitamin D status categories are presented in Table 1.
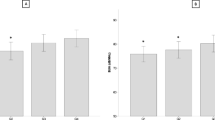
The age-specific prevalence of different categories of vitamin D status is presented in Fig. 2. Mean levels of 25(OH)D, PTH, BUA, SOS, SI, by age group, BMI categories, seasons of examination, and area of residence are presented in Table 2. According to the age group, serum vitamin D is higher in the 18–50 age group in relation to ≥ 65 years (p = 0.032). Mean serum PTH is lower in subjects 18–50 years in relation to 51–65 and ≥ 65 years and the 51–65 age group had lower PTH than ≥65 years (p = 0.00). Mean SI and BUA are higher in the 18–50 age group in relation to 51–65 and ≥ 65 years and in 51–65 in relation to ≥ 65 years (p = 0.00). Mean SOS is higher in the 18–50 age group in relation to ≥ 65 years (p = 0.00).
According to the weight status, mean serum 25(OH)D was higher in normal-weight subjects than in obese (p = 0.01).
Underweight subjects had lower serum PTH than obese and normal-weight subjects had lower PTH than overweight and obese subjects (p < 0.05).
Mean serum 25(OH)D levels differ between individuals depending on the season of blood sampling. Those measured in winter-spring period had lower mean serum 25(OH)D than those who were measured in summer-autumn (p = 0.007). Serum PTH was also lower in individuals measured in summer and autumn than those measured in winter and spring (p < 0.001).
The 72.51% of the total population lived in urban areas. Serum 25(OH)D levels were higher in urban than in rural areas (20.39 ng/mL vs 18.96 ng/mL, p = 0.005) as presented in Table 2, but the difference did not exist after adjustment for BMI, season of sampling, sex, and age (data not shown). Urban residents had lower SOS (1549.14 vs 1564.51, p = 0.031) and SI (90.37 vs 94.32, p = 0.037) values than rural residents (Table 2). The relationship remained after adjustment for PTH, age, sex, and organized physical activity, only for SOS parameter (B = 17.881, p = 0.016) (data not shown).
The binary logistic regression with serum 25(OH)D, < 20 ng/mL and ≥ 20 ng/mL as dependent variable and obesity and age groups (18–50, 51–65, and ≥ 65) as independent categorical variables, revealed that obese individuals had 1.458 times increased risk to have 25(OH)D < 20 ng/mL (p = 0.006). People 51–65 years had a 1.75 times increased risk of vitamin D insufficiency in relation to those of 18–50 years (data not shown).
The SOS parameter was significantly different in all categories of vitamin D, with individuals who had 25(OH)D ≥ 20 ng/mL presenting higher SOS measurements than those with 25(OH)D < 20 ng/mL (1561.5 ± 37 and 1553.2 ± 46.6, respectively, p = 0.008), and this significance remained after adjustment for age (B = 13.04, p = 0.04) (data not shown).
The correlation among different characteristics of the population and 25(OH)D, PTH, BUA, SOS, SI, is presented in Table 3.
In order to determine which factors had contributed to vitamin D status, multiple variables that could possibly influence 25(OH)D levels were included into a stepwise multiple linear regression analysis model. Female sex, season, the hours of summer sun exposure, and participation in organized physical activity had a significant effect; Age presented a significant negative effect only among obese subjects (Table 4). This model explains the 5.3% of the variability of 25(OH)D in population.
Discussion
The present study recruited a large number of subjects from the general healthy population from several regions of Greece. Previous studies investigating vitamin D status in Greece had smaller samples from specific areas and age groups [7,8,9]. This is the first epidemiological study providing the association of QUS and other lifestyle parameters with vitamin D status in a large sample of Greek women and men [21, 22].
