Abstract
Hydrogeochemistry and factor analysis were conducted together to assess the distribution and the major geochemical processes in fluoride-contaminated shallow groundwater in the Yuncheng Basin. Spatially, fluoride concentration was low (< 1.5 mg/L) in the southern piedmont plain, medium (< 4 mg/L) in the central basin, and high (up to 14.1 mg/L) in Kaolao lowland areas in shallow aquifers. A three-factor principal component analysis model explained over 75.1% of the total variance. Sediment weathering leaching and evapotranspiration were recognized as the first primary hydrochemical processes response for the groundwater chemistry and explained the largest portion (42.1%) of the total variance. Factor two reflects the negative influence of human activities, with a positive loading of NO3− and HCO3−, and negative loading of well depth. Fluoride-bearing mineral dissolution and alkaline condition was ranked as the third factors responding for groundwater chemistry and explained 11.2% of the total variance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Groundwater plays a significant role in the water supply and economic development in China, especially in the arid and semi-arid areas in northern China. About one-third of the total population in China relies on groundwater for drinking water supply. However, potentially toxic elements, e.g. fluorine and arsenic, may reach hazardous concentrations in groundwater as a result of specific hydrogeological and geochemical processes. F-rich groundwaters are found in many areas around the world, including India (Choubisa 2013; Jacks et al. 2005; Rao 2001; Reddy et al. 2010; Srikanth et al. 2013; Vikas et al. 2013), Brazil (Souza et al. 2013), Mexico (Aguilar-Diaz et al. 2011; Daessle et al. 2008; Irigoyen et al. 1995; Reyes-Gómez et al. 2015), and China (Amini et al. 2008; Ando et al. 2001; Cao et al. 1997; Gao et al. 2007; Smedley et al. 2003), yet notably in Asia and Africa (Appelo and Postma 2005; Hu et al. 2013; Mondal et al. 2014; Sajil-Kumar et al. 2015).
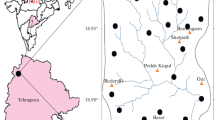
The Yuncheng Basin in North China is one of the representative fluoride-polluted areas. The basin is surrounded by mountains to the south, east, and north, forming a NE–SW semi-closed rifted basin. Zhongtiao Mountain lies down the south and east boundary, with elevation at the main peaks of 1200–1900 m. To the northeast, north, and northwest boundary of the Yuncheng Basin are Zijin Mountain, Jiwang Mountain, and Gufeng Mountain, respectively. To the west is the Yellow River (Fig. 1). As a semi-closed inter-mountainous basin, Yuncheng Basin is subject to numerous environmental issues such as shortage of water resources and deterioration of groundwater quality, especially the widely distributed F-rich groundwater.
A simple geomorphology map of the Yuncheng basin, China (→ groundwater flow direction; Line AB, position of cross-section in Fig. 2)
Endemic fluorosis was first confirmed at Yuncheng Basin in the 1980s. In 2005, Gao et al. focused on the occurrence of fluoride in rocks as the original source of fluoride in the groundwater. Then, Gao et al. (2007) studied the effect of salt lake water intrusion on shallow groundwater, which result in fluoride elevations. A study by Zhang (2010) revealed the leacheability of fluoride in soils from the Yuncheng Basin. Currell et al. (2010, 2011) demonstrated natural mineral sources of fluoride in groundwater in the central basin with some limited fieldwork. As a further work, the hydrochemistry of F-rich groundwater was reported by Khair et al. (2014), Li et al. (2015, 2016, 2018), Luo et al. (2018) and Li and Gao (2018).
