Abstract
Fluoride (F−) is the most important element for human health. Hydrogeochemical survey was conducted to probe into the controlling factors of F− in the groundwater of a semi-arid part of South India. The study area comprises of F−-bearing minerals (hornblende, biotite, and apatite) in the Precambrian rocks, sandstones in the Upper Gondwana rocks, and clays in the Quaternary formations. Forty-seven percent of the total groundwater samples have the higher F− content than its permissible limit of 1.50 mg/L prescribed for drinking purpose. The chemical data of the groundwater is analyzed to assess the geochemical processes dominating the F−-bearing groundwater, using Piper and Gibbs diagrams, correlations, saturation indices, ionic ratios, and multivariate analysis. Piper diagram shows that the groundwater quality is characterized by Na+-HCO\( {}_3^{-} \) and Na+-Cl−types, while the Gibbs diagrams suggest that the groundwater chemistry is mainly controlled by water-rock interactions and is subsequently modified by human activities. The F− groundwater is positively correlated with pH, total dissolved solids, Mg2+, Na+, K+, HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), and NO\( {}_3^{-} \) and negatively with Ca2+, representing the influences of geogenic and anthropogenic origins on the groundwater system. Geochemical ratios and saturation indices indicate that the processes of mineral dissolution, ion exchange, and evaporation mainly govern the high F− groundwater, while the chemical fertilizers cause for the enrichment of F−in the groundwater. Cluster and principal component analyses further support the above findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Normally, the small amount of fluoride (F−) is essential for development of bones and formation of dental enamel (Handa 1975). However, low concentration of F− (< 0.60 mg/L) in drinking water results in dental decay, while an excess of it (> 1.50 mg/L) causes dental fluorosis (WHO 2011; BIS 2012). Drinking water with F− content in between 0.60 and 1.50 mg/L is, thus, stipulated as safe limit for human health.
In the world, more than 260 million people suffer from fluorosis (Amini et al. 2008) due to drinking of water with higher F−content (> 1.50 mg/L). Contamination of groundwater with F−is widespread especially in Africa, China, India, Iran, Kenya, Nigeria, Pakistan, South America, and Srilanka (Ayoob and Gupta 2006), posing high health risk to people (Wu and Sun 2016; Li et al. 2016a). In India, the first case of endemic fluorosis was reported due to higher concentration of F− in groundwater as long as 1937 in the Prakasam district (erstwhile Guntur District), Andhra Pradesh (Short et al. 1937). About 61 million people suffer from ill-health in India caused by drinking water with consumption of F− contamination in 20 states, including Andhra Pradesh, Assam, Bihar, Chhatisgarh, Delhi, Gujarat, Haryana, Jammu and Kashmir, Jharkhand, Karnataka, Kerala, Madhya Pradesh, Maharashtra, Orissa, Punjab, Rajasthan, Tamil Nadu, Telangana, Uttar Pradesh, and West Bengal (Susheella 1999; CGWB 2015).
A lot of research on occurrence of F− in groundwater has been carried out in different parts of the world: Harrison (2005) discussed the impact of F− content on human health in the UK. Ayoob and Gupta (2006) and Ozsvath (2009) carried out a literature review on health impacts, sources, and mobility of F−, which provides a comprehensive insight into the relation among F−, human health, and the environment. Chae et al. (2007) studied the hydrogeochemistry of bed rocks in order to evaluate the geological and geochemical controls on the concentration of F− in groundwater in South Korea. Fordyce et al. (2007) and Wen et al. (2013) assessed the areas of high F− waters and their health effects in Central Europe and China. Madhnure et al. (2007) stated that the long residence time of water in deeper aquifers than in shallow aquifers of Maharashtra (India) was responsible for higher concentration of F− in groundwater. Ayenew (2008) reported the high concentration of F− caused by low concentration of Ca2+ under alkaline environment in Ethiopian groundwater. Hudak (2009) observed the contamination of F− groundwater, especially in public areas as well as in irrigated areas in West Texas. Suman et al. (2009) studied the controlling factors of F− content in groundwater in a semi-arid region (Sirsa City, Haryana) of India, using statistical and multivariate techniques. Li et al. (2010) performed a geochemical model to explain an evolution mechanism of F− in groundwater in Pengyang County, China. Fluorite, apatite, biotite, hornblende, and clay are the minerals, which have the greatest effect on geochemistry of F− in groundwater (Dar et al. 2011), when they are contacted with groundwater and dissolved, leading to increase of F− in groundwater. Li et al. (2011, 2014, 2017) concluded in their study conducted in different parts of China that the high F− was controlled by hydrogeological conditions, but man-made activities and mixing of different recharge waters were also important reasons. Ghrefat et al. (2014) found that the geochemical environment is the main controlling factor of F− content in groundwater from the region of Midyan Basin, Saudi Arabia. Wu et al. (2015) assessed the hydrochemical characterization of drinking groundwater with reference to F− content in an arid area of China.
Handa (1975), Jacks et al. (1993, 2005), Shaji et al. (2007), Mamatha and Rao (2010), Reddy et al. (2010, 2015), Reddy (2014), Brindha et al. (2011), Chidambaram et al. (2013), Kumar et al. (2014), Rao et al. (2014), Singaraja et al. (2014), Dhanya Raj and Shaji (2017), and Satyanarayana et al. (2017) examined the factors responsible for higher concentration of F− in groundwater of different states (Andhra Pradesh, Karnataka, Kerala, Tamil Nadu, Telangana) of South India and found that the major source of F− is caused by dissolution of F−-bearing minerals (fluorite, apatite, hornblende, and biotite), ion exchange, and evaporation and also the usage of chemical fertilizers.
From the cited literature reports, it is clear that the geochemical environment of groundwater is the main controlling factor of F− migration, while the anthropogenic activities like application of chemical fertilizers are the adding factors for enrichment of F− in groundwater. However, the intensity and activity of the chemical reactions are generally different from place to place, depending upon the availability of the source material, movement of groundwater flow, and climatic conditions. These result in the varying of F− content. In view of the increased importance worldwide on the concentration of F− in relation to its effects on human health, the present research has been carried out to assess the controlling factors of F− in the groundwater, using Piper and Gibbs diagrams, correlations, saturation indices, ionic ratios, and multivariate analysis, in a part of Prakasam district, Andhra Pradesh, South India. Hence, the present study will be of great significant to the scientific community.