The majority of Greek adults (54%) had vitamin D deficiency (< 20 ng/mL) and only 12.3% had levels above 30 ng/mL, with males having higher serum vitamin D than females. This percentage is similar to Germany, a northern country (11.8%) [23]. Similar results were derived from other northern countries like Denmark, Poland, Bosnia [24,25,26], and South Australia [27]. Specifically, the 25.9% of Bosnia and Herzegovina population had severe vitamin D deficiency, greater than 8% of current population, but only 18% and 12% of Bosnia and Greek population respectively had sufficient levels of 25(OH)D. The percentage of vitamin D adequacy in our Greek healthy population is very low compared to the Bosnia and Herzegovina study which however corresponds to a patients’ population. In a recent study where 1075 adults were studied from seven European countries, including Greece, about 34% of adults had 25(OH)D < 20 ng/mL [28]. In Canada, a large proportion οf the population (40.8%) is reported to have 25(OH)D > 30 ng/mL [29]. This may be due to food fortification, a possible cause of the difference of vitamin D status among Canada and the other countries. In a Canadian study, fortification of milk, yogurt, and cheese at 6.75 μg (270 IU)/serving led to more than doubling of vitamin D intake across all sex and age groups and a drop in the prevalence of dietary inadequacy from > 80 to < 50% in all groups. Furthermore, no intakes approached the upper level (UL) under any fortification scenario in any sex and age group [30].
In our study, vitamin D < 20 ng/mL was related to lower SOS values. As it was showed in other studies, these two conditions may increase the risk of fracture [31, 32]. Serum 25(OH)D levels were previously reported to be an independent determinant of SOS [33]. In contrast, another study found that QUS measurements did not differ between Arabian women with serum 25(OH)D < or ≥ 30 ng/mL [34]. Although our study is lacking histomorphometric data, it is possible that the defective collagen mineralization among vitamin D-deficient patients might be a reason for the lower SOS. This finding provides evidence for clinical use of QUS in subjects with low serum 25(OH)D levels.
Vitamin D status was influenced by BMI, so obese people had lower levels from non-obese. Obese individuals had 1.458 times increased risk to having 25(OH)D < 20 ng/mL (p = 0.006), independently of the age group. In a Polish obese population, mean 25(OH)D levels were 15.8 ± 8.5 ng/mL [25]. Mean serum 25(OH)D levels of general population in Portugal was 22 ng/mL, where the 48% of population had vitamin D deficiency. In the same population, obese people had lower 25(OH)D levels than not obese [35]. Healthy Italian adults had 25(OH)D mean levels 34.3 ng/mL [36]. In another Greek study of postmenopausal non-osteoporotic women, serum 25(OH)D levels were inversely associated with body fat mass, as measured using dual-energy x-ray absorptiometry [37]. Obviously, the explanation of this expected finding resides on the fat-soluble property of vitamin D.
Hours of summer sun exposure proved to have a beneficial effect on serum 25(OH)D as also expected, and our population had higher levels in summer and autumn than winter and spring. The beneficial effect of summer sun irradiation was in agreement with the results of a Swedish study [38] and of a Norwegian adolescent population [39]. A Danish study concludes that sunbathing and whole body sun exposure of healthy perimenopausal women leads to 13.2% and 27.6% respectively increase in serum 25(OH)D levels [24].
Participation in organized moderate or vigorous physical activity seems to have a beneficial effect on serum 25(OH)D. Similar are the results in an Australian population where those with higher levels of physical activity had less risk of 25(OH)D below 50 or 75 nmol/L [27]. As well as in a European study, engagement in ≥ 30 min per day of moderate- and vigorous-intensity physical activity was associated with higher odds for maintaining sufficient (≥ 50 nmol/L) 25(OH)D3 concentrations [28]. In US population, an increase of 10 min of objectively measured and self-reported moderate-to-vigorous activities per day was associated with an increase in circulating 25(OH)D of 0.32 ng/mL (95% CI 0.17, 0.48) and of 0.18 ng/mL (95% CI 0.12, 0.23), respectively. Associations were not due to sun exposure during activity because it was similar for outdoor and indoor activities [40].
According to the determinants of vitamin D status, the female sex, the winter-spring season, and age only in obese subjects influence serum 25(OH)D levels negatively. Hours of summer sun exposure and organized physical activity have positive effects on serum 25(OH)D. Aging did not affect the vitamin D status, but only in obese people. Similarly, a relevant review mentioned that people with outdoor lifestyle prevented 25(OH) decline during aging [41]. In another study, vitamin D status was unrelated to age [42].
Conclusion
In conclusion, this study highlights the emerging issue of hypovitaminosis D in Greece. It also detected major determinants of serum 25(OH)D, obesity, poor exposure to sunlight, age, and physical activity. Moreover, subjects with 25OHD levels < 20 ng/mL had lower SOS values. With the evidence that vitamin D sufficiency may be linked to the prevention of multiple extra-skeletal conditions, further studies are needed to detect other environmental parameters, such as nutrition and clinical and genetic factors which might influence vitamin D status even in sunny countries, as is Greece.