Based on the previous researches, several hydrogeochemical processes, including water–rock interaction, evaporation, saline water intrusion, and polluted water leakage were considered to be the major sources for fluoride in groundwater in the basin. Additionally, alkaline pH values are favorable for fluoride desorption from the sediments (Jacks et al. 2005; Smedley et al. 2005; Bhattacharya et al. 2006). The mechanisms may include: 1) high pH could affect the ion charge of F− and properties of solid surface, and further to promote the adsorption of anions; 2) considerable OH− in groundwater could precipitate Ca2+, Fe3+, and Al3+, preventing F− from complexing with cations, which results in copious release of F− in groundwater; and 3) under alkaline condition, OH− could be exchanged by F− adsorbed in clay minerals, humus, and soil colloid. However, further understanding the extent of the hydrogeochemical processes involved requires an understanding of the contributions of these major geochemical factors, which are not well stated in the studies mentioned above. Multivariate statistical method (Principal Component Analysis) is a useful tool in providing additional information on groundwater chemistry interpretation. Principal Component Analysis reduces a large data matrix to a new matrix with fewer dimensions where the new references axes are the main variation directions. The geological interpretation of components yields insight into the main hydrogeochemical processes which may govern the distribution of hydrochemical variables (Belkhiri et al. 2010; Cloutier et al. 2008; Dassi 2011; Galazoulas and Petalas 2014; Gao et al. 2011; Masoud 2014; Singh et al. 2013; Suk and Lee 1999; Wang et al. 2001).
The aims of this study were: (1) to investigate the hydrochemistry of groundwater in the Yuncheng Basin; (2) to evaluate the distribution of F− in groundwater in the Yuncheng Basin; and (3) to identify and assess the potential contribution of major hydrochemical processes on hydrochemistry of fluoride contaminated groundwater.
2 Geology and hydrogeology
Basin geology and hydrogeology is discussed by Currell et al. (2010) and Li et al. (2015), hence only a brief summary is given here. The Yuncheng Basin comprises a 300–500 m thick Quaternary sediments (Q1–Q4), which are composed of aeolian loess, lacustrine clays, fluvial sands, and gravels. Basement rock outcrops in the south of the area are Archean metamorphic rock. This rock formation (Arsm) is composed of granite, biotite plagioclase gneiss, quartzite, and migmatite; the major minerals in the rocks include feldspar, biotite, quartz, chlorite, and so on. Elsewhere, sedimentary rocks, mainly Neogene mudstone and Cambro-Ordivician limestone, underlie the Quaternary sediments.
Due to the thick loose sediments accumulated in the Yuncheng Basin, groundwater is stored in the Quaternary alluvium that forms several aquifers (Fig. 2). On account of the differences in runoff conditions, shallow groundwater flows from the mountain fronts towards the center of the basin. The hydraulic gradient is 6–12‰ in the mountain front, 7‰ in the Emei tableland, and 3‰ in the flatlands (Chen et al. 1993). Phreatic and artesian aquifers are the main water resources for exploitation in the study area. Based on the distribution characteristics of aquifers and the hydraulic features, pore waters in the Yuncheng Basin could be classified into three types: (1) phreatic aquifers (5–70 m) which are supplied by precipitation, canal seepage, infiltrating of irrigation return flow, reservoir seepage, and lateral recharge, whose discharge is dominated by artificial exploitation and by evaporation; (2) intermediate semi-confined artesian aquifers (70–120 m), which are recharged mainly by lateral runoff from mountain fronts, and discharged dominantly by artificial exploitation; (3) semi-confined phreatic-artesian aquifers (130–500 m), which are remotely recharged and their hydrogeological conditions are controlled by leakage through aquitards.
3 Sampling and methods
A total of 79 samples, including one rainwater, six surface water, 22 groundwater from shallow aquifers, 9 groundwater from intermediate aquifers and 41 groundwater from deep aquifers were collected across the Yuncheng Basin in August 2013. When sampling, water samples were collected only after the in situ physicochemical parameters, including temperature, pH, and electrical conductivity (EC) were stable and all these parameters were measured within several minutes by portable meters.
