Abstract
Mollugo nudicaulis Lam., commonly known as John’s folly or naked-stem carpetweed, is an ephemeral species of tropical regions. The plant is ideal to study the eco-physiological adaptations of C3–C4 intermediate plants. In the present report, in vitro growth profiling of the plant and comparative leaf anatomy under in vitro and ex vitro conditions were studied. In vitro propagation of the plant was carried out on Murashige and Skoog (MS) basal medium augmented with additives and solidified with 0.8% (w/v) agar-agar or 0.16% (w/v) Phytagel™. The concentration of plant growth regulators (PGRs) in the basal medium was optimized for callus induction, callus proliferation, shoot regeneration, and in vitro rooting. The optimum callus induction was obtained from M. nudicaulis seedling hypocotyls. The highest regeneration induction of about 88% or nearly 41 shoots with about 142 leaves per culture vessel was observed from friable callus on MS basal medium solidified with Phytagel™ and containing 4.44 μM 6-benzylaminopurine, 4.65 μM kinetin, 2.69 μM naphthaleneacetic acid, and 0.91 μM thidiazuron. In leaf anatomy, differences related to photosynthetic tissue organization were observed in leaves of in vitro and ex vitro plants, which indicated that changes in the environment affected the anatomy of subsequent leaves in plants. This is the first report of an efficient micropropagation protocol for M. nudicaulis, using an indirect organogenesis method. Efforts were made to optimize the concentrations of various PGRs and organic compounds for in vitro growth of regenerated shoots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The C3 and C4 systems are well-known pathways of photosynthesis that assimilate the carbon dioxide (CO2) from the air in green leaf cells. By their physio-ecological adaptation to high temperature and low availability of water, nitrogen, and CO2, plant species with the C4 photosynthesis system are comparatively more advanced and efficient than C3 species (Rawsthorne 1992 ; Christin and Osborne 2014; Stata et al. 2014). Besides C3 and C4 plants, there are certain plant species that have CO2 compensation points between those of C3 and C4 plants and are thus known as intermediates or C2 types (Rawsthorne 1992; Sage et al. 2014; Schlüter and Weber 2016). Such C3–C4 intermediate species are geographically rare in comparison to both C3 and C4 species (Nicholson 2011; Christin and Osborne 2014). These C3–C4 intermediate plants may have evolved to adapt to climatic conditions interlinking C3 and C4 species in the same genus or may have a separate origin (Voznesenskaya et al. 2010; Gowik and Westhoff 2011). At present, C3–C4 intermediate species have been reported in different clades of 17 genera of flowering plants, with one to two species in each genus (Sage et al. 2011). To date, only two C3–C4 intermediate species with a cosmopolitan distribution in warm climates, Mollugo nudicaulis Lam. and Mollugo verticillata L., have been reported (Vincent 2003). These plants are suitable for studying the function of warm climate C3–C4 intermediates.
In a desert climate, a variety of plant species are ephemeral and complete their life cycle during the rainy season (Nicholson 2011). These plants are adapted to either high or low temperatures at anatomical, biochemical, and physiological levels. According to The International Plant Names Index 2012, Mollugo nudicaulis Lam., also known by its synonym Paramollugo nudicaulis (Lam.) Thulin (Thulin et al. 2016), is an ephemeral species, belonging to the family Molluginaceae, order Caryophyallales (Bhandari 1990; Sukhorukov and Kushunina 2016). The plant possesses a characteristic feature of rosette leaves with many inflorescence stalks (Fig. 1a–c). This species is characterized as a C2 type I intermediate, with low levels of C4 metabolism (Christin et al. 2011; Sage et al. 2014). Additionally, M. nudicaulis possesses hepatoprotective properties (Rajamanikandan et al. 2012), and its extract is also used to cure eye and skin infections, whooping cough, and typhoid in traditional medicine (Ignacimuthu et al. 2008; Rameshkumar and Sivasudha 2012).