Study area
Location
The present study area (15o 24′−15o-39′30″ N and 80o 15′−80o 35″ E) is located in a part of Prakasam district, Andhra Pradesh, India (Fig. 1). It covers an area about 325 km2, falling in the Survey of India toposheets 66 A/2 and 66 A/3. Ongole is the main city. The population is about 5,00,000. The area experiences a semi-arid climate, with minimum and maximum temperatures of 28 and 39 °C, respectively. The annual rainfall is about 910 mm. Southwest monsoon (June to September) contributes 69%, northeast monsoon (October to December) 29%, and the remaining seasons (winter and summer) 12% of the total rainfall. The river Gundlakamma flows through the area, which is an ephemeral nature. The drainage pattern is dendritic to sub-dendritic. The drainage density is generally less than 0.4 km/km2 (CGWB 2013).
Geology
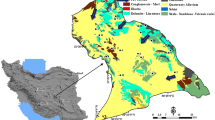
Topographically, the slope of the study area is towards southeast. The silty clay soils are the dominant type with the occurrence of calcareous concretions (CaCO3) locally known as kankar, which is a characteristic feature of the semi-arid climate. Geologically, the study area comprises of the Precambrian formations (Charnockites of the Eastern Ghats Super-group and unclassified Metamorphic Super-group of the Archaeans with younger intrusive bodies), Upper Gondwana sequence of Lower Cretaceous age, and Quaternary formations (Fig. 2). Pyroxene granulites come under the Charnockite group (CGWB 2013). Migmatized quartzo-felspathic-gneiss/schist associated with the amphibolites comes under the unclassified metamorphic rocks. Intrusive body comprising gabbro/norite occurs. The rocks are intensely migmatized at places. They constitute essentially of quartz, plagioclase and orthoclase feldspars, hornblende, hypersthene, sillimanite, garnet, apatite, and biotite. The rocks show a strike from NE–SW to NW–SE, with moderate to steep dip. Sandstones belong to the Upper Gondwana formations. Laterite, brown silty clay, black silty clay, brown silty sand, brown sand, and white to gray sand come under the Quaternary deposits, which occur over the basement rocks.
Geology of the study area (after CGWB 2013). Dotted lines denote the major fractures
Hydrogeological conditions
The rocks of Precambrians and Gondwanas come under the consolidated formations, which occupy the large part of the study area (Fig. 2). They are not generally porous and permeable. But, they become aquifers due to weathering of the rocks and development of fractures in the rocks. However, the clay products, resulting from the highly weathering activity in the upper part of the bedrock, reduce the permeability to some extent. Thickness of the weathered rock portion varies from 3 to 6 m and the fractured rock portion from 5 to 35 m, depending upon the topography and rock type. The Quaternary deposits are the aquifers by virtue of their unconsolidated formations.
Groundwater is extracted through shallow wells and deep wells for domestic and irrigation purposes. The former wells are mainly confined to the weathered zone under unconfined conditions, while the latter wells extend into the fractured zone under semi-confined conditions. The depth to water levels is in between 3 and 16 m below ground level, fluctuating within the weathered and fractured rock portions. Spatial distribution of depth to groundwater level shows that the water levels are higher in northwestern side (> 15 m) and lower in southeastern side (< 5 m; Fig. 1), depending upon the porosity and permeability characters of lithology and slope of the area.
The present study area (325 km2) is a part of the regional hydrogeological set-up of the Prakasam district, spreading over an area of 17,626 km2. Depending upon the porosity and permeability characters of the geological formations present in the study area, the yield of the wells varies from place to place. The average yield of the wells located in the weathered and fractured rock portions is 50 and 100 m3/day, respectively, while it is 425 and 250 m3/day in the river alluvium and coastal zone, respectively (CGWB 2013). The irrigation wells are pumped at a discharge of about 4 L/s to meet the demand of high agricultural production (Reddy et al. 2015). The quality of groundwater is not generally potable, which is mostly brackish. Fluorosis is observed in this region during the field work.
Agriculture is the main occupation of the people. Irrigation is intensive and long-term practice in the study area. Soil amendments (gypsum) are used to alter the physical and chemical properties of soil permeability. The application of uncontrolled usage of chemical fertilizers (potash, phosphate, nitrogen and sulphate varieties) for higher crop yields is a common practice in the study area. Flood irrigation is very limited. Important crops are rice, groundnuts, chilies, tobacco, cotton, etc. Drainage conditions (dumping of wastes on the street sides and empty places, flowing of waste waters through leaked channels, leakage of septic tanks, etc) are poor.
Materials and methods
Sample collection and analysis
A total of 30 groundwater samples were monitored during summer 2012 and their locations are shown in Fig. 1. Most of them are located in the inland area. Very few are located along the coast, but they are far off from the influence of sea water. The groundwater samples were collected in half-liter polythene bottles, after the bottles were soaked in 1:1 HCl for 24 h and were rinsed with distilled water followed by deionized water. The bottles were tightly capped without air gap. Immediately, after collection of the groundwater, the samples are shifted to laboratory for the chemical analysis, following the standard water quality procedures of American Public Health Association (APHA 2012).
The groundwater samples were analyzed for pH, electrical conductivity (EC), total dissolved solids (TDS), total hardness (TH), calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), bicarbonate (HCO\( {}_3^{-} \)), chloride (Cl−), sulphate (SO\( {}_4^{2-} \)), nitrate (NO\( {}_3^{-} \)), and fluoride (F−). The pH and EC were measured in the field, using portable pH and EC meters. The EC was used to calculate the concentration of TDS, following the procedure of Hem (1991). The TH (as CaCO3) and Ca2+ were analyzed volumetrically, using standard EDTA. The Mg2+ was computed, taking the difference between TH (as CaCO3) and Ca2+. A flame photometer was used for estimation of Na+ and K+ ions. The HCO\( {}_3^{-} \) was estimated by titrating with standard HCl. The Cl− was analyzed by titrating with standard AgNO3. The SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F− were determined, using turbidimetric procedure, colorimeteric method, and ion analyzer, respectively. The units of all chemical variables (except pH) are expressed in milligrams per liter (mg/L).
Ionic-balance-error
Ionic-balance-error between the total concentrations of cations (Ca2+, Mg2+, Na+, and K+) and the total concentrations of anions (HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F−) was checked for accuracy of each groundwater sample, following the formula (Eq. 1), where all ions are expressed in milliequivalent per liter (meq/L). The calculated ionic-balance-error was observed to be within the acceptable limit of ± 5% (Domenico and Schwartz 1990).
Chloro-alkaline indices
Chloro-alkaline (CA) indices viz. CA1 and CA2 proposed by Schoeller (1965) were calculated, following the formulas (2 and 3), where all concentrations of ions are expressed in meq/L. They are widely used to assess the possible mechanism of ion exchange between the groundwater and host rocks (Marghade et al. 2012; Li et al. 2013a; Wu and Sun 2016).
Saturation index
The intensity of soluble minerals is expressed as saturation index (SI). Thus, it is used to evaluate the degree of equilibrium between water and respective mineral (Garrels and Christ 1965; Fetter 1990; Li et al. 2010), which is calculated as (4).
where IAP is the ion activity product and SP is the solubility product.
If SI is zero, it indicates saturated state (equilibrium) with respect to the particular mineral. If SI is less than zero, it shows under-saturated state (dissolution) with respect to the concerned mineral. If SI is more than zero, it suggests over-saturated state (precipitation) with respect to the particular mineral (Li et al. 2010).
Statistical analysis
The chemical data of the groundwater obtained from the chemical analysis are subjected to statistical analysis to (a) measure the distribution and central tendency of the data set, (b) know about the interrelationship of the chemical variables, and (c) assess the sources of chemical species. Multivariate statistical analysis was also carried out to find out the possible sources of chemical variables (Ca2+, Mg2+, Na+, K+, HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \) and F−), using Statistic (version 6) software (StatSoft Inc. 2001).
In R-mode analysis, principal component analysis (PCA) was performed with varimax orthogonal rotation and Kaiser normalization to reduce the data set and also to explain the possible sources of controlling processes of chemical variables (Kaiser 1958; Davis 1986; Suman et al. 2009; Nosratic and Eechhaut 2012; Kim and Park 2016; Ravikumar and Somashekar 2017).
Results and discussion
Groundwater quality
The chemical composition of the groundwater is shown in Table 1 and Fig. 3. The pH varies from 7.2 to 8.3, with an average of 7.81, indicating an alkaline condition. This is within the recommended limit of 6.5 to 8.5 for drinking water in all groundwater samples (WHO 2011; BIS 2012). The TDS is in the range of 995 to 3290 mg/L and its average is 1884.27 mg/L, showing a wide variation in the degree of water quality. As per the TDS classification (Fetter 1990), about 97% of the total samples (1 to 6 and 8 to 30) come under the brackish water type (1000 to 10,000 mg/L) and the remaining 3% (sample 7) under the freshwater type (< 1000 mg/L). In all groundwater samples, the concentration of TDS is more than its permissible limit of 500 mg/L prescribed for drinking water. This causes a gastrointestinal irritation (WHO 2011; BIS 2012).
Among cations, the Ca2+ varies from 60 to 75 mg/L and the Mg2+ from 42 to 105 mg/L, being averages of 65.67 and 76.87 mg/L, respectively (Table 1). The concentration of Ca2+ is within the safe limit of 75 mg/L, except in two water samples ( 8 and 23), while that of Mg2+ is higher than that of its prescribed limit (30 mg/L) recommended for drinking water in all water samples (WHO 2011; BIS 2012). It leads to a scale formation in water distribution structures. The Na+ is in between 189 and 823 mg/L with its average of 444.73 mg/L. The concentration of Na+ is higher than its allowable limit of 200 mg/L for drinking water (BIS 2012) in 90% of the total water samples (1, 4 to 6 and 8 to 30). It effects on human health, who suffer from the hypertension. The concentration of K+ varies from 12 to 121 mg/L, being an average of 54.33 mg/L. Generally, it is less than 10 mg/L in groundwater (Davis and Dewiest 1966). The higher concentration of K+ than that of 10 mg/L is, thus, considered to be a man-made pollution.
Among anions, the HCO\( {}_3^{-} \)ranges from 480 to 1025 mg/L and its average is 731.33 mg/L (Table 1). The threshold limit of HCO\( {}_3^{-} \)is 300 mg/L in groundwater (BIS 2012). In all groundwater samples, the concentration of HCO\( {}_3^{-} \)exceeds its recommended limit. The concentration of Cl− is from 215 to 1060 with an average of 568.83 mg/L. This is more than its safe limit of 250 mg/L prescribed for drinking water (BIS 2012) in 90% of the total groundwater samples (1, 2, 4 to 6, 8, 9, and 11 to 30). It develops salt water, which is a common phenomenon in the study area. The SO\( {}_4^{2-} \)content varies from 22 to 125 and its average is 71.47 mg/L. In all groundwater samples, the concentration of SO\( {}_4^{2-} \)is within its safe limit of 200 mg/L allowed for drinking water (BIS 2012). The NO\( {}_3^{-} \) is in between 31 and 97 me/L, being an average of 56.20 mg/L. The NO\( {}_3^{-} \) is higher than its recommended limit of 45 mg/L permitted for drinking water (BIS 2012) in 73% of the total groundwater samples (1, 5, 6, 8 to 11, 13, 14, 16 to 18, and 20 to 30). This causes a blue baby disease. However, it is not observed from the study area. The higher concentration of NO\( {}_3^{-} \), which is more than 10 mg/L, indicates an anthropogenic pollution (Cushing et al. 1973). The concentration of F− is from 0.70 to 2.80 mg/L with an average of 1.34 mg/L. Spatial distribution of F− is shown in Fig. 4. In 47% of the total groundwater samples (4, 8, 9, 11, 12, 21 to 28 and 30), the F− content is higher than its safe limit of 1.50 mg/L assigned for drinking water (WHO 2011; BIS, 2012). It causes a fluorosis, which is observed from the study region during the field work.
From Table 1, it is noted that the chemical variables show a wide range of standard deviations (SD). The TDS, Cl−, HCO\( {}_3^{-} \), and Na+ have the higher values of SD (781.93, 303.48, 200.97, and 228.72, respectively) compared to the other chemical variables (pH, Ca2+, Mg2+, K+, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F−). The values of SD in these chemical parameters vary from 0.40 to 28.49. It is also significant to note that the K+ (28.49) and SO\( {}_4^{2-} \)(28.12) as well as Mg2+ (18.87) and NO\( {}_3^{-} \)(18.91) have almost the similar values of SD, followed by Ca2+ (8.88), while the pH (0.40) and F− (0.56) have the lowest values of SD. The difference in the values of SD among chemical variables clearly suggests that there is a wide dispersion of salts in the groundwater system. This is a result of differences in (a) the availability of sources of salts and (b) the processes of mineral dissolution, mineral solubility, ion exchange, and evaporation over the control of water chemistry (Ghrefat et al. 2014). Further, the values of coefficient of variation (CV) vary from 5.12 to 67.81%. The difference in the CV among chemical variables also clearly supports the spatial variation of chemical composition of groundwater.
Hydrogeochemical evolution
A trilinear diagram (Piper 1953) helps for characterization of hydrogeochemical evolution (Fig. 5). This diagram consists of three distinct fields, including two triangular fields on the lower side to plot cations (left side) and anions (right side) and a diamond-shaped field on upper side of these two triangular fields to plot an overall chemical quality of groundwater for characterization of hydrogeochemical evolution through assessment of various water types viz. zone-5 (carbonate hardness or temporary harness or freshwater), zone-6 (non-carbonate hardness or permanent hardness), zone-7 (non-carbonate alkali or saline water), zone-8 (carbonate alkali or excess alkalinity), and zone-9 (mixed or transition water).
Hydrogeochemical evolution (after Piper 1953). Zone-5 denotes the carbonate hardness (temporary harness or freshwater), zone-6 non-carbonate hardness (permanent hardness), zone-7 non-carbonate alkali (saline water), zone-8 carbonate alkali (excess alkalinity) and zone-9 mixed (transition) water. Arrow mark denotes the flow path of groundwater
As shown in Fig. 5, the chemical data of the groundwater sampling points fall in the right lower corner of the cation triangle, which reveals that the Na+ is a dominant ion in the groundwater. Similarly, the groundwater samples are observed from the central lower part of the anion triangle, indicating the higher concentrations of both HCO\( {}_3^{-} \)and Cl− ions in the groundwater. Most groundwater sampling points fall in the central part of the diamond-shaped field and their trend direction is towards the right side from the left side, following the flow path. It suggests that the freshwater (zone-5) moves towards the saline water (zone-7) through the mixed water (zone-9). The changing of Na+-HCO\( {}_3^{-} \)type gradually towards the Na+-Cl− type is a result of influence of anthropogenic activity along the flow path.
The characterization of hydrogeochemical evolution obviously explains the change in degree and type of mineralization of groundwater due to different stages of interaction of recharged water through soil cover and weathered/fractured rock portions with the contaminated water caused by human activities. During its initial stage of movement, the water exchanges Ca2+ for Na+ by ion exchange process (Hem 1991). When the groundwater is dominated by Na+, it should have HCO\( {}_3^{-} \) as a predominant ion due to CO2, which releases from the decay of organic and root respiration in soil zone (Rogers 1989). This combines with H2O to form HCO\( {}_3^{-} \) (Eqs. 5 and 6). As a result, the groundwater shows Na+-HCO\( {}_3^{-} \)type water. It causes the higher concentration of F− in the groundwater (Eq. 7), which is in consistency with the results of Chae et al. (2007). In the latter stage, the groundwater shows Na+-Cl− type water due to influence of man-made pollution on the fresh groundwater system. These two ions increase the TDS, which includes F− content in the groundwater.
Mechanism controlling groundwater chemistry
The hypothesis explained in the hydrogeochemical characterization is further verified by Na+ + K+:Na+ + K+ + Ca2+ and Cl−:Cl− + HCO\( {}_3^{-} \) ratios, where they are plotted against TDS (Fig. 6), following the procedure of Gibbs (1970). The diagrams of Gibbs explain the three mechanisms that control the water chemistry with respect to the influences of precipitation (rainwater), rock (soil and/or lithology), and evaporation (climate).
Mechanisms (precipitation, rock, and evaporation) controlling the groundwater chemistry (after Gibbs 1970)
In the present study, the chemical data of the groundwater sampling points fall in rock and evaporation domains (Fig. 6). No single sample is observed from the domain of precipitation (TDS < 100 mg/L). Falling of a few groundwater samples in the domain of rock indicates that the geogenic origin is the main responsible factor for the increase of ions from the domain of precipitation towards the domain of rock (TDS 100 to 1000 mg/L). This is a result of interaction of water with soils and/or weathering of rock forming minerals, before reaching the water table.
A general reaction for water-rock interaction is shown in Eq. (8). According to this reaction, the groundwater should have the Na+ and HCO\( {}_3^{-} \)as the dominant ions. However, the influence of sources of evaporation and/or anthropogenic origin increases the concentrations of Na+ and Cl− ions (Todd 1980; Hem 1991; Drever 1997), which make the higher mineralization, i.e., TDS (> 1000 mg/L) in the groundwater. As a result, the chemical data of the groundwater samples moves towards the domain of evaporation from the domain of rock in the study area (Fig. 6).
Perel’man (1977) reported that the higher concentration of TDS enhances the ionic strength. This leads to an increase of the solubility of CaF2. This causes the higher concentration of F− in groundwater, as observed in the present study area (Table 1). Kumar et al. (2014) and Li et al. (2014) also observed the similar results from the regions of Vellore district (India) and Weihe River (China), respectively.
Controlling factors of F− content
Correlation coefficient analysis
Pearson’s correlation coefficient (r) is widely used tool to get information on associations between individual chemical variables (Jiang et al. 2009; Suman et al. 2009; Vasanthavigar et al. 2013). It demonstrates the overall data set that indicate their controlling factors (Li et al. 2011, 2012, 2014). The calculated correlation coefficient matrix is presented in Table 2. The binary plots were plotted in Fig. 7 to assess the possible controlling factors of F− content in the groundwater. If the geogenic origin is the only source for dissolved ions, their spreading points are close to the linear trend. If there is any deviation in the spreading points from the linear trend, it indicates the involved of other sources (like anthropogenic origin) for the dissolved contents.
The positive correlation of pH with F− (r = 0.64; Fig. 7a and Table 2) suggests that the F− tends to be higher in the higher level of pH due to precipitation of Ca2+ and dissolution and/or replacement of F− with OH− (Li et al. 2014). Further, the Ca2+ and Mg2+ ions have positive correlations with F−. However, the Ca2+ shows low correlation with F− (r = 0.30; Fig. 7b), while the Mg2+ has good correlation with F− (r = 0.72; Fig. 7c). This difference can be explained in a general perspective with respect to CaF2 and MgF2 minerals. For instance, the SI of CaF2 is 3.11 and of MgF2 is 1.56 (Table 3), indicating that these salts are in the precipitated state. The higher precipitation of CaF2 than that of MgF2 may be caused by differences in their solubility (Hem 1991). On the other hand, high positive correlation is observed between HCO\( {}_3^{-} \) and Mg2+ (r = 0.76) and low correlation between HCO\( {}_3^{-} \) and Ca2+ (r = 0.20). This suggests that MgCO3 is the main source of these ions rather than the CaCO3, which is further supported by positive SI of CaCO3 (0.93) and negative SI of MgCO3 (−0.20). The natural occurrence of calcareous concretions in the soils is the direct evidence of precipitation of CaCO3 due to semi-arid climate of the study area, supporting the above finding.
Significantly, it is noted that the concentration of Ca2+ (average 65.67 mg/L) is lower than that of Na+ (average 444.73 m/L). The present study area (325 km2) is a discharge part of the regional hydrogeological set-up of the Prakasam district (17,627 km2). Therefore, the difference in the values of Ca2+ and Na+ is a result of the discharge area. Similar observation has been made by Wu et al. (2015) in an arid area of China. Further, the higher Na+ than the Ca2+ in the groundwater is a result of two geochemical processes—(a) ion exchange, which leads to an increase of Na+ by a decrease of Ca2+, and (b) precipitation of CaCO3, which reduces the concentration of Ca2+ due to semi-arid climate (Li et al. 2016b, c). Jack et al. (1993) stated that the rate of evaporation increases the concentration of HCO\( {}_3^{-} \), while the precipitation of carbonates occurs.
The Mg2+ salts do not precipitate readily as the Ca2+ salts due to differences in their solubility (Hem 1991). As a result, very low correlation exists between Na+ and Ca2+ (r = 0.10) compared to that between Na+ and Mg2+ (r = 0.79; Table 2). On the other hand, the solubility of F− is low (Nordstrom and Jenne 1977). However, it increases with the increase of Na+ in groundwater (Apambire et al. 1997). Since the Na+ is the dominant ion in the groundwater (Table 1) due to rock weathering (Stumm and Morgan 1996), it favors the release of F− (Li et al. 2014). Consequently, good correlation is observed between Na+ and F− (r = 0.85; Fig. 7d). Because of the rock weathering, the HCO\( {}_3^{-} \) can also release along the Na+ into the groundwater (Eq. 8). This accelerates the dissolution of F−-bearing minerals more effectively (Shyam and Kalwania 2012), which favors the release of F−. This pollutes the groundwater. A high positive correlation is, thus, observed between HCO\( {}_3^{-} \)and F− (r = 0.94; Fig. 7e).
Sodium has good positive correlation with Cl− (r = 0.98; Table 2). This indicates that these two ions are derived from the same source and also they have the higher solubility conditions (Hem 1991; Li et al. 2016b). Therefore, the SI of NaCl shows the negative value (−2.06; Table 3), which indicates the dissolution state of Na+ and Cl− ions in the groundwater. However, the calculated ratio of Cl− to Na+ is less than unity (0.83; Table 3). If the groundwater is contaminated with sea water, the ratio between Cl− and Na+ should be more than one, because the sea water contains more concentration of Cl− (19,000 mg/L) than that of Na+ (10,500 mg/L; Hem 1991). But, the influence of sea water on the groundwater system is not so happened in the present study area so that the sources of Na+ and Cl− ions should be related to other origins. Thus, the dissolution of sodium feldspars is the primary source of Na+. Further, the clays are also the sources of both Na+ and Cl− ions in addition to the drainage wastes (Todd 1980; Hem 1991).
Since the clays occur in the present study area, it is important to assess the role of ion exchange between Ca2++Mg2+ and Na++K+, which control the geochemistry of groundwater. Chloro-alkaline (CA) indices viz. CA1 and CA2 proposed by Schoeller (1965) are widely used to evaluate the mechanism of ion exchange in groundwater (Marghade et al. 2012; Li et al. 2016c). If CA is positive, it indicates an exchange of Na+ and K+ from the water with Ca+ and Mg2+ of the rocks. It is called base-exchange reaction. If CA is negative, it specifies an exchange of Ca2+ and Mg2+ of the water with Na+ and K+ of the rocks. It is called cation-anion exchange reaction. In the present study area, the CA shows negative values (CA1 = −0.29 and CA2 = −0.31; Table 3). This indicates that the Ca2++Mg2+ in the groundwater exchanged for Na++K+ from aquifer materials, which is also supported by the ratio Na+:Ca2+ (3.04; Table 3). Further, the negative trend of Ca+ + Mg2+ − HCO\( {}_3^{-} \) + SO\( {}_4^{2-} \) vs Na+ + K+ − Cl− also indicates the existence of reverse cation-anion exchange phenomenon in the groundwater (Fig. 8). Marghade et al. (2012), Ahmed et al. (2013), Li et al. (2013b), and Wu et al. (2015) also reported the similar observations in India (Nagpur city), Egypt (Eastern Nile Delta), and China (Ordos Basin and Shizuishan city).
It is significant to note that the F− shows a good positive correlation with SO\( {}_4^{2-} \)(r = 0.74; Fig. 7f), K+ (r = 0.71; Fig. 7g), and NO\( {}_3^{-} \)(r = 0.69; Fig. 7h) and their correlation values are more or less the same. This indicates a common source of these ions. The chemical fertilizers (potash, phosphate, nitrogen, and sulphate varieties) are the main sources of K+, Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F− ions, as reported by Jiang et al. (2009), Chidambaram et al. (2013), and Li et al. (2016c). Further, F− also shows a good positive correlation with TDS (r = 0.86; Table 2). Perel’man (1977) and Rogers (1989) stated that the TDS increases the ionic strength, which increases the solubility of CaF2, causing the higher concentration of F− in the groundwater (Table 1). Furthermore, the TDS has a good correlation with Mg2+ (r = 0.84), Na+ (r = 0.99), K+ (r = 81), Cl− (r = 0.98), SO\( {}_4^{2-} \)(r = 0.90), and NO\( {}_3^{-} \)(r = 0.77). It represents the influences of geogenic and anthropogenic sources on the groundwater system. Otherwise, such relations should not have among these chemical parameters.
Multivariate analysis
Hierarchical cluster analysis
Hierarchical cluster analysis (HCA) classifies the chemical variables into clusters or groups, which provide a preface of water quality, depending upon the intensity of chemical variables (Suman et al. 2009). The groups are similar to each other group, but distinct from the other groups (Li et al. 2013b; Wu et al. 2014).
From the cluster analysis, all chemical variables (Ca2+, Mg2+, Na+, K+, HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F−) can be divided into two major groups. Group I is related to Ca2+, Mg2+, K+, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F− ions, while group II is associated with Na+, HCO\( {}_3^{-} \), and Cl− ions (Fig. 9). The former group implies the weathering of plagioclase feldspar (Eq. 9), hornblende (Eq. 10), biotite (Eq. 11), and apatite (Eq. 12) minerals (Ca2+, Mg2+, Na+, K+, SO\( {}_4^{2-} \), and F−) and the influences of chemical fertilizers and drainage wastes (Mg2+, Na+, K+, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F−) on the groundwater body. Further, the occurrence of sandstone is also responsible for F− content in the groundwater, as it contains 350 mg/L of F− (Rao et al. 2014). The latter group represents the weathering of plagioclase feldspar mineral (Na+ and HCO\( {}_3^{-} \); Eq. 9) and also the influence of soil salts developed by evaporation (Na+, HCO\( {}_3^{-} \), and Cl−) on the groundwater system. Similar observations have been reported from the other regions (Raju et al. 2009; Zhang et al. 2013; Christou et al. 2014; Rao et al. 2014; Reddy et al. 2010, 2015; Reddy 2014; Singaraja et al. 2014; Satyanarayana et al. 2017).
As explained earlier, the HCO\( {}_3^{-} \) favors the solubility of F−-bearing minerals more effectively. As a result, these two ions are interdependent with each other. However, such relation is not observed from Fig. 9. For example, the F− is associated with group I and HCO\( {}_3^{-} \) with group II. This difference can be explained by HCA, which pools multiple (> 1) variables into groups based on distance measures (StatSoft Inc. 2001). Each group is sub-divided in a hierarchical manner. It is a pseudo-dimension reduction technique. The advantage is looking into small number of groups, each containing several variables. Thus, F− and HCO\( {}_3^{-} \) fall into two different groups in the present study area.
Principal component analysis
Principal component analysis (PCA) is widely used for identification of influence of sources of chemical variables on variance in data matrix (Dalton and Upchurch 1978; Davis 1986; Jiang et al. 2009; Kim and Park 2016; Ravikumar and Somashekar 2017). In the present study, two principal components (PCs) are observed from the transformation of the chemical data of Ca2+, Mg2+, Na+, K+, HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F−, using varimax orthogonal rotation and Kaiser normalization, where the eigenvalue is more than one (Kaiser 1958; Davis 1986). The PC1 is related to the largest eigenvalues and is able to explain the greatest amount of variance in the data set, while the PC2 (orthogonal and uncorrelated with the PC1) explains the most of the remaining variance. These two PCs have a significant effect on variability of the groundwater chemistry.
Table 4 presents the results of principal component analysis. The total variation is 85.108%. The PC1 accounts for 72.824% of the total variation with high positive loadings of SO\( {}_4^{2-} \) (0.965), Na+ (0.943), Cl− (0.920), K+ (0.908), NO\( {}_3^{-} \) (0.879), HCO\( {}_3^{-} \) (0.862), Mg2+ (0.854), and F− (0.853). The PC2, which accounts for 12.284% of the total variation, is dominated by high positive loading of Ca2+ (0.961).
In PC1, the Mg2+, Na+, K+, and F− ions represent the contribution of weathering of plagioclase and orthoclase feldspars, hornblende, biotite, and apatite; the Mg2+, Na+, K+, Cl−, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F− ions reflect the influences of chemical fertilizers and drainage wastes; the Na+ and Cl− ions indicate the impact of evaporation; and the Na+, HCO\( {}_3^{-} \), and Cl− ions are the result of soil salts (Todd 1980; Hem 1991; Drever 1997; Manish et al. 2007; Suman et al. 2009; Chidambaram et al. 2013;Singaraja et al. 2014). In the PC2, the Ca2+ is a result of weathering of calcium feldspars (Hem 1991).
Since the Ca2+, Mg2+, Na+, K+, HCO\( {}_3^{-} \), SO\( {}_4^{2-} \), and F− are both the sources of lithological (geogenic) and non-lithological (anthropogenic) origins and the Cl− and NO\( {}_3^{-} \) are the sources of non-lithological origin, the PC1 can be explained as a result of both the geogenic and anthropogenic sources, while the PC2 is caused by the geogenic origin.
Conceptual model of geochemistry of fluoride
Based on the field conditions and hydrogeochemical data, it is clear that the source of F− in the groundwater is due to weathering of F−-bearing minerals (hornblende, biotite, and apatite) and sandstones. The processes of ion exchange (between Na+ and Ca2+) and evaporation (precipitation of CaCO3) enhance the concentration of Na+ by reduction of Ca2+, while the process of evaporation increases the concentration of HCO\( {}_3^{-} \). The Na+ and HCO\( {}_3^{-} \)ions, thus, favor the mineral weathering and dissolution and increase the solubility of F−-bearing minerals more effectively from the source. As a result, the F− shows a strong positive correlation with Na+ and HCO\( {}_3^{-} \). Further, the chemical fertilizers are the sources of K+, SO\( {}_4^{2-} \), NO\( {}_3^{-} \), and F− ions so that the F− shows a good positive correlation with K+, SO\( {}_4^{2-} \), and NO\( {}_3^{-} \). Therefore, the conceptual model explains the geogenic origin as the primary source for the occurrence of F− content and the anthropogenic input as the secondary source for further enrichment of F− in the groundwater.
Conclusions
Piper and Gibbs diagrams, correlations, saturation indices, ionic ratios, and multivariate analysis have been used to assess the controlling factors of F− in the groundwater in a part of South India. The following conclusions can be summarized.
-
The concentration of F− varies from 0.70 to 2.80 mg/L. It is higher (1.60 to 2.80 mg/L) than its safe limit of 1.50 mg/L recommended for drinking water in 47% of the total groundwater samples.
-
Piper and Gibbs diagrams suggest that the groundwater quality is characterized by Na+-HCO\( {}_3^{-} \)- and Na+-Cl−-type waters caused by water-rock interactions and anthropogenic sources, respectively.
-
A positive correlation of F− with pH, TDS, Mg2+, Na+, K+, HCO\( {}_3^{-} \), Cl−, SO\( {}_4^{2-} \), and NO\( {}_3^{-} \) and low with Ca+2, saturation indices, chloro-alkaline indices, and multivariate statistical analysis suggest that the mineral dissolution, ion exchange, and evaporation play a significant role as primary processes for the concentration of F− in the groundwater, while the influence of chemical fertilizers is the secondary factor for further enrichment of F− in the groundwater.
References
Ahmed MA, Abdel Samie SG, Badawy HA (2013) Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the Eastern Nile Delta, Egypt. Environ Earth Sci 68(2):369–394
Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Moller KN, Sarr M, Johnson CA (2008) Statistical modelling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42:3662–3668
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis and health implications of fluoriferous groundwaters in the upper regions of Ghanna. Environ Geol 33:13–24
APHA (2012) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Ayenew T (2008) The distribution and hydrogeological controls of fluoride in the groundwater of central Ethiopian rift and adjacent highlands. Environ Geol 54:1313–1324
Ayoob S, Gupta AK (2006). Fluoride in drinking water: a review on the status and stress effects. Critical Reviews in Environ Sci Technol 36: 433–487
BIS (2012) Indian standard specifications for drinking water, IS:10500, Bureau of Indian Standards, New Delhi
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda district, Andhra Pradesh, India. Environ Mon Assess 172:481–492
CGWB (2013) Groundwater brochure, Prakasam district, Central Ground Water Board, Government of India, New Delhi
CGWB (2015) Groundwater quality scenario in India. Central Ground Water Board, Government of India, New Delhi
Chae GY, Seong TM, Bernhard K, Kyoung-Ho K, Seong-Young K (2007). Fluorine geochemistry in bed rock groundwater of South Korea. Sci Total Environ 385: 272–283
Chidambaram S, Bala Krishna Prasad M, manivannan R, Karmegam U, Singaraja C, Anandhan P, Prasanna MV, Manikandan S (2013) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul district, Tamilnadu (India). Environ Earth Sci 68:333–342
Christou A, Eliadou E, Michael C, Hapeshi E, Fatta-Kassinos D (2014) Assessment of long-term wastewater irrigation impacts on the soil geochemical properties and the bioaccumulation of heavy metals to the agricultural products. Environ Monit Assess 186:4857–4870
Cushing EM, Kantrowitz IH, Taylor KR (1973) Water resources of the Delmarva Peninsular. U. S. Geological Survey Professional Paper 822, Washington DC, 58 p
Dalton MG, Upchurch SB (1978) Interpretation of hydrochemical facies by factor analysis. Ground Water 16:228–233
Dar MA, Sankar K, Dar IA (2011) Fluorine contamination in groundwater: a major challenge. Environ Mon Assess 173:955–968
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Davis SN, Dewiest RJM (1966) Hydrogeology. John Wiley and Sons Inc, New York, p 463
Dhanya Raj, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Fron 8:117–124
Domenico PA, Schwartz FW (1990). Physical and chemical hydrogeology. New York: Wiley. 824 p
Drever JI (1997) The geochemistry of natural waters. Prentice Hall, Englewood, p 436
Fetter CW (1990) Applied hydrogeology. CBS Publishers & Distributors, New Delhi, p 592
Fordyce FM, Vrana K, Zhovinsky E, Povoroznuk V, Toth G, Hope BC, Iljinsky U, Baker J (2007) A health risk assessment for fluoride in Central Europe. Environ Geoche Health 29:83–102
Garrels RM, Christ CL (1965) Solutions, minerals and equilibria (p. 450). New York, USA: Harper & Row 450 p
Ghrefat H, Nazzal Y, Batayneh A, Zumlot H, Elawadi E, Laboun A, Mogren S, Qaisy S (2014) Geochemical assessment of groundwater contamination with special emphasizes on fluoride, a case study from Midyan Basin, northwestern Saudi Arabia. Environ Earth Sc 71:1495–1505
Gibbs RJ (1970) Mechanism controlling world’s water chemistry. Science 170:1088–1090
Handa BK (1975) Geochemistry and genesis of fluoride-containing groundwaters in India. Ground Water 13:275–281
Harrison PTC (2005) Fluoride in water: a UK perspective. J Fluoride Chem 126:1448–1456
Hem JD (1991) Study and interpretation of the chemical characteristics of natural water. United States Geological Survey Professional Paper 2254, Scientific Pub. Jodhpur, India
Hudak PF (2009) Elevated fluoride and selenium in West Texas groundwater. Bull Environ Contam Toxi 82:39–42
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228
Jacks G, Rajagopalan K, Alveteg T, Johnsson M (1993) Genesis of high-F groundwaters, southern India. Appl Geochem (Suppl) 2:241–244
Jiang Y, Wu Y, Groves C, Yun D, Kambesis P (2009) Natural and anthropogenic factors affecting the groundwater quality in the Nandong Karst underground river system in Yunan, Vhina. J Contamin Hydrol 109:49–61
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika 23:187–200
Kim H, Park S (2016) Hydrogeochemical characteristics of groundwater highly polluted with nitrate in an agricultural area of Hongseong, Korea. Water 8:7–18
Kumar PJS, Jegathambal P, James EJ (2014) Factors influencing the high fluoride concentration in groundwater of Vellore District, South India. Environ Earth Sci 72:2437–2446
Li P, Li X, Meng X, Li M, Zhang Y (2016a) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health 8:361–379
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013a) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32:195–206
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71:3133–3145
Li P, Qian H, Wu JH, Ding J (2010) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3:282–291
Li P, Tian R, Xue C, Wu J (2017) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Poll Research 24:13224–13234
Li P, Wu J, Qian H (2012) Groundwater quality assessment based on rough sets attributes reduction and TOPSIS method in a semi-arid area, China. Environ Mon Assess 184:4841–4854
Li P, Wu J, Qian H (2013b) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225
Li P, Wu J, Qian H (2016b) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab Jour Geosci 9(1):15. https://doi.org/10.1007/s12517-015-2059-1
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016c) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8:331–348
Li X, Hou X, Zhou Z, Liu L (2011) Geochemical provenance and spatial distribution of fluoride in groundwater of Taiyuan basin, China. Environ Earth Sci 62:1635–1642
Madhnure P, Sirsikar DY, Tiwari AN, Ranjan B, Malpe DB (2007) Occurrence of fluoride in the groundwaters of Pandharkawada area, Yavatmal District, Maharashtra, India. Curr Sci 92:675–679
Mamatha P, Rao S (2010) Geochemistry of fluoride rich groundwater in Kolar and Tumkur Districts of Karnataka. Environ Earth Sci 61:131–142
Manish K, Kalpana K, Ramanathan AL, Rajinder S (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Marghade D, Malpe DB, Zade AB (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418
Nordstrom DK, Jenne EA (1977) Fluoride solubility in selected geothermal waters. Geoch Coschim Acta 41:175–188
Nosrati K, Eeckhaut VN (2012) Assessment of groundwater quality using multivariate statistical techniques in Hashtgerd Plain, Iran. Environ Earth Sci 65:331–344
Ozsvath DL (2009) Fluoride and environmental health: a review. Rev Environ Sci Biotech 8:59–79
Perel’man AI (1977) Geochemistry of elements in supergene zone. Keter Pub. House, Jerusalem
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. United States Geological Survey, Washington
Raju JN, Dey S, Das K (2009) Fluoride contamination in groundwaters of Sonbhadra District, Uttar Pradesh, India. Curr Sci 96:979–985
Rao PN, Rao AD, Bhargav JS, Siva Sankar K, Sudarshan G (2014) Regional appraisal of the fluoride occurrence in grondwaters of Andhra pradesh. J Geol Soci India 84:483–493
Ravikumar P, Somashekar RK (2017) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi River Basin, Karnataka state, India. Appl Water Sci 7:745–755
Reddy AGS (2014) Geochemical evaluation of nitrate and fluoride contamination in varied hydrogeological environs of Prakasam district, southern India. Environ Earth Sci 71:4473–4495
Reddy DV, Nagabhushanam P, Sukhija BS, Reddy AGS, Smedley PL (2010) Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chem Geol 269:278–289
Reddy DV, Nagabhushanam P, Madhav T, Chandrakala P, Reddy AGS (2015). Characterization of groundwater contaminant sources in the coastal sand dune aquifer, Prakasam district, A.P., India. Environ Earth Sci 74: 3453–3466
Rogers RJ (1989) Geochemical comparison of groundwater in areas of New England, New York and Pennsylvania. Ground Water 27:690–712
Satyanarayana E, Dhakate R, Laxman Kumar D, Ravindar P, Muralidhar M (2017) Hydrochemical characteristics of groundwater quality with special reference to fluoride concentration in parts of Mulugu-Venkatapur Mandals, Warangal District, Telangana. J Geol Soc India 89:247–258
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and Techniques of groundwater investigation and development. Water Research Series, vol 33. UNESCO, Paris
Shaji E, Bindu Viju J, Thambi DS (2007) High fluoride in groundwater of Palghat District, Kerala. Curr Sci 92:240–245
Shortt HE, McRobert GR, Barnard TW, Mannadinayer AS (1937) Endemic fluorosis in Madras Presidency, India. Indian J Med Res 25:553–561
Shyam R, Kalwania GS (2012) Health risk assessment of fluoride with other parameters in ground water of Sikar city (India). Environ Earth Sci 65:1275–1282
Singaraja C, Chidambaram S, Anandhan P, Prasanna MV, Thivya C, Thilagavathi R, Sarathidasan J (2014) Geochemical evaluation of fluoride contamination of groundwater in the Thoothukudi District of Tamilnadu, India. Appl Water Sci 4:241–250
StatSoft Inc (2001) STATISTICA version 6. StatSoft Inc., USA
Suman M, Surender S, Poonam Y, Versha R, Pushapa R, Monika S, Gurmeet S, Khaiwal R (2009) Appraisal of salinity and fluoride in a semi-arid region of India using statistical and multivariate techniques. Environ Geochem Health 31:643–655
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York
Susheela AK (1999) A treatise on fluorosis. Fluorosis Research and Rural Development Foundation, 2nd Edition, New Delhi
Todd DK (1980) Groundwater hydrology. Wiley, New York
Vasanthavigar M, Srinivasamoorthy K, Prasana MV (2013) Identification of groundwater contamination zones and its sources by using multivariate statistical approach in Thirumanimuthar sub-basin, Tamil Nadu, India. Envrion Earth Sci 68:1783–1795
Wen DG, Zhang FC, Zhang EY, Wang C, Han SB, Zheng Y (2013) Arsenic, fluoride and iodine in groundwater of China. J Geochem Explor 135:1–21
WHO (2011) Guidelines for drinking water quality. World Health Organization, Geneva
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73:8575–8588
Wu J, Li P, Qian H, Duan Z, Zhang X (2014) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: case study in Laoheba phosphorite mine in Sichuan, China. Arab Jour Geosci 7:3973–3982
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8:311–329
Zhang X, Xu Z, Sun X, Dong W, Ballantine D (2013) Nitrate in shallow groundwater in typical agricultural and forest ecosystems in China, 2004–2010. J Environ Sci 25:1007–1014
Acknowledgements
The author is thankful to the University Grants Commission, Government of India, New Delhi, for financial support to carry out this work under major research project (F.No.39-132/2010 (SR). The author also thanks Prof. R. Sambasiva Rao, Department of Chemistry, Andhra University, for his assistance in data analysis. Thanks are due to Abdullah M. Al-Amri, Editor-in-Chief, Arabian Journal of Geosciences, and anonymous reviewers for their valuable and constructive suggestions in bringing the manuscript to its present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subba Rao, N. Controlling factors of fluoride in groundwater in a part of South India. Arab J Geosci 10, 524 (2017). https://doi.org/10.1007/s12517-017-3291-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3291-7