Each sample was collected in three polyethylene bottles, one for anion analysis, one for cation analysis and the rest kept in a refrigerator for future experimental use. The total alkalinity was measured on the sampling day using the Gran titration method with the triple repetition analyze error < ± 2%. The concentrations of HCO3− and CO32− were computed by the software PHREEQC 2.8. Hydrochemical analyses were performed at the Key Laboratory of Biological and Environmental Geology, China University of Geosciences (Wuhan, China). The concentrations of Cl−, SO42−, and NO3− were determined using ion chromatography (IC, Dionex 120, Dionex, Sunnyvale, CA, USA). For cation analysis, reagent-quality HNO3 was added to one of these polyethylene bottles until the pH of samples was less than 2. Major cations, K+, Na+, Ca2+, and Mg2+, were measured using inductively coupled plasma-atomic emission spectrometry (ICP-AES, IRIS Intrepid XSP, Thermo Elemental, Madison, WI, USA). The analytical precision for the measurements of cations and anions is indicated by the ionic balance error, which is observed to be within the standard limit of ± 5%.
Box plot and principal component analyses of the water hydrochemical data were performed using the SPSS software version 9.0 (SPSS Inc. 2008). Factor extraction was carried out by principal component analysis. Varimax rotation was applied to obtain unrelated components (Kaiser 1958) and a three-factor model is determined. In this present study, the dataset was made up, including 79 samples and 11 chemical parameters (pH, well depth, F−, NO3−, Cl−, HCO3−, SO42−, Ca2+, K+, Mg2+, and Na+).
4 Results and discussions
4.1 Groundwater chemistry
The major properties and hydrochemistry of all water samples are summarized in Table 1. The chemistry of the groundwater in the area shows a wide variation in concentration ranges (Fig. 3). SO42− is found to be an extremely variable component, ranging between 17.5 and 8295 mg/L, followed by Cl− (6.73 – 3044 mg/L) and HCO3− (66.45 – 1094 mg/L). Concentrations of Na+, the major dominant cation, span over a wide range of 8.28–4967 mg/L. Other cations, including Ca2+, Mg2+, and K+, are relatively stable. Ca2+ and Mg2+ are two secondary dominant cations in groundwater, with average concentrations of 51.53 and 75.17 mg/L, respectively. K+, having had little impact on groundwater quality, is detected at a low concentration with an average value of 3.96 mg/L.
Based on the burial depth and regional hydrogeological conditions, groundwater samples were divided into three categories: shallow groundwater, intermediate groundwater, and deep groundwater. Shallow groundwater from the Kaolao lowland and the southern piedmont plain belong to Na-HCO3 type with low F contents (Fig. 2). While shallow groundwater from the central basin area gives the highest TDS values, up to 17,452 mg/L. These groundwaters belong to Na–SO4–HCO3/Cl or Na–Cl–SO4 type (Fig. 4), with Na+ as the dominant cation and SO42− and/or Cl− as the dominant anions. The concentrations of Na+, Cl− and SO42− are up to 4967, 3044 and 8295 mg/L, respectively, in these higher TDS groundwaters. Evaporation tends to concentrate all the species in the shallow groundwater (Li et al. 2015), therefore, strong evaporation is believed to be one of the major factors inducing the enrichment of sulfate and chloride concentration in shallow groundwater at Yuncheng Basin. Gypsum dissolution and anthropogenic activities, like agricultural fertilizers, may also contribute to the groundwater chemistry. Soluble gypsum is widely distributed in the middle Pleistocene and, hence, can be regarded as one of the primary sources of sulfate in shallow groundwater in the Yuncheng Basin (Gao et al. 2007; Shanxi Province Geological Survey 1982). More than 70% of the shallow groundwater contains NO3− in excess of the WHO standard for drinking water (10 mg/L). On account of the lack of natural nitrate in most geologic formations, NO3− concentrations > 5 mg/L are generally indicative of water contamination by animal wastes, fertilizers, and/or effluents (Heaton 1986). The highest NO3− content (67.9 mg/L) is observed in shallow groundwater, implying an anthropogenic source in this area.