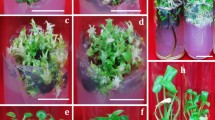
Depiction of in vitro responses of Mollugo nudicaulis Lam. (a) Ex vitro plant in its natural habitat; (b) flower; (c) mature seeds; (d) in vitro seed germination; (e) callus induction (indicated by arrow) from hypocotyl; (f) callus proliferation on 2,4-dicholorophenoxyacetic acid alone; (g) proliferated green friable regenerative callus on optimum medium combination; (h, i) shoot regeneration from callus; (j, k, l) multiplication of shoot clumps; scale bars = 10 mm.
The development of micropropagation protocols for plants helps in conserving the germplasm and provides disease-free, synchronized plant material in a short time (Mosaleeyanon et al. 2004). Additionally, these techniques can constitute an essential first step toward effective transgenic plant engineering and crop manipulation (Jauhar 2006) and an informative means for studying gene function (Thole and Rawsthorne 2003). Nagesh and Shanthamma (2011) reported a preliminary study regarding the micropropagation of M. nudicaulis using shoot tip explants and explored for its antioxidant properties.
The present paper analyzes the in vitro growth profile of M. nudicaulis to achieve effective regeneration of plants throughout the year. During in vitro conditions, plants are subjected to a saturated environment, which often leads to the modification of anatomical features. These phenomena could be exploited for studying the comparative leaf anatomy of these C3–C4 intermediate plants at in vitro, as well as ex vitro, levels. This is the first report of a comparative study on anatomical intermediate characteristics found in ex vitro and in vitro-grown leaves of M. nudicaulis.
Materials and methods
Plant material
Whole plants of M. nudicaulis were collected at flowering and fruiting stages in early August to late October from natural and cultivated areas in Jodhpur, India (26.350932 N, 73.046042 E). These plants were grown under greenhouse conditions in an aeroponic chamber made up of styrofoam sheets according to Mehandru et al. (2014), where moisture was maintained through water mist applied to the basal parts of the plants at regular intervals of 60-s misting with an 800-s pause between consecutive misting. In this setup, misting of water on basal parts of roots was carried out through nine 50-μ-high pressure nozzles connected to 50-mm diameter polyvinyl chloride (PVC) pipes (Prince piping system, Mumbai, India) through which 125 mL of water per nozzle was pumped by a 0.373-kW electric motor (Crompton Greaves, Mumbai, India) at a pressure of 4.21 kg cm−2.
To establish the in vitro culture, two different types of explant were selected, juvenile explants and mature leaves. Seeds and mature leaf tissue were surface sterilized with 0.1% (w/v) Bavistin® (a fungicide containing 50% (w/w) Carbendazim, BASF India Limited, Mumbai, India) for 10 min, washed three to four times with autoclaved, distilled water, and then sterilized with 0.1% (w/v) HgCl2 (Sigma-Aldrich®, St. Louis, MO) for 3 min, then again thoroughly washed three to four times with autoclaved water.
Nutrient medium preparation
Murashige and Skoog (MS; Murashige and Skoog 1962) basal salts augmented with 50 mg L−1 ascorbic acid, 25 mg L−1 each of arginine, adenine sulfate, and citric acid (Hi-Media®, Mumbai, India); and 3% (w/v) sucrose (Qualigens Fine Chemicals, Mumbai, India) was used as a basal medium for in vitro studies. Two different gelling agents, 0.8% (w/v) agar-agar (Bacteriological grade, Qualigens Fine Chemicals) and 0.16% (w/v) Phytagel™ (Sigma-Aldrich®), were compared. The pH was adjusted to 5.8 ± 0.02 using 1 N KOH or HCl (Loba Chemie, Mumbai, India) prior to autoclaving at 121°C and 1.1 kg cm−2 for 15 min.
Callus induction and proliferation
The sterilized seeds were inoculated onto half-strength MS medium (macro and micro nutrients of MS basal reduced to half) with 0.2% (w/v) activated charcoal, supplemented with 500 μM gibberellic acid (GA3; Sigma-Aldrich®) or 0.9 μM 2,4-dichlorophenoxyacetic acid (2,4-D; Sigma-Aldrich®). After seed germination (Fig. 1d), whole seedling, or their hypocotyl, epicotyl, cotyledonary leaves, and roots, or leaf explants from mature plants (cut into 0.5 mm to 1 cm sections) were used for callus initiation. All these explants were placed onto MS basal medium supplemented with 2.27, 4.53, 9.06, or 13.59 μM 2,4-D. The cultures were incubated for 4 wk in a culture room at 26 ± 2°C with a 55–60% relative humidity (RH), and a light intensity of 40–50 μmol m−2 s−1 photon flux density (PFD) from white fluorescent tubes (Philips, India Ltd.; Mumbai, India) with a 12-h photoperiod.