References
Report of a WHO Study Group (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 843:1–129
Moayyeri A, Adams JE, Adler RA, Krieg MA, Hans D, Compston J, Lewiecki EM (2012) Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int 23(1):143–153
Marin F, González-Macías J, Díez-Pérez A, Palma S, Delgado-Rodríguez M (2006) Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res 21:1457–1463
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930
Lapatsanis D, Moulas A, Cholevas V, Soukakos P, Papadopoulou ZL, Challa A (2005) Vitamin D: a necessity for children and adolescents in Greece. Calcif Tissue Int 77:348–355
Papapetrou PD, Triantaphyllopoulou M, Karga H, Zagarelos P, Aloumanis K, Kostakioti E, Vaiopoulos G (2007) Vitamin D deficiency in the elderly in Athens, Greece. J. Bone Miner Metab 25:198–203
Pazaitou-Panayiotou K, Papapetrou PD, Chrisoulidou A, Konstantinidou S, Doumala E, Georgiou E, Panagiotou V, Sotiriadou E, Mavroudi E, Apostolaki-Christopoulou M (2012) Height, whole body surface area, gender, working outdoors, and sunbathing in previous summer are important determinants of serum 25-hydroxyvitamin D levels. Exp Clin Endocrinol Diabetes 120(1):14–22
International Physical Activity Questionnaire Available at: http://www.ipaq.ki.se/. Accessed 12 Dec 2009
Webb AR, Kline L, Holick MF (1988) Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894:i–xii 1–253
Tai SS, Bedner M, Phinney KW (2011) Development of a candidate reference measurement procedure for the determination of 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem 82:1942–1948
Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM (2011) Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem 57:441–448
Stöckl D, Sluss PM, Thienpont LM (2009) Specifications for trueness and precision of a reference measurement system for serum/plasma 25-hydroxyvitamin D analysis. Clin Chim Acta 408:8–13
Flöter M, Bittar CK, Zabeu JL, Carneiro AC (2011) Review of comparative studies between bone densitometry and quantitative ultrasound of the calcaneus in osteoporosis. Acta Reumatol Port 36(4):327–335
Huopio J, Kröger H, Honkanen R, Jurvelin J, Saarikoski S, Alhava E (2004) Calcaneal ultrasound predicts early postmenopausal fractures as well as axial BMD. A prospective study of 422 women. Osteoporos Int 15(3):190–195
Hans D, Dargent-Molina P, Schott AM, Sebert JL, Cormier C, Kotzki PO, Delmas PD, Pouilles JM, Breart G, Meunier PJ (1996) Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 348(9026):511–514
Trovas G, Tsekoura M, Galanos A, Dionyssiotis Y, Dontas I, Lyritis G, Papaioanou N (2009) Quantitative ultrasound of the calcaneus in Greek women: normative data are different from the manufacturer's normal range. J Clin Densitom 12(3):353–359
Babaroutsi E, Magkos F, Manios Y, Sidossis LS (2005) Lifestyle factors affecting heel ultrasound in Greek females across different life stages. Osteoporos Int 16(5):552–561
Babaroutsi E, Magkos F, Manios Y, Sidossis LS (2005) Body mass index, calcium intake, and physical activity affect calcaneal ultrasound in healthy Greek males in an age-dependent and parameter-specific manner. J Bone Miner Metab 23(2):157–166
Rabenberg M, Scheidt-Nave C, Busch MA, Rieckmann N, Hintzpeter B, Mensink GB (2010) Vitamin D status among adults in Germany--results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 15:641. https://doi.org/10.1186/s12889-015-2016-7
Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sørensen OH (2001) Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr 86(Suppl 1):S97–S103
Płudowski P, Ducki C, Konstantynowicz J, Jaworski M (2016) Vitamin D status in Poland. Pol Arch Med Wewn 126(7–8):530–539
Sokolovic S, Alimanovic-Alagic R, Dzananovic L, Cavaljuga S, Beslic N, Ferhatbegovic-Opankovic E (2017) Vitamin D status in Bosnia and Herzegovina: the cross-sectional epidemiological analysis. Osteoporos Int 28(3):1021–1025
Gill TK, Hill CL, Shanahan EM, Taylor AW, Appleton SL, Grant JF, Shi Z, Dal Grande E, Price K, Adams RJ (2014) Vitamin D levels in an Australian population. BMC Public Health 14:1001