Intermediate and deep groundwaters are all alkaline, with a pH range of 7.3–8.6. Most of them are freshwaters with TDS lower than 1000 mg/L and belong to Na-HCO3 type water. About 30% of intermediate and deep groundwater collected from the Kaolao lowland and central basin areas belong to Na–SO4–HCO3/Cl or Na–Cl–SO4 type with TDS > 1000 mg/L. The sources of salt in these waters may either come from evaporite (e.g. gypsum, halite, and mirabilite) dissolution (Li et al. 2015) or leakage of saline shallow groundwater. High NO3− concentrations are also observed in intermediate and deep groundwaters. Due to the low background nitrate concentrations in intermediate and deep groundwater samples, the elevated NO3− concentrations in these groundwaters indicate the downwards vertical leakage of irrigated water and/or polluted shallow groundwater.
4.2 Fluoride pollution in groundwater
Rainwater, surface water and groundwater are collected for fluoride were analyzed in this case study. Rainwater contains a low fluoride concentration of 0.32 mg/L. Generally, fluoride content in precipitation is lower than the detection limit, but seawater spray and atmospheric pollutants are the major sources of fluoride in precipitation. As an inland catchment, the fluoride in rainwater at the Yuncheng Basin mainly derives from atmospheric pollution from local industries and coal consumption (Luo et al. 2018).
Concentrations of fluoride in surface waters vary between 0.32 and 11.2 mg/L with an average value of 4.1 mg/L. Fluoride concentration is low in the surface water samples collected from mountain areas and the piedmont plain areas and higher in lakes and reservoir where human activities are frequent and intensive. This suggests that human activities are partly responsible for the enrichment of fluoride in these waters.
Groundwater has severely suffered from fluoride pollution in Yuncheng Basin (Cao 2005; Gao et al. 2007; Li et al. 2015). Over 68% of the shallow wells contain fluoride concentrations above the WHO provisional drinking water guide value of 1.5 mg/L (WHO 2004). The concentration of fluoride in groundwater is not uniform in the study area. In the case of shallow aquifer, fluoride concentrations range between 0.32 and 14.1 mg/L with an arithmetical mean value of 4.27 mg/L. The fluoride concentration is low in the shallow groundwater from the south piedmont plain areas (Fig. 2), whereas the highest fluoride concentration (14.1 mg/L) is found in shallow groundwater from the Kaolao lowland area in the Shushui river basin. Increase of fluoride content is supposed to be controlled by hydrological and geochemical processes in this area (e.g., irrigation leaching, dissolution of fluorine minerals, cation exchange, and evaporation). Shallow groundwater that has medium to high fluoride contents with elevated Ca and TDS contents are observed from the north part of the salt lake.
Intermediate and deep groundwater samples are characterized by low to medium F− concentration values, ranging from 0.19 to 3.2 mg/L and 0.1 to 2.9 mg/L, respectively. Forty percent of the intermediate wells and thirty percent of the deep wells fall above the 1.5 mg/L threshold. The wide distribution of F-rich groundwater in intermediate and deep groundwater suggests that the fluoride pollution is a non-point source pollution.
The spatial variation of the fluoride concentration is presented in a spatial distribution map (Fig. 2). It can be seen that high fluoride groundwater is concentrated mostly in the central areas rather than in the margins (Figs. 1, 2).
4.3 Assessment of major geochemical processes using PCA
In general, PCA (principle component analysis) extracts correlations and reduces the amount of data into components that explain a portion of the total variances between chemical parameters. These variances are mainly related to the chemical parameters showing the highest loading factors obtained by using the varimax rotation. Those high loaded components are further regarded as references for identifying the geochemical processes involved. In this case study, three factors are defined and 75.11% of the total cumulative variances are obtained (Tables 2, 3; Fig. S1).