For callus proliferation, the friable, competent, young calluses obtained from the hypocotyl part of young seedlings were transferred to medium containing different concentrations (Table 2) of 2,4-D, indole-3-acetic acid (IAA; Sigma-Aldrich®), or naphthaleneacetic acid (NAA; Sigma-Aldrich®), alone or in combination with 6-benzylaminopurine (BAP; Sigma-Aldrich®), and/or 6-furfuryl aminopurine (kinetin; Kin), and/or N6-(2-isopentenyl) adenine (2iP; Sigma-Aldrich®). The optimum proficient callus was sub-cultured three to four times onto fresh, identical medium at 4-wk intervals. Borosilicate (Borosil Glass Works Ltd., India) culture tubes (60 mL, 25 mm × 150 mm), Erlenmeyer conical flasks (100 mL; 64 × 105 mm; 150 mL, 72 × 124 mm and 250 mL; 85 × 145 mm), and screw-cap glass bottles (420 mL, 70 × 130 mm; Siddhivinayak Glass Concepts, Firozabad, India) were used to analyze the effect of culture vessel size and shape on callus proliferation and regeneration. About 15 mL of optimized callus proliferation medium was dispensed into 25 × 150-mm culture tubes, 20 to 50 mL dispensed into 100, 150, and 250-mL Erlenmeyer conical flasks and about 50 mL into each 420-mL screwed glass bottles.
Shoot regeneration from callus
The friable callus was transferred onto MS basal medium solidified with agar-agar or Phytagel™ and fortified with different combinations of BAP, Kin, and/or TDZ with 2,4-D or NAA. Proliferating callus (approximately 200 to 400 mg per callus) was fragmented into 8 to 12 pieces and used for shoot regeneration. After regeneration of shoots, the clumps of callus were subcultured onto the same medium after an interval of 4 wk.
Effect of PGRs and medium composition on regenerated shoots
In vitro regenerated shoots were harvested into clumps of 2 to 3 shoots and further cultured for analyzing the effects of culturing on the growth of shoots and the shape and size of leaves after 4 wk of culture on MS medium containing 2.22 μM BAP, 0.9 μM thidiazuron (TDZ, Sigma-Aldrich®), or 100 to 500 μM GA3; full or half-strength MS basal salts; and agar-agar or Phytagel™.
In vitro rooting
Regenerated shoots were inoculated into quarter-, half-, or full-strength MS basal medium supplemented with 0.2% (w/v) activated charcoal (AC; Sigma-Aldrich®). Various concentrations of indole-3-butyric acid (IBA; Sigma-Aldrich®), 2,4-D, NAA, or IAA listed in Table 5, were added to the medium. For in vitro rooting, a pulse treatment method, in which shoot bases were treated with 490 μM IBA for 1 to 2 min and inoculated on MS medium containing 0.45 μM 2,4-D, 0.54 μM NAA, or 0.57 μM IAA or quarter-, half-, or full-strength MS basal medium was also used.
Transfer to ex vitro and hardening
In vitro produced plantlets were taken out from culture vessels and transferred to glass bottles containing soil-rite (horticulture grade perlite with Irish peat moss mixture and exfoliated vermiculite (Kel Perlite, Bangalore, India)) and moistened with quarter strength of MS basal salt solution. The glass bottles were covered with polycarbonate caps and placed near the cooling pad end in a greenhouse where there was a high relative humidity (80–90%) and low temperature (28 ± 2°C). After 7 to 8 d, the caps of the bottles containing rooted plantlets were loosened and finally removed to acclimatize the in vitro grown plantlets to the external environment. Simultaneously, bottles were gradually shifted to the fan end of the greenhouse for further acclimatization where there was a low relative humidity (45–55%) and high temperature (36 ± 2°C). After 40 to 50 d, successfully hardened plants were transferred to polybags (Lodha Plastic Industries, Jodhpur, India) containing a mixture of soil-rite and field soil (1:1) and kept in greenhouse condition for 30 to 40 d and finally transferred to a nursery.