Manios Y, Moschonis G, Lambrinou CP, Mavrogianni C, Tsirigoti L, Hoeller U, Roos FF, Bendik I, Eggersdorfer M, Celis-Morales C, Livingstone KM, Marsaux CF, Macready AL, Fallaize R, O’Donovan CB, Woolhead C, Forster H, Walsh MC, Navas-Carretero S, San-Cristobal R, Kolossa S, Hallmann J, Jarosz M, Surwiłło A, Traczyk I, Drevon CA, van Ommen B, Grimaldi K, Matthews JN, Daniel H, Martinez JA, Lovegrove JA, Gibney ER, Brennan L, Saris WH, Gibney M, Mathers JC, Food4Me Study (2017) Associations of vitamin D status with dietary intakes and physical activity levels among adults from seven European countries: the Food4Me study. Eur J Nutr 57:1357–1368. https://doi.org/10.1007/s00394-017-1415-1
Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, Poliquin S, Krieger J, Richards JB, Goltzman D, CaMos Research Group (2011) 25-hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int 22(5):1389–1399
Shakur YA, Lou W, L’Abbe MR (2014) Examining the effects of increased vitamin D fortification on dietary inadequacy in Canada. Can J Public Health 105(2):e127–e132
Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF (2015) Vitamin D: update 2013 from rickets prophylaxis to general preventive healthcare. Dermatoendocrinol 5(3):331–347
Kauppi M, Impivaara O, Mäki J, Heliövaara M, Jula A (2013) Quantitative ultrasound measurements and vitamin D status in the assessment of hip fracture risk in a nationally representative population sample. Osteoporos Int 24(10):2611–2618
Gianoudis J, Bailey CA, Daly RM (2015) Associations between sedentary behavior and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int 26:571–579
Kauppi M, Impivaara O, Mäki J, Heliövaara M, Marniemi J, Montonen J, Jula A (2009) Vitamin D status and common risk factors for bone fragility as determinants of quantitative ultrasound variables in a nationally representative population sample. Bone 45(1):119–124
Bettencourt A, Boleixa D, Reis J, Oliveira JC, Mendonça D, Costa PP, Silva BM, Marinho A, Silva AM (2018) Serum 25-hydroxyvitamin D levels in a healthy population from the North of Portugal. J Steroid Biochem Mol Biol 175:97–101
Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D (2018) Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels an observational case-control study. Medicine 97:e11185
Moschonis G, Tanagra S, Koutsikas K, Nikolaidou A, Androutsos O, Manios Y (2009) Association between serum 25-hydroxyvitamin D levels and body composition in postmenopausal women: the postmenopausal health study. Menopause 16(4):701–707. https://doi.org/10.1097/gme.0b013e318199d5d5
Klingberg E, Oleröd G, Konar J, Petzold M, Hammarsten O (2015) Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 49(3):800–808. https://doi.org/10.1007/s12020-015-0548-3
Oberg J, Jorde R, Almås B, Emaus N, Grimnes G (2014) Vitamin D deficiency and lifestyle risk factors in a Norwegian adolescent population. Scand J Public Health 42(7):593–602. https://doi.org/10.1177/1403494814541593
Wanner M, Richard A, Martin B, Linseisen J, Rohrmann S (2015) Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control 26(6):881–891. https://doi.org/10.1007/s10552-015-0563-y
Morris HA (2005) Vitamin D: a hormone for all seasons--how much is enough? Clin Biochem Rev 26(1):21–32
Morris HA, Morrison GW, Burr M, Thomas DW, Nordin BE (1984) Vitamin D and femoral neck fractures in elderly South Australian women. Med J Aust 140(9):519–521
Acknowledgments
The authors thank the Hellenic Society for the Support of Patients with Osteoporosis and all the dietitians and clinicians for their contribution to the project.
Special thanks to post doc researcher Maria Stathopoulou for her contribution to the study design and the dietitian Evangelia Bitsani for the subject recruitment.
Funding
This study was partially supported by a research grant from the Hellenic Society for the Study of Bone Metabolism.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(DOC 53 kb)
Rights and permissions
About this article
Cite this article
Grigoriou, E.V., Trovas, G., Papaioannou, N. et al. Serum 25-hydroxyvitamin D status, quantitative ultrasound parameters, and their determinants in Greek population. Arch Osteoporos 13, 111 (2018). https://doi.org/10.1007/s11657-018-0526-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-018-0526-5