Factor one, associated with high positive loadings for Cl−, SO42−, Ca2+, Mg2+, and Na+, explains the largest portion of the total variance (42.1%). This factor can be explained by chemical weathering and evapotranspiration processes. In general, groundwater in the Yuncheng basin is recharged by precipitation and discharged by abstraction and evapotranspiration. During the movement of groundwater from the margins to the central areas, the chemical constituents in groundwater are naturally sourced from the dissolution of sediment/rock minerals. The major minerals in sediments are quartz, albite, k-feldspar, mica, calcite, chlorite, and some amphibole and dolomite in the Yuncheng Basin (Table 4, Li et al. 2015). Minor contents of gypsum, mirabilite, and halite are also reported for sediments from the basin (Gao et al. 2007; Shanxi Province Geological Survey 1982). As a consequence, the weathering leaching of these minerals yields high contents of HCO3−, SO42−, Na+, Cl−, Ca2+, and Mg2+ in Quaternary groundwater.
However, the involvement of additional hydrochemical processes in this stage is suggested by the low loading of HCO3− in factor one. Evapotranspiration is regarded as another crucial factor affecting groundwater chemistry. If groundwater undergoes the evapotranspiration process, there would be a consistency of (Na + Ca + Mg)/Cl ratios of samples with increasing TDS value. As shown in Fig. 5, the distribution of the water samples shows two trends: (1) parallel to the X-axis reflecting the influence of evapotranspiration and (2) close to the Y-axis indicating a sustained increase of Na/Ca/Mg-bearing mineral dissolution. Block groundwater samples, collected from intermediate and deep aquifers, located close to the Y-axis showed a low TDS value and increased (Na + Ca + Mg)/Cl ratio. Some surface water and shallow groundwater samples, collected from the piedmont plain areas, also dropped into this group. The increasing (Na + Ca + Mg)/Cl ratio and low TDS in this group suggest that water chemistry components here were obtained by natural mineral weathering leaching process, which was controlled by the kinetic dissolution rate of minerals.
Two surface water samples and several shallow groundwater samples, collected from the central basin, displayed a significant increase of TDS value with little change in (Na + Ca + Mg)/Cl ratio. Several deep groundwater samples were classified into this group too. Evapotranspiration generally increases the concentration of major ions and TDS in groundwater without making a significant change of the ion ratios. The constant (Na + Ca + Mg)/Cl ratio and the elevated TDS value in these waters suggest a significant influence of evapotranspiration on groundwater chemistry. Considering the fact that deep groundwater is not affected by evapotranspiration, deep samples that appeared in this group may be due to the leakage of shallow groundwater or surface water subjected to evapotranspiration.
Factor two, which explains 19.997% of the total variance, is dominated by well depth (− 0.702), NO3− (0.685), and HCO3− (0.668). The negative loading of well depth and positive loading of HCO3− and NO3− suggest a reverse correlation between burying depth and groundwater chemistry components. Basically, deep burying depth stands for a weak surface leakage and/or interference of human activities. Hence, high concentrations found in wells with lower depth indicates heavier human activity influence.
Due to the lack of natural source in the geologic formations in the area, high loading value of NO3− indicates a significant human activity input, e.g. animal wastes, agricultural fertilizers and/or effluents. The highest NO3− content (67.9 mg/L, Table 1) observed in shallow groundwater implies an anthropogenic source in this area. Given the low background nitrate concentrations in deep groundwater, the high NO3− concentrations in some deep samples with depth over 200 m may be due to mixing with irrigation return water and/or shallow polluted groundwater.
The common source of HCO3− is derived from carbonate weathering dissolution due to the lack of organic sources in the sediment aquifers (Currell et al. 2011). But the negative loading of Ca2+ and low loading of Mg2+ suggest that carbonate minerals dissolution may be masked by a high degree of cation exchange. Briefly, the association of well depth, NO3−, and HCO3− reflects the influence of anthropogenic processes on pollution of groundwater and can thus be termed as the ‘anthropogenic factor’.