Leaf anatomy
For anatomical comparisons, 25 to 30 leaves of approximately the same size of M. nudicaulis were taken from the 2nd tier of leaves from the base of 2 to 3-mo-old wild mature plants, from 4- to 5-wk-old aeroponically grown plants, and from plants still in in vitro cultures. Transverse handmade cross sections were cut from fresh leaves and stained with 0.1% (w/v) safranin O or toluene blue (Sigma-Aldrich®) for 30 to 60 s and mounted on slides with distilled water. Photomicrographs were taken with a digital camera mounted on the CH20i binocular microscope (Olympus Corp., Tokyo, Japan) at 50× and 100× magnification.
Data recording and analysis
All experiments were set up in a completely randomized block design (RBD) for single factor experiments (Compton and Mize 1999) and repeated three times with 15 replicates in each treatment. Percent response of callus, color and texture of callus, number of shoots, and number and shape of leaves were observed, and data was scored. Percent proliferation was measured by observing visual increase in volume of initial callus versus days. The data were analyzed statistically by analysis of variance test (ANOVA; Gomez and Gomez 1984) and differences among mean values were compared by Duncan’s multiple range test (Duncan 1955) at P < 0.05 and alpha at 0.05 using Agricolae software package of R (Mendiburu 2016).
Results and discussion
Callus induction and proliferation
Callus was induced from different types of juvenile explants as well as from mature leaf explants on various concentrations of 2,4-D (Table 1). The best responses were obtained with 9.06 μM 2,4-D applied to hypocotyl explants, cotyledonary leaves, and whole seedling, with 98%, 92%, and 90% of callus initiation, respectively (Table 1). However, hypocotyl explants produced the preferable white and friable callus (Fig. 1e), and cotyledonary leaves induced fast growing, but watery callus whereas callus obtained from whole seedling was friable whitish creamy and rapid growing (Table 1) but unsynchronized due to origin sites being mixed. In all explants, reduced response was observed at 13.59 μM 2,4-D and only roots were induced from cells of calluses in primary culture at this concentration (Table 1). For other plant species, the desired effect of 2,4-D for callus induction has been noted in previous reports (Chaâbani et al. 2015; Mohlakola et al. 2017).
For optimization of hypocotyl-induced callus proliferation, different plant growth regulators (PGRs), alone or in combination, were added to the medium. When PGRs were used independently, maximum proliferation of callus (86%) was observed with 11.33 μM 2,4-D, with rapidly growing, creamy, and friable callus (Table 2). No callus proliferation response was observed on concentrations of IAA below 17.13 μM; however, among different NAA concentrations, optimum response (49%) was recorded with 16.11 μM NAA, proliferating a fast growing, white, and loose callus (Table 2).
Among different combinations of PGRs, the optimum response was observed with a combination of 11.33 μM 2,4-D, 2.22 μM BAP, 2.33 μM Kin, and 0.98 μM 2iP in terms of percent proliferation (98.33 ± 2.36%) with minimum of 4 d to respond (Table 2). The callus grown on medium containing both 2,4-D alone was cream colored, rooty, and friable (Fig. 1f) whereas the optimum proliferating callus obtained from the combined effect of 2,4-D, BAP, Kin, and 2iP was fast growing, pale-yellowish, and granular in appearance (Fig. 1g). Rathore et al. (2013) also found a positive effect of 2iP-inclusion with other PGRs on callus proliferation of in Leptadenia reticulata (Retz.) Wight & Arn.
Regarding the glass vessel effect, the best callus proliferation was observed in 250-mL Erlenmeyer flasks. Callus proliferated rapidly in comparison to other glassware used. The wider space of the conical flask might have favored the cell division due to better aeration and subsequent dilution of the ethylene effect. Glassware types affect the in vitro growth of many plants (Patel et al. 2016).
Shoot regeneration
The optimization of the plant hormones in growth medium is necessary for long-term in vitro culturing and regeneration (Morini et al. 2000; Stobbe et al. 2002; Ziv and Altman 2003; Khan et al. 2009). For shoot regeneration from proliferating callus, different PGR combinations (Table 3) were used. Maximum regeneration percent response of 100% was observed on MS basal medium supplemented with 4.44 μM BAP, 4.65 μM Kin, and 2.69 μM NAA and solidified with Phytagel™. The maximum response in terms of the number of shoots (36.50 ± 1.05) was observed on a regeneration medium containing 4.44 μM BAP, 4.65 μM Kin, 2.69 μM NAA, and 0.91 μM TDZ (Table 3; Fig. 1h). Thidiazuron, an active cytokinin-like phytohormone, has been reported in many plants to mimic the functions of both auxin and cytokinin, when added in minute concentrations to the medium (Huetteman and Preece 1993; Morini et al. 2000; Sharma et al. 2017). The exogenous levels of plant hormones in culture medium have been observed to be a significant factor for induction of organogenesis in friable callus (Stobbe et al. 2002).
Use of 0.16% (w/v) Phytagel™ instead of agar-agar in the above combination enhanced the response in terms of the number of shoots (41.17 ± 1.6), as well as number of leaves (141.50 ± 4.85) per culture vessel, with an overall shoot regeneration percentage of 87.50% (Table 3; Fig. 1i). The superiority of Phytagel™ over other gelling agents for most plant culture systems may be due to its consistent texture and high purity (Huang et al. 1995).
Total biomass production in terms of number of shoots and leaves per treatment was compared for different PGR combinations (Fig. 2) and demonstrated that including a low concentration of TDZ in the medium increased the total biomass production by encouraging axillary bud formation, which is consistent with the finding that TDZ prompts axillary proliferation, callus formation, shoot organogenesis, and somatic embryogenesis in many tissue-cultured species (Huetteman and Preece 1993; Morini et al. 2000; Yancheva et al. 2003; Sharma et al. 2017). The average of total biomass production in each category of treatments was increased two- to three-fold when Phytagel™ solidified medium supplemented with TDZ with other PGRs were used. The superior effect of Phytagel™ over agar-agar on in vitro shoot multiplication and growth has been reported in Chlorophytum borivilianum (Kumar et al. 2010).
Effect of the PGRs and medium composition on regenerated shoots
Regenerated shoots were cultured on MS medium containing different PGRs, and their growth responses were noted (Table 4). The number of shoot buds were two- to three-fold higher upon the addition of 2.22 μM BAP to the medium (Table 4). In vitro flowering was observed when GA3 was added to the medium (Fig. 3a, b). Huh et al. (2017) reported similar effects of GA3 and BAP in Passiflora edulis Sims. When cultured on 2.22 μM BAP, shoots produced a higher number of axillary buds, whereas on 0.9 μM TDZ-containing medium, shoots were hyper hydrated/vitrified and produced adventitious shoots (Table 4; Fig. 3f, g).
Comparative effects of different combinations of plant growth regulators on Mollugo nudicaulis Lam. shoot and leaf production. Dark box, total number of shoots per treatment. Light box, total number of leaves per treatment. Medium was solidified with agar-agar unless otherwise indicated. BK, 6-benzylaminopurine (BAP) + kinetin (Kin); BKD, BAP + Kin + 2,4-dicholorophenoxyacetic acid (2,4-D); BKN, BAP + Kin + naphthaleneacetic acid (NAA); BKNP, BAP + Kin + NAA + Phytagel™ (solidifying agent); BKNT, BAP + Kin + NAA + thidiazuron (TDZ); and BKNTP, BAP + Kin + NAA + TDZ + Phytagel™. Whiskers as box bar = Lowest and highest observed value, center line in box = median.
In order to analyze the effect of MS medium strength, the control shoots were cultured on MS basal and half-strength MS medium without any PGR, and they started drying after 1 wk of culture and showed no significant growth (Table 4; Fig. 3c). Addition of BAP, GA3, or TDZ to medium increased the survivability and growth of the in vitro raised plantlets.
In vitro rooting
Rooting was induced in the regenerated plantlets using medium containing various combinations of auxins with 0.2% (w/v) AC. In vitro rooting was only obtained after a pulse treatment of 490 μM IBA was applied to shoot basal sections for 2 to 3 min. Two weeks after the IBA treatment, root development could be observed (Fig. 3d, e). The effectiveness of the synthetic auxin IBA for ex and in vitro rooting might be due to its preferential transportation in the acropetal direction by plant cells and its ability to activate the successively interacting genes necessary for rooting (Ludwig-Müller 2000; Rufai et al. 2016; Sharma et al. 2017). However, the best response was observed on both lower concentrations of 2,4-D and half-strength MS medium in terms of number and size of roots (Table 5). When 0.45 μM 2,4-D was incorporated into MS basal medium, in vitro rooting was established within 2 wk, whereas on pulse treated half-strength hormone free (HF) MS medium roots were induced after more than 3 wk. In vitro rooted plantlets were transferred to the greenhouse with successful hardening and the plantlets completed their life cycle after seed formation within 1 to 2 mo (based on observation); this was probably due to the plant’s annual habit (Fig. 3h).
In the present study of this C3–C4 intermediate species, it was observed that once in vitro shoots regenerated and formed plantlets in M. nudicaulis, they utilized the available resources as fast as they could as evidenced by rapid production of in vitro flowering and seed setting in 3 to 4 wk in in vitro conditions, compared to the ex vitro grown plants of M. nudicaulis that flowered and set seed in 4 to 6 wk after emergence of seedlings.
Leaf anatomy
In physiological adaptations, leaf anatomy is a key indicator for the plant’s photosynthetic type and its capability to thrive in a specific environment (Terashima et al. 2011; Tholen et al. 2012). Determination of gross leaf anatomy under the light microscope allowed the identification of the anatomical adaptations at an initial level regarding photosynthetic tissue differentiation and organization in leaves.
Under the microscope, the leaf anatomy of natural habitat plants showed more compact tissue organization, compared to aeroponically grown and in vitro grown leaves (Fig 4). In naturally grown leaves, this study revealed the distinct differentiation of mesophyll tissues into two-layered palisade cells and compact spongy cells with abundant inclusion of chloroplasts (leaves were dark green; Fig. 4d), as generally found in C3 plants under direct sunlight (Stata et al. 2014). Furthermore, a clear Kranz anatomy (concentrically arranged bundle sheath cells and mesophyll cells around a vascular bundle) indicative of C4 plants, as reported by Kennedy et al. (1980) in Mollugo spp., was less evident in these leaves. In contrast, the transverse sections of aeroponically grown leaves (Fig. 4c) showed the presence of single-layered palisade cells, loose spongy mesophyll cells, and the presence of a distinct Kranz morphology with a single layer of bundle sheath cells, including centripetally arranged chloroplasts (green color accumulated toward center) around the vascular bundle. These leaves were a light-green color, likely due to growing in a controlled greenhouse environment.
Depiction of responses of in vitro raised Mollugo nudicaulis Lam. plantlets. (a) Effects of gibberellic acid (GA3): adventitious bud activation and in vitro flowering. (b) Combined effect of 6-benzylaminopurine (BAP) and GA3: typical leaf shape and thickness, in vitro flowering, and seed formation. (c) Effect of Murashige and Skoog (MS; Murashige and Skoog 1962) strength medium on shoot growth. (d) Rooted shoots on MS medium containing 0.45 μM 2,4-dicholorophenoxyacetic acid after indole-3-butyric acid (IBA) pulse treatment. (e) Rooting on half strength MS with activated charcoal after IBA pulse treatment. (f) Direct shoot formation on 0.91 μM thidiazuron and Phytagel™. (g) Growth on MS supplemented with 2.22 μM BAP. (h) Plantlets under hardening process. Scale bars = 5 mm.
Comparison of leaf anatomy of free hand transverse sections of (a, b) in vitro, (c) aeroponically grown, and (d) mature ex vitro leaf of Mollugo nudicaulis Lam. observed under light microscope at 50× (a) and 100× magnification (inset, b, c and d). Inset shows enlarge view of a vascular bundle of in vitro leaf section. (a) In vitro leaf at 50× showed loosely arranged mesophyll cells with single layer of palisade (PMS), but bundle sheath (BS; arrow, inset), and (b) with centripetal arranged chloroplast was present around a vascular bundle, whereas (c) the aeroponic leaf section showed distinct single layer of palisade mesophyll cells (PMC; arrow), compact spongy mesophyll cells (SMC; arrow), and clear bundle sheath (BS; arrow) around vascular bundle (VB; arrow) with centripetal chloroplasts; by comparison, (d) the natural habitat leaf was dark green in color and showed two layers of palisade cells (PMC; branched arrow), compact spongy mesophyll cells (SMC; arrow), and bundle sheath (BS; arrow) with chloroplasts compactly surrounded by mesophyll cells. 50× = 5 × 10 and 100× = 10 × 10 (power of objective × ocular lens).
An in vitro environment consists of constant temperature, low photosynthetic photon flux density, large diurnal fluctuation in CO2 concentration, high relative humidity level, high concentrations of sugar, salts, PGRs, and nutrients in the medium, and the absence of microorganisms (Xiao et al. 2011; Kaur 2015), and thus often leads to low rates of transpiration, photosynthesis, and water, nutrient, and CO2 uptake, all of which can result in poor growth and physiological adaptations in the in vitro-grown plant (Xiao et al. 2011). In the present study, microscopic examination of transverse handmade sections of M. nudicaulis from in vitro conditions (Fig. 4a, b) revealed similar findings as less compact and less differentiate mesophyll cells (present as uncleared spongy mesophyll and single layer of palisade cells), an underdeveloped cuticle, and overall, the leaves were a very delicate and lighter green, compared to other two leaf types observed. In previous studies, Hazarika (2006) reviewed that the poor development of photosynthetic apparatus in in vitro leaves may be due to the continuous exogenous supply of high sucrose and salt in media and poor light conditions. However, in M. nudicaulis, the presence of bundle sheath cells with chloroplast arranged centripetally around the vascular bundles (Kranz system) was observed in the in vitro-derived leaves (Fig. 4a, b) which is indicative of C3-C4 intermediacy.
The presence of all types of photosynthetic tissues (palisade and spongy mesophyll cells, and Kranz system with dense chloroplasts in bundle sheath cells) in a leaf is considered as characteristic for C3–C4 plant intermediates at the anatomical level (Kennedy et al. 1980). The bundle sheath cells typically demark the inner compartment of cells where the enzyme Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is localized and high levels of CO2 are found (Sage et al. 2014; Stata et al. 2014). Thus, in this comparison, leaves from both the aeroponically and in vitro-grown plants showed characteristics closer to C4 species compared to ex vitro-grown M. nudicaulis. However, an empirical experiment will be needed to observe an effect on physiological adaptations in aeroponically and in vitro-grown plants compared to naturally grown C3 or C4 plants. This present study is first to report the comparison of M. nudicaulis in vitro and ex vitro (both, native habitat and aeroponic) derived leaves as demonstrating anatomical evidence for this C3–C4 intermediate species.
Conclusions
For the first time, the in vitro growth responses and simple comparative leaf anatomy in the C3–C4 intermediate medicinal plant M. nudicaulis were studied. From the results, it can be concluded that the hypocotyl was best for callus induction in M. nudicaulis and that callus induction required a high concentration of auxin. At the leaf anatomy level, in vitro- and aeroponically grown M. nudicaulis plants showed increased intermediate characteristics, compared to plants grown in their natural habitat. This tissue culture protocol could be used for year-round availability of the plant, as well as allowing for comparative analysis of the functional anatomy of the aeroponic- and in vitro-grown plants of other C3 and C4 species.
References
Bhandari MM (1990) Flora of the Indian desert. MPS Repros, Jodhpur, pp 159–160
Chaâbani G, Tabart J, Kevers C, Dommes J, Khan MI, Zaoