Abstract
In vitro culture in combination with aeroponics is observed to be an efficient means for mass propagation of Sarcostemma acidum in the present investigation. S. acidum is a rare leafless xerophytic shrub of the family Apocynaceae, commonly known as Som-lata or khir-kheep, a source of medicines and the religious drink “Somaras.” Aeroponic technique was used for the induction of adventitious roots from stem cuttings of field-grown plants for the development of quality plants in the greenhouse as a source of explants for micropropagation. Among various concentrations of IBA, NAA and NOA used for induction of adventitious rooting from stem cuttings 3.0 g L−1 NAA were found to be most suitable with average 90% induction response and 14 adventitious roots. Surface-sterilized nodal shoot segments of 6 to 7 cm length were inoculated on basic and modified Murashige and Skoog’s medium containing 3% (w/v) sucrose, 0.8% (w/v) agar, and various concentrations of BAP and kinetin for activation of axillary shoot buds. Shoots were mass multiplied on a modified MS (MMS) medium containing 3.5 mg L−1 BAP and 0.01 mg L−1 IAA. In vitro multiplied shoots were rooted in vitro on half-strength MS medium containing 0.25 mg L−1 NAA. Ninety percent of in vitro regenerated plantlets were hardened successfully on Soilrite in the greenhouse and survived on garden and field soils. This protocol will be useful for in vitro propagation of S. acidum for mass plantations in the desert ecosystem for prospects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcostemma acidum (Roxb) Voigt is one of the rare medicinal plants of the Indian Thar Desert. It is a leafless, straggling, xerophytic succulent shrub of the family Apocynaceae, commonly known as Som-lata and Khir-kheep. The plant grows naturally in the rocky sites in Rajasthan, Bihar, West Bengal, and southern India (Bhandari 1990). The plant was used to prepare “Somaras,” a religious drink as recorded in Vedic literature. The rural and tribal people have been using the herb in order to cure various disorders. Pandey et al. (2017) in their survey reported that indigenous communities in various states of India have been using this whole plant as traditional medicine (TM) for treating 40 different kinds of ailments including snake bites and rheumatism (Senthilkumar et al. 2006). Whole-plant extracts have shown antimicrobial activities against gram-positive bacteria as well as an anti-inflammatory (Shailesh et al. 2011), anti-fertility (Verma et al. 2002), anti-ulcer (Gulshan et al. 2017), anxiolytic, anti-psychotic, and central nervous system (CNS) inhibitory properties were already reported by Ittiyavirah and Rahees (2013). Dhivya and Kalaichelvi (2017) have reported the presence of about forty bioactive compounds in the GC–MS analysis of ethanolic shoot extract of S. acidum such as alkaloids, glycosides, flavonoids, phenols, steroids, amino acids, tannins, terpenoids, quinines, and coumarin. Mass propagation of S. acidum is needed for conservation and fulfillment of the increasing medicinal need. Plant tissue culture is a major technique of mass propagation of rare and threatened plants having lower seed setting, viability, and germination ability in nature. The plants can be regenerated using somatic cells, tissues, and organs of selected genotypes of this plant to meet the increasing demand of the herbal industry due to its immense medicinal uses. Although Rathore and Shekhawat (2013) reported in vitro regeneration of Sarcostemma acidum through callus differentiation, this is the first report of mass multiplication of S. acidum through mature nodal explants achieved by using a combination of plant tissue culture with aeroponic technique, in which in vitro micropropagation of S. acidum from nodal shoots was efficiently performed by using aeroponically developed plants. Plant development through adventitious root induction from stem cuttings is a common practice in plant breeding for many horticultural and forestry plant species. Aeroponics is an efficient means of induction of roots from shoot segments in the air by a regular supply of nutrients and water in the form of mist. The potential of aeroponics for the mass propagation of threatened medicinal plants such as Caralluma edulis, Leptadenia reticulata, and Tylophora indica has also been reported by Mehandru et al. (2014). Due to the rare availability of S. acidum plants in natural habitats, the aeroponic technique can be a useful means for the development of rare and threatened plants in the greenhouse as a source of explants for mass propagation by tissue culture mechanism. It minimizes the dependency on rare and threatened plants growing in natural habitats.
The present investigation was aimed at (i) induction of adventitious roots from stem cutting through the aeroponic technique; (ii) establishment of rooted stem cutting (developed through aeroponic technique) in the greenhouse to raise quality source plants for in vitro plantlet regeneration; (iii) standardization of basal culture medium and establishment of in vitro culture of shoot segments from plants maintained in the greenhouse; (iv) induction of in vitro rooting from in vitro produced shoots; (v) hardening of in vitro regenerated plantlets. This is the first report on the use of the aeroponic technique in combination with plant tissue culture for micropropagation of S. acidum from Indian arid zone.
Materials and methods
Establishment of source plants in greenhouse through aeroponics
Mature stem cuttings of 25 to 30 cm length were collected from the rocky areas of the Machia Safari Park, Jodhpur, during September 2017 (Fig. 1a and b). Half of the explants from these were used directly for in vitro culture establishment and the rest of the shoot cuttings were treated with various concentrations (0.0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 gL−1) of 1-naphthoxyacetic acid (NOA), 1-naphthaleneacetic acid (NAA), and indole-3-butyric acid (IBA) (Sigma-Aldrich®, St. Louis MO) for 5 min and control (without treated cuttings) were inserted vertically in the holes of the styrofoam sheet of the aeroponic system (Fig. 1c) in the greenhouse for induction of adventitious roots (AR). Basal-treated part of the vertically inverted shoots was kept moist by misting of water. Misting was uninterruptedly applied (60 s of regular misting phase with the pause of 600 s between two misting phases) by nine 50-µm high-pressure nozzles connected to 50-mm diameter polyvinyl chloride (PVC) pipes (Prince Piping System, Mumbai, India) through which 125 mL of water per nozzle was pumped by a 0.373-kW electric motor (Barupal et al. 2018). Data were observed after 3 to 4 wk of insertion of basal-treated part of shoots in the holes of aeroponic system. The parameters used for observation were based on percentage of adventitious rooting, number of adventitious roots per shoot segment, and average length of adventitious roots. All the aeroponically rooted plantlets were transferred into the polybags containing garden soil and kept for 25 to 30 d in greenhouse and then transferred in nursery. These established plants were used as a source of explants after 5 to 6 mo of aeroponic rooting (7 to 8 mo of shoot segment treatment with auxins) when these were fully matured for in vitro multiplication of shoots and regeneration of plantlets.
Sarcostemma acidum (Roxb) Voigt. (a) In a natural habitat. (b) Inflorescence. (c) Stem cuttings treated with 3.0 g L−1 NAA and inserted vertically in an aeroponic chamber. (d) Adventitious root induction from stem cuttings in an aeroponic chamber. (e) Shoot cuttings with adventitious roots induced (scale bar, 2.5 cm) (NAA 1-naphthaleneacetic acid)
Establishment of in vitro culture and shoot activation
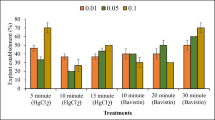
Plants that were collected directly from the field and aeroponically raised in the greenhouse were selected as a source of explants. The medium consisted of Murashige and Skoog (MS) (Murashige and Skoog 1962) basal salts and modified MS with high nitrates (5.325 g L−1 NH4NO3 and KNO3 supplement (Hi-Media®, Mumbai, India)), reduced concentration of calcium chloride (220 mg L−1 CaCl2 (Hi-Media®)) and 200 mg L−1 meso-inositol (Loba Chemie Pvt.Ltd., Mumbai, India), 3% (w/v) sucrose, and 0.8% (w/v) agar–agar (Qualigens Fine Chemicals, Mumbai, India). The medium was supplemented with various concentrations (0.0, 0.5, 2.0, 4.0, 6.0, 8.0, 10.0 mg L−1) of 6-benzylaminopurine (BAP) and 6-furfurylaminopurine (kinetin; Kin) (Hi-Media®) for axillary shoot bud activation. The pH of medium was adjusted to 5.8 using 1 M KOH or 0.1 N HCl (Loba Chemie Pvt.Ltd., Mumbai, India) before autoclaving at 121°C temperature and 1.1 kg cm−2 pressure for 15 min. Nodal shoot cuttings of 6 to 7 cm length were taken from aeroponically developed plants (maintained in the nursery) and used for activation of axillary shoot buds in vitro for large-scale shoot multiplication. Excised nodal shoot segments were pretreated first with 70% ethanol for 10 to 15 s and then surface sterilization of the nodal shoots was done by treating with 0.1% (w/v) Bavistin® (a fungicide containing 50% (w/w) Carbendazim, BASF India Limited, Mumbai, India) for 5 min followed by 0.1% (w/v) HgCl2 (Sigma-Aldrich®) for 4 min followed by thorough rinses of shoot cuttings with autoclaved distilled water for 4 to 5 times. Both the ends of these surface-sterilized shoots were trimmed with sharp scissors and inoculated on a culture medium. The cultures were kept in a growth room at 28° ± 2°C, 10-h photoperiod of 40 to 50 µmol m−2 s−1 photon flux density (PFD) per day, and 50 to 60% relative humidity (RH). Shoots were regularly transferred on fresh culture medium after 25 to 30 d of inoculation.
Shoot multiplication
In vitro produced axillary shoots of 3 to 4 wk were excised from mother explants and used same as mother explant (nodal part of the previously activated axillary shoot with trimmed tip) for further mass multiplication of shoots. Various concentrations (0.00, 0.01, 0.25, and 0.5 mg L−1) of indole-3-acetic acid (IAA; Sigma-Aldrich®) and indole-3-butyric acid (IBA; Sigma-Aldrich®) were used along with BAP for optimization shoot multiplication. Different types and sizes of culture vessels were used for mass multiplication of shoots, which are borosilicate (Borosil Glass Works Ltd., Mumbai, Maharashtra, India) culture tubes (60 mL; 25 mm × 150 mm), and Erlenmeyer conical flasks (150 mL, 72 × 124 mm; and 250 mL, 85 mm × 145 mm).
In vitro root induction
In vitro multiplied shoots were excised from mother explants and inoculated on one-half and one-fourth strength basal and slightly modified (half strength of nitrates and double strength of calcium chloride of full-strength MS medium) MS medium containing 100 mg L−1 activated charcoal (AC; Sigma-Aldrich®), and 3% sucrose and 0.8% agar–agar, supplemented with NOA, IBA, and NAA (0.00, 0.01, 0.025, 0.05, 0.10, 0.25, and 0.50 mg L−1) for induction of in vitro rooting from shoots. Cultures were kept in dark initially until the roots were initiated and then shifted in the light.
Hardening and acclimatization in vitro regenerated plantlets
In vitro rooted shoots (plantlets) of S. acidum were taken out from the medium and washed thoroughly with autoclaved water to remove agar from roots. Rooted parts of plantlets were inserted in autoclaved Soilrite (horticulture-grade perlite and Irish peat moss mixture) and exfoliated vermiculite (Kel Perlite, Bangalore, India) in glass bottles and moistened with one-fourth strength of MS basal salts. Bottles were tightly kept covered with polycarbonate caps for an initial 1 wk, after that caps were loosened and finally removed. Simultaneously, for the acclimatization of the in vitro generated plantlet to the external environment, bottles with plantlets were gradually shifted from high to low relative humidity and low to high temperature (from cooling pad to fan sections) in the greenhouse. Hardened plantlets were further transferred in a mixture of Soilrite and garden soil (1:1) in polybags (Lodha Plastic Industries, Jodhpur, India) for field transfer.
Statistical analysis
Present experiments were conducted using the randomized block design method for single-factor experiments (Compton and Mize 1999) and repeated three times. Each value of induction of adventitious roots from stem cuttings, activation of axillary bud from nodal shoot segments, mass multiplication of shoots, and in vitro rooting represents an average number of five replicates using 35, 35, 20, and 35 explants, respectively. The observed data were analyzed statistically by analysis of variance test (Gomez and Gomez 1984) through SPSS v.17 software (SPSS, Chicago, IL). The significance among mean differences was calculated by Duncan’s multiple range test (DMRT) (Duncan 1955) at P < 0.05.
Results and Discussion
Induction of adventitious roots from mature shoot segments through aeroponic system
Adventitious roots were induced from all stem segments after 1 wk of insertion in the aeroponic chamber (Fig. 1d and e) including the control (Fig. 2a). In plants, growth and development processes are directly or indirectly regulated by both environmental and endogenous factors (Gehlot et al. 2014). Endogenous factors like plant growth regulators (PGRs) play an important role in adventitious root development. The endogenous auxin concentration is responsible for the developmental stages in the root. High endogenous auxin concentration is directly proportional to the high rate of root initiation. (Heloir et al. 1996; Blazkova et al. 1997; Caboni et al. 1997). Endogenous level of auxin increases in exogenously treated shoot cuttings with auxin, which is corresponding to the root initiation at the treated zone (Gaspar et al. 1996; Gatineau et al. 1997). Auxin interacts with other signaling pathways such as cytokinin, ethylene, nutrient, and stress to regulate branching and growth of roots (Murphy et al. 2016; Veloccia et al. 2016; Qu et al. 2017).
Induction of adventitious root from stem cuttings of Sarcostemma acidum (Roxb) Voigt through aeroponics, stem cuttings pretreated with 3.0 g L−1 auxins: (a) control, (b) NOA, (c) IBA, (d) NAA, (e) rooted shoots in polybags containing garden soil, (f) plants developed through rooted stem cuttings in greenhouse (scale bar, 2.5 cm) (NOA 1- naphthoxyacetic acid, IBA indole-3-butyric acid, NAA 1-naphthaleneacetic acid)
Among all the auxin treated shoots used for inductions of adventitious roots, NAA (3.00 g L−1) was the most suitable for S. acidum with an average 90% induction response and average 14.27 ± 1.01 ARs per cutting (Table 1; Fig. 2d) followed by IBA (Fig. 2c), NOA (Fig. 2b), and control (Fig. 2a). NAA has been reported the most suitable auxin for the induction of adventitious roots from the stem segments of Passiflora pahlii on semisolid medium (Simão et al. 2016). All the rooted shoots were successfully established on garden soil in polybags (Fig. 2e) and pots (Fig. 2f) and maintained in the nursery.
In vitro shoot bud activation
Between explants collected directly from the field and aeroponically raised in the nursery, those from aeroponically raised plants were found more responsive for the in vitro culture establishment. Furthermore, a modified MS medium with high nitrates (1.5X of MS medium) and low calcium chloride (half of MS medium) was found to be the most appropriate basal media for the activation of S. acidum axillary shoot buds from mature shoot segments and shoot multiplication in comparison to unmodified basal MS medium. Axillary buds were induced after 1 wk after inoculation of nodal shoots, Among various concentrations of BAP and Kin used for activation of axillary buds, BAP (4.0 mg L−1) was observed to be more suitable than Kin in both types of explants (Fig. 3; and Fig. 6a). BAP has also been proved to be more efficient for shoot proliferation of medicinal plants in Pongamia pinnata (Sugla et al. 2007), Andrographis paniculata (Purkayastha et al. 2008), Rhodiola imbricata (Bhardwaj et al. 2018), and Salvia sclarae (Grigoriadou et al. 2020) and in ornamental shrub Physocarpus opulifolius L. maxim cultivar (Jagiello-Kubiec et al. 2021). In field-collected explants, 45.00 ± 3.00% of axillary shoot buds were activated on a basal MS medium, whereas approximately 84.00 ± 2.00% bud activation was observed on modified MS (MMS) medium containing 4.0 mg L−1 BAP (Fig. 4). However, in the aeroponically raised explants, 78.33 ± 2.89% of axillary shoot buds were activated on basal MS medium and approximately 93.00 ± 3.00% bud activation was observed with 3.67-cm length of axillary shoots on modified MS (MMS) medium containing 4.0 mg L−1 BAP (Figs. 3 and 5). Some difficulties were faced during the in vitro culture establishment of this plant. The first problem observed was the high contamination rate and the lesser response of axillary bud activation, which was overcome by the use of aeroponically raised plants as a source for in vitro culture establishment.
Comparative effect on axillary bud activation due to explant type on normal Murashige and Skoog basal media (field collected and aeroponically raised) in Sarcostemma acidum (Roxb) Voigt. Highest 78.33 ± 2.89% axillary buds activated in aeroponically raised explant, whereas 45.00 ± 3.00% axillary buds activated in field explants at 4.0 mg L−1 BAP (6-benzylaminopurine)
Comparative effect of basal medium on axillary bud activation in mature explants of Sarcostemma acidum (Roxb) Voigt. Normal Murashige and Skoog medium (MS) and modified MS (MMS) medium. Maximum axillary bud activation percentage recorded at MS basal medium was 78.33 ± 2.89 whereas on MMS medium it was 93.00 ± 3.00% (BAP 6-benzylaminopurine, Kin 6-furfuryle aminopurine)
Comparative effect of BAP and Kin and basal medium (MS and MMS) on length of axillary bud of Sarcostemma acidum. Maximum length of axillary shoot observed was 3.67 cm at 4.0 mg L−1 BAP in aeroponically raised explant (BAP 6-benzylaminopurine, Kin 6-furfuryle aminopurine, MS Murashige and Skoog medium, MMS modified Murashige and Skoog medium)
The establishment of mother plants in the greenhouse ensures better growth and development of mother plants and helps in the reduction of contaminations during the establishment of aseptic culture (Debergh and Maene 1981). Another problem encountered was the appearance of huge callus formation at the basal part of the explant during axillary bud activation; this was reduced by doing some modifications in macronutrients of basal MS media. Callusing at basal part of explants was minimized by decreasing the calcium concentration in unmodified MS media because calcium enhances the cell division and higher nitrogen level was found to be more appropriate for activation of axillary shoot buds from mature shoot segments of S. acidum, in comparison to basal MS medium in the present investigation. Kang and McMahan (2014) also reported a moderate (150 to 300 mg L−1) level of calcium for emergence of single, elongated shoots in guayule tissue culture. Although MS medium has high inorganic nitrogen concentrations and is being used widely in many plant species, in some plant cultures the concentration of inorganic nitrogen in MS medium is far more the amount required for the normal growth of plantlets in vitro (Zhang et al. 2019). Induction of callus during axillary bud activation from nodal shoot segments was observed to be the major hindrance for clonal propagation of S. acidum from field-grown mature plants. Although callus induced from nodal shoots can be an alternative source of in vitro plant regeneration as reported by Rathore and Shekhawat (2013), there may be chances of genetic variability during de-differentiation and re-differentiation of cells. Hence, plants regenerated through axillary shoots of nodal shoots are preferred over callus differentiation for efficient mass propagation of mature plants.
In vitro shoot multiplication
The highest shoot multiplication was achieved by the addition of IAA to MMS medium containing BAP (Table 2). Shoots were multiplied suitably on a modified MS medium containing 3.5 mg L−1 BAP and 0.01 mg L−1 IAA. An average of 12.00 ± 1.00 shoots per node with 3.67 ± 0.39 cm length was observed on modified MS medium containing 3.5 mg L−1 BAP and 0.01 mg L−1 IAA ( Table 2; Fig. 6b). BAP in combination with auxin has been proved to be best for mass multiplication of shoots in Thalictrum foliosum (Mishra et al. 2020). Arya et al. (2003) used IAA in combination with BA for in vitro multiplication of shoot of Leptadenia reticulata. This IAA and BAP combination has also been reported as the best combination by Nayak et al. (2007) and Makunga and van Staden (2008) in Aegle marmelos and Salvia africana-lutea respectively. Comparatively longer shoots were observed in the medium supplemented with BAP along with IBA, and the highest average length (3.87 ± 0.12 cm) of shoots was observed at 3.5 mg L−1 BAP and 0.25 mg L−1 IBA containing medium (Table 2).
In vitro regeneration of Sarcostemma acidum (Roxb) Voigt. (a) Axillary bud activation from nodal shoot segments on MMS (modified Murashige and Skoog) medium containing 4.0 mg L−1BAP. (b) Shoot multiplication on MMS medium containing 3.5 mg L−1 BAP and 0.01 mg L−1 IAA. (c) In vitro root induction from in vitro multiplied shoots on half-strength MS medium containing 0.25 mg L−1 NAA and 100 mg L−1 activated charcoal. (d) In vitro rooted plantlet. (e and f) Acclimatized plant on Soilrite in greenhouse (scale bar, 1 cm) (BAP 6-benzylaminopurine, NAA 1-naphthaleneacetic acid)
Among all types of culture vessels tested for shoot multiplication responses, 250-mL conical flasks were found to be more suitable than culture tubes and 150-mL flasks for in vitro mass multiplication of shoots due to more headspace. It is important to choose the optimal size of culture vessel for any plant species, and ensure the proper gas exchange between the medium and the atmosphere in the vessel for the shoot proliferation (Monette 1983).
In vitro rooting and acclimatization of in vitro regenerated plantlets
Among various concentrations of NAA, IBA, and NOA, and strengths of MS salts used for induction of in vitro rooting, NAA followed by IBA and NOA in half-strength MS medium was found to be more appropriate. NAA was also found to be suitable for induction of rooting in micro-cuttings of Cornus alba (Ilczuk and Jacygrad 2016) and Achyranthes asper (Pai et al. 2018). Low-strength MS salts generally promote root induction in shoots (Bopana and Saxena 2008). Half-strength MS macronutrient was also reported to be suitable for rooting of shoots in Dendrobium candidum (Zhao et al. 2007). Low-strength MS medium has also been proved to be more effective for induction of roots from shoots of Dendrocalamus longispathus (Saxena and Bhojwani 1993), Lavendula vera (Andrade et al. 1999), and Cicer microphyllum (Singh et al. 2019). Reduced concentration of nitrogen ions in low-strength MS medium generally promotes root formation (Driver and Suttle 1987).
Activated charcoal also plays an important role in in vitro root induction. There are many reports proving the increased rooting responses shown by activated charcoal when added to the rooting media. Firoozabady et al. (2006) reported enhanced rooting ability in transgenic shoots of pineapple (Ananas comosus) by the addition of activated charcoal. Agrawal et al. (2002) also reported rooting in nodal segment-derived explants of Simmondsia chinensis (jojoba), when MS medium supplemented with activated charcoal; according to the author, activated charcoal not only increased the percentage of rooting response but also reduced the callusing of the explants. In vitro rooting was observed after 25 to 30 d of inoculation of shoots. The highest 86.67% rooting and average 5.93 roots per shoot with 4.95-cm length were observed on a half-strength MS medium containing 0.25 mg L−1 NAA (Table 3; Fig. 6c and d) and in vitro rooting in half-strength MS medium supplemented with NAA, Kin, and activated charcoal in Fortunella crasifolia (Yang et al. 2006). Regeneration protocol standardized in the present investigation using the aeroponic technique in combination with plant tissue culture technique can be an efficient means of micropropagation for the prospects of large-scale plantation and conservation of this rare and endangered plant species of the Indian arid zone. Light green shoots turned dark green during hardening (Fig. 6e and f). Approximately 90% of in vitro regenerated plantlets successfully hardened in the greenhouse within 6 to 8 wk and survived on garden and field soils.
Conclusions
The aeroponic technique was found to be a very useful technique for induction of adventitious root and development of quality plants in greenhouse. The plants developed in greenhouse through aeroponics are proved to be a better source of explants for efficient in vitro plantlets regeneration and conservation of S. acidum.
References
Agrawal V, Prakash S, Gupta SC (2002) Effective protocol for in vitro shoot production through nodal explants of Simmondsia chinensis. Biol Plant 45:449–453
Andrade LB, Echeverrigaray S, Fracaro F, Pauletti GF, Rota L (1999) The effect of growth regulators on shoot propagation and rooting of common levender (Lavandula vera DC). Plant Cell Tiss Org Cult 56:79–89
Arya V, Shekhawat NS, Singh RP (2003) Micropropagation of Leptadenia reticulata - a medicinal plant. In Vitro Cell Dev Biol - Plant 39:180–185
Barupal M, Kataria V, Shekhawat NS (2018) In vitro growth profile and comparative leaf anatomy of the C3–C4 intermediate plant Mollugo nodicaulis. In Vitro Cell Dev Biol-Plant 54:689–700. https://doi.org/10.1007/s11627-018-9945-7
Bhandari MM (1990) Flora of Indian desert. MPS Repros, Jodhpur
Bhardwaj AK, Singh B, Kaur K, Roshan P, Sharma A, Dolker D, Naryal A, Saxena S, Pati PK, Chaurasia OP (2018) In vitro propagation, clonal fidelity and phytochemical analysis of Rhodiola imbricata Edgew: a rare trans-Himalayan medicinal plant. Plant Cell Tiss Org Cult 135:499–513. https://doi.org/10.1007/s11240-018-1482-x
Blazkova A, Sotta B, Tranvan H, Maldiney R, Bonnet M, Einhorn J, Kerhoas L, Miginiac E (1997) Auxin metabolism and rooting in young and mature clones of Sequoia sempervirens. Physiol Plant 99:73–80. https://doi.org/10.1111/j.1399-3054.1997.tb03433.x
Bopana N, Saxena S (2008) In vitro propagation of a high value medicinal plant: Asparagus racemosus Willd. In Vitro Cell Dev Biol - Plant 44:525–532. https://doi.org/10.1007/s11627-008-9137-y
Caboni E, Tonelli MG, Lauri P, Iacovacci P, Kevers C, Damiano C, Gaspar T (1997) Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol Plant 39:91–97
Compton ME, Mize CW (1999) Statistical considerations for in vitro research: I — birth of an idea to collecting data. In Vitro Cell Dev Biol - Plant 35:115–121. https://doi.org/10.1007/s11627-999-0020-2
Debergh PC, Maene LJ (1981) A scheme for commercial propagation of ornamental plants by tissue culture. Sci Hortic 14:335–345. https://doi.org/10.1016/0304-4238(81)90047-9
Dhivya SM, Kalaichelvi K (2017) Phytochemical studies and gas chromatography-mass spectrometry analysis of Sarcostemma brevistigma, Wight & Arn. Asian J Pharm Clin Res 10:462–466. https://doi.org/10.22159/ajpcr.2017.v10i3.16538
Driver JA, Suttle GRL (1987) Nursery handling of propagules. In: Bonga JM, Durzan DJ (eds) Cell and tissue culture in forestry. Springer, Netherlands, Dordrecht, pp 320–335
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Firoozabady E, Heckert M, Gutterson N (2006) Transformation and regeneration of pineapple. Plant Cell Tiss Org Cult 84:1–16
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol - Plant 32:272–289. https://doi.org/10.1007/s11240-005-1371-y
Gatineau F, Fouché JG, Kevers C, Hausman JF, Gaspar T (1997) Quantitative variations of indolyl compounds including IAA, IAA-aspartate and serotonin in walnut microcuttings during root induction. Biol Plant 39:131–137
Gehlot A, Gupta RK, Tripathi A, Tripathi A, Arya ID, Aryan S (2014) Vegetative propagation of Azadirachta indica: effect of auxin and rooting media on adventitious root induction in mini-cuttings. Adv For Sci 1:1–9
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley & Sons
Grigoriadou K, Trikka FA, Tsoktouridis G, Krigas N, Sarropoulou V, Papanastasi K, Maloupa E, Makris AM (2020) Μicropropagation and cultivation of Salvia sclarea for essential oil and sclareol production in northern Greece. In Vitro Cell Dev Biol - Plant 56:51–59. https://doi.org/10.1007/s11627-019-10040-4
Gulshan MD, Chandrasekhar G, Kumar BV, Ramarao N (2017) Anti-ulcer activity of ethanolic Sarcostemma acidum stem extract. Int Res J Pharm 8:91–94
Heloir M-C, Kevers C, Hausman J-F, Gaspar T (1996) Changes in the concentrations of auxins and polyamines during rooting of in-vitro-propagated walnut shoots. Tree Physiol 16:515–519. https://doi.org/10.1093/treephys/16.5.515
Ilczuk A, Jacygrad E (2016) In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. using RAPD and ISSR markers. In Vitro Cell Dev Biol - Plant 52:379–390. https://doi.org/10.1007/s11627-016-9781-6
Ittiyavirah SP, Rahees T (2013) Evaluation of psychopharmacological activity of ethyl acetate extract of Sarcostemma acidum (Roxb). Voigt J Phytopharmacol 2:1–7
Jagiello-Kubiec K, Nowakowska K, Ilczuk A, Lukaszewska AJ (2021) Optimizing micropropagation conditions for a recalcitrant ninebark (Physocarpus opulifolius L. maxim.) cultiver. In Vitro Cell Dev Biol - Plant 57:281–295
Kang BG, McMahan C (2014) Medium and calcium nitrate influence on shoot regeneration from leaf explants of guayule. Ind Crops 60:92–98
Makunga NP, van Staden J (2008) An efficient system for the production of clonal plantlets of the medicinally important aromatic plant: Salvia africana-lutea L. Plant Cell Tiss Org Cult 92:63–72. https://doi.org/10.1007/s11240-007-9305-5
Mehandru P, Shekhawat NS, Rai MK, Kataria V, Gehlot HS (2014) Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica- three threatened medicinal Asclepiads. Physol Mol Biol Plant 20:365–373
Mishra MK, Pandey S, Misra P, Niranjan A, Srivastava A (2020) An efficient protocol for clonal regeneration and excised root culture with enhanced alkaloid content in Thalictrum foliolosum DC.— an endemic and important medicinal plant of temperate Himalayan region. Ind Crops Prod 152:112504. https://doi.org/10.1016/j.indcrop.2020.112504
Monette PL (1983) Influence of size of culture vessel on in vitro proliferation of grape in a liquid medium. Plant Cell Tiss Org Cult 2:327–332. https://doi.org/10.1007/BF00039879
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:437–497
Murphy E, Vu LD, Van den Broeck L, Cotte BVD, Gaudinier A, Goh T, Slane D, Beeckman T, Inze D, Brady SM, Fukaki H, Smet ID (2016) RALFL34 regulates formative cell divisions in Arabidopsis pericycle during lateral root initiation. J Exp Bot 67:4863–4875. https://doi.org/10.1093/jxb/erw281
Nayak P, Behera PR, Manikkannan T (2007) High frequency plantlet regeneration from cotyledonary node cultures of Aegle marmelos (L.) Corr. In Vitro Cell Dev Biol - Plant 43:231–236. https://doi.org/10.1007/s11627-006-9013-6
Pai SR, Upadhya V, Hedge HV, Joshi RK, Kholkute SD (2018) In vitro rapid multiplication and determination of triterpenoids in callus cultures of Achyranthes aspera Linn. Indian J Biotechnol 17:151–159
Pandey S, Shukla A, Pandey S, Pandey A (2017) Morphology, chemical composition and therapeutic potential of somlata (Sarcostemma acidum Wight. & Arn.). Pharma Sci Monit 8:54–60
Purkayastha J, Sugla T, Paul A, Solleti S, Sahoo L (2008) Rapid in vitro multiplication and plant regeneration from nodal explants of Andrographis paniculata: a valuable medicinal plant. In Vitro Cell Dev Biol - Plant 44:442–447. https://doi.org/10.1007/s11627-008-9156-8
Qu Y, Wang Q, Guo J, Wang P, Song P, Jia Q, Zhang X, Kudla J, Zhang W, Zhang Q (2017) Peroxisomal CuAOζ and its product H2O2 regulate the distribution of auxin and IBA-dependent lateral root development in Arabidopsis. J Exp Bot 68:4851–4867. https://doi.org/10.1093/jxb/erx290
Rathore MS, Shekhawat NS (2013) In vitro regeneration in Sarcostemma acidum (Roxb.) –an important medicinal plant of semi-arid ecosystem of Rajasthan. India Physiol Mol Biol Plant 19:269–275. https://doi.org/10.1007/s12298-012-0158-y
Saxena S, Bhojwani SS (1993) In vitro clonal multiplication of 4-year-old plants of the bamboo, Dendrocalamus longispathus Kurz. In Vitro Cell Dev Biol - Plant 29:135–142
Senthilkumar M, Gurumoorthi P, Janardhanan K (2006) Some medicinal plants used by Irular, the tribal people of Marudhamalai hills, Coimbatore, Tamil Nadu. Nat Prod Radiance 5:382–388
Shailesh G, Seema K, Dwivedi S (2011) In-vitro anti-inflammatory activity of Sarcostemma acidum Wight. & Arn. Indian herb by human red blood cell membrane stabilization method. Int J Pharm Teach Pract 2:184–188
Simão MJ, Fonseca E, Garcia R, Mansur E, Pacheco G (2016) Effects of auxins and different culture systems on the adventitious root development of Passiflora pohlii Mast. and their ability to produce antioxidant compounds. Plant Cell Tiss Org Cult 124:419–430. https://doi.org/10.1007/s11240-015-0904-2
Singh RK, Anandhan S, García-Pérez LM, Ruiz-May E, Perez EN, Quiro-Figueroa FR (2019) An efficient protocol for in vitro propagation of the wild legume Cicer microphyllum Benth., a crop wild relative of chickpea (Cicer arietinum L.). In Vitro Cell Dev Biol - Plant 55:9–14. https://doi.org/10.1007/s11627-018-09958-y
Sugla T, Purkayastha J, Singh SK, Solleti SK, Sahoo L (2007) Micropropagation of Pongamia pinnata through enhanced axillary branching. In Vitro Cell Dev Biol - Plant 43:409–414. https://doi.org/10.1007/s11627-007-9086-x
Veloccia A, Fattorini L, Della Rovere F, Sofo A, Angeli SD, Betti C, Falasca G, Altamura MM (2016) Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J Exp Bot 67:6445–6458. https://doi.org/10.1093/jxb/erw415
Verma PK, Sharma A, Mathur A, Sharma P, Gupta RS, Joshi SC, Dixit VP (2002) Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J Androl 4:43–48
Yang L, Xu CJ, Hu G-B, Chen KS (2006) Direct shoot organogenesis and plant regeneration in Fortunella crassifolia. Biol Plant 50:729–732. https://doi.org/10.1007/s10535-006-0117-y
Zhang K, Wu Y, Hang H (2019) Differential contribution of NO3-/ NH4- to nitrogen use in response to a variable inorganic nitrogen supply in plants of two Brassicaceae species in vitro. Plant Meth 15:86. https://doi.org/10.1186/s13007-019-0473-1
Zhao P, Wang W, Feng FS, Wu F, Yang ZQ, Wang WJ (2007) High-frequency shoot regeneration through transverse thin cell layer culture in Dendrobium Candidum Wall Ex Lindl. Plant Cell Tiss Org Cult 90:131–139. https://doi.org/10.1007/s11240-006-9181-4
Funding
The first author Preeti Choudhary received from the University Grants Commission, India, the Research Fellowship (Ref. no. 21/12/2014 (ii) EU-V).
Author information
Authors and Affiliations
Contributions
Author VK made conception and designed experiment present investigation and author PC performed experiments, collected data, and analyzed scientifically.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Choudhary, P., Kataria, V. In vitro culture in combination with aeroponics is an efficient means of mass propagation of Sarcostemma acidum: a rare medicinal plant of Indian arid zone. In Vitro Cell.Dev.Biol.-Plant 58, 372–381 (2022). https://doi.org/10.1007/s11627-021-10245-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10245-6