Fluoride bearing mineral dissolution and alkaline condition was ranked as the third factors responding for groundwater chemistry. Factor three, containing only two high loading factors F− (0.804) and pH (0.785), reflects the influence of fluorine as a pollutant in the area, and could be defined as the ‘F pollution factor’. Natural water–rock interactions and hydrogeological processes are the key factors controlling groundwater fluoride mobilization. In general, F− is preferentially adsorbed to sediment mineral surfaces under neutral conditions (Smedley et al. 2005; Bhattacharya et al. 2006). pH is one of the major factors that govern the liberation and mobility of fluoride into groundwater. Thus, pH is considered to be the most important factor causing F mobilization (Osei et al. 2016; Tang and Zhang 2016; Zhang et al. 2015). Nonetheless, as a mutable parameter in groundwater, pH is not an independent parameter and it is closely associated with the relevant hydrogeochemical processes mentioned above.
5 Conclusion
Groundwater in the Yuncheng Basin showed wide variations in major ions and fluoride concentration. [F] is low in the shallow groundwater from the south piedmont plain areas, whereas the highest fluoride concentration (14.1 mg/L) is found in shallow aquifers from the Kaolao lowland area in the Shushui river basin. Intermediate and deep groundwater are characterized by low to medium F− concentrations (0.19 ~ 3.2 mg/L).
Integrated hydrogeochemistry and principal component analysis provide important clues for understanding the major processes controlling the hydrochemistry of fluoride contaminated groundwater at Yuncheng Basin. Natural water–rock interactions including dissolution of sediment/rock minerals (e.g., fluoride bearing minerals, evaporites) and evapotranspiration were recognized as the prime process impacting the high fluoride groundwater. Anthropogenic activities have affected the hydrochemistry of fluoride contaminated shallow groundwater, as indicated by high NO3− contents, the negative loading of well depth and positive loading of HCO3− and NO3− in Factor 2. Alkaline condition is considered to be the most important factor leading to F mobilization.
References
Aguilar-Diaz FC, Irigoyen-Camacho ME, Borges-Yanez SA (2011) Oral-health-related quality of life on schoolchildren in an endemic fluorosis area of Mexico. Qual Life Res 20(2):1699–1706
Amini M, Mueller K, Abbaspour KC (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42(10):3662–3668
Ando M, Tadano M, Yamamoto S (2001) Health effects of fluoride pollution caused by coal burning. Sci Total Environ 271(1):107–116
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. Amsterdam
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—A case study: Ain Azel plain (Algeria). Geoderma 159(2010):390–398
Bhattacharya P, Claesson M, Bundschuh J, Sracek O, Fagerberg J, Jacks G, Martin RA, Stoniolo AR, Thir JM (2006) Distribution and mobility of arsenic in the Rio Dulce alluvial aquifers in Santiago del Estero Province, Argentina. Sci Total Environ 358:97–120
Cao XH (2005) Study of the confined groundwater system of middle-deep layers in Sushui catchment. Shanxi Hydrotech Bull 3(8):41–43 (in Chinese)
Cao J, Zhao Y, Liu J (1997) Brick tea consumption as the cause of dental fluorosis among children from Mongol, Kazak and Yugu populations in China. Food Chem Toxicol 35(8):827–833
Chen CX, Lin M, Zhu WW (1993) Groundwater Salinization in Yuncheng City: predicated by a three dimensional numerical simulation. Earth Sci: China Univ Geosci 1:48–59 (in Chinese with English Abstract)
Choubisa SL (2013) Fluorotoxicosis in diverse species of domestic animals inhabiting areas with high fluoride in drinking water of Rajasthan, India. Proc Nat Acad Sci India Sect B-Biol Sci 83(3):317–321
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(2008):294–313
Currell MJ, Cartwright I, Bradley DC, Han DM (2010) Recharge history and controls on groundwater quality in the Yuncheng Basin, north China. J Hydrol 385(1):216–229
Currell MJ, Cartwright I, Raveggi M (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin, China. Appl Geochem 26(4):540–552
Daessle LW, Ruzi-Montoya L, Tobschall HJ, Chandrajith R, Camacho-Ibar VF, Mendoza-Espinosa LG (2008) Fluoride, nitrate and water hardness in groundwater supplied to the rural communities of Ensenada County, Baja California, Mexico. Environ Geol 58(2):419–429
Dassi L (2011) Investigation by multivariate analysis of groundwater composition in a multilayer aquifer system from North Africa: a multi-tracer approach. Appl Geochem 26(1):1386–1398
Galazoulas EC, Petalas CP (2014) Application of multivariate statistical procedures on major ions and trace elements in a multilayered coastal aquifer: the case of the south Rhodope coastal aquifer. Environ Earth Sci 72(10):4191–4205
Gao XB, Wang YX, Li YL (2007) Enrichment of fluoride in groundwater under the impact of saline water intrusion at the salt lake area of Yuncheng Basin, northern China. Environ Geol 53(4):795–803
Gao XB, Wang YX, Ma T, Hu QH, Xing XL, Yu Q (2011) Anthropogenic effects on hydrochemistry of Niangziguan karst water. Proc ICE Water Manag 164(10):495–510
Heaton TH (1986) Nitrate pollution of groundwater. S Afr J Sci 82(1):279–287
Hu S, Luo T, Jing CY (2013) Principal component analysis of fluoride geochemistry of groundwater in Shanxi and Inner Mongolia, China. J Geochem Explor 135:124–129
Irigoyen DE, Monlina N, Luengas I (1995) Prevalence and severity of dental fluorosis in a Mexican community with above-optimal fluoride concentration in drinking water. Commun Dent Oral Epidemiol 24(4):243–245
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20(2):221–228
Kaiser HF (1958) The varimax criteria for analytical rotation in factor analysis. Psychometrika 23(3):187–200
Khair AM, Li CC, Hu QH, Gao XB, Wang YX (2014) Fluoride and arsenic hydrogeochemistry of groundwater at Yuncheng Basin, northern China. Geochem Int 52(10):868–881
Li CC, Gao XB (2018) Assessment of groundwater quality at Yuncheng Basin: denotation for the water management in China. Groundwater
Li CC, Gao XB, Wang YX (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165
Li CC, Liu T, Xu S, Gao XB, Wang YX (2016) Groundwater salinization in shallow aquifers adjacent to a low-altitude inland salt lake: a case study at Yuncheng Basin, northern China. Environ Earth Sci 75:370
Li DN, Gao XB, Wang YX, Luo WT (2018) Diverse mechanisms drive fluoride enrichment in groundwater in two neighboring sites in northern China. Environ Pollut 237:430–441
Luo WT, Gao XB, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—An area with complex hydrogeochemical conditions. PLoS ONE 13:e0199082
Masoud AA (2014) Groundwater quality assessment of the shallow aquifers west of the Nile Delta (Egypt) using multivariate statistical and geostatistical techniques. J Afr Earth Sci 95:123–137
Mondal D, Gupta S, Reddy DV, Nagabhushanam P (2014) Geochemical controls on fluoride concentrations in groundwater from alluvial aquifers of the Birbhum district, West Bengal, India. J Geochem Explor 145:190–206
Osei J, Gawu SK, Schäfer AI, Atipoka FA, Momade FW (2016) Impact of laterite characteristics on fluoride removal from water. J Chem Technol Biotechnol 91:911–920
Rao NS (2001) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41(5):552–562
Reddy AG, Reddy DV, Rao PN, Prasad KM (2010) Hydrogeochemical characterization of fluoride rich groundwater of Wailpalli watershed, Nalgonda District, Andhra Pradesh, India. Environ Monit Assess 171(1):561–577
Reyes-Gómez VM, Alarcón-Herrera MT, Gutiérrez M, Núñez-López D (2015) Arsenic and fluoride contamination in groundwater of an endorheic basin undergoing land use changes. Arch Environ Contam Toxicol 68(2):292–304
Sajil-Kumar PJ, Jegathambal P, Nair S, James EJ (2015) Temperature and pH dependent geochemical modeling of fluoride mobilization in the groundwater of a crystalline aquifer in southern India. J Geochem Explor 156:1–9
Shanxi Province Geological Survey (1982) Hydrological and Geological maps and explanations for the Yuncheng region, 1:100,000, Special Report 1982 (in Chinese)
Singh E, Gupta A, Singh NR (2013) Groundwater quality in Imphal West district, Manipur, India, with multivariate statistical analysis of data. Environ Sci Pollut Res 20(4):2421–2434
Smedley PL, Zhang M, Zhang G, Luo Z (2003) Mobilization of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia. Appl Geochem 18(9):1453–1477
Smedley PL, Kinniurgh DG, Macdonald DMJ, Nicolli HB, Barros AJ, Tullio JO, Pearce JM, Alonso MS (2005) Arsenic associations in sediments from the loess aquifer of La Pampa, Argentina. Appl Geochem 20(5):989–1016
Souza CF, Lima JF, Adriano MS, Carvalho FG, Forte FD, Oliveira R (2013) Assessment of groundwater quality in a region of endemic fluorosis in the northeast of Brazil. Environ Monit Assess 185(6):4735–4743
Srikanth R, Gautam A, Jaiswal SC, Singh P (2013) Urinary fluoride as a monitoring tool for assessing successful intervention in the provision of safe drinking water supply in five fluoride-affected villages in Dhar district, Madhya Pradesh, India. Environ Monit Assess 185(3):2343–2350
Su CL, Wang YX, Xie XJ, Li JX (2013) Aqueous geochemistry of high-fluoride groundwater in Datong Basin, Northern China. J Geochem Explor 135(2013):79–92
Suk H, Lee KK (1999) Characterization of a ground water hydrochemical system through multivariate analysis: clustering into ground water zones. Ground Water 37(3):358–366
Tang D, Zhang G (2016) Efficient removal of fluoride by hierarchical Ce–Fe bimetal oxides adsorbent: thermodynamics, kinetics and mechanism. Chem Eng J 283:721–729
Vikas C, Kushwaha R, Ahmad W, Prasannakumar V, Reghunath R (2013) Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environ Earth Sci 68(1):289–305
Wang Y, Ma T, Luo Z (2001) Geostatistical and geochemical analysis of surface water leakage into groundwater on a regional scale: a case study in the Liulin karst system, northwestern China. J Hydrol 246(1–4):223–234
Wen D, Zhang F, Zhang E, Wang C, Han S, Zheng Y (2013) Arsenic, fluoride and iodine in groundwater of China. J Geochem Explor 135(2013):1–21
WHO (2004) Fluoride in drinking water-background document for development of WHO guidelines for drinking water quality. WHO, Geneva, p 2004
Zhang HM (2010) A dynamic experimental study of the fluorine transport regularity in soil of Yuncheng Basin. Geol China 37(3):686–689
Zhang K, Wu S, Wang X, He J, Sun B, Jia Y, Shen W (2015) Wide pH range for fluoride removal from water by MHS-MgO/MgCO3 adsorbent: kinetic, thermodynamic and mechanism studies. J Colloid Interface Sci 446:194–202
Acknowledgements
The authors would like to thank the anonymous reviewers for their helpful and constructive comments that greatly contributed to improving the final version of the paper. We would also like to thank the Editors for their generous comments and support during the review process. Finally, this research was financially supported by the National Natural Science Foundation of China (41877204), Foundation for Innovative Research Groups of the National Natural Science Foundation of China (41521001) and the China Postdoctoral Science Foundation (2018M642944).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, T., Gao, X., Zhang, X. et al. Distribution and assessment of hydrogeochemical processes of F-rich groundwater using PCA model: a case study in the Yuncheng Basin, China. Acta Geochim 39, 216–225 (2020). https://doi.org/10.1007/s11631-019-00374-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-019-00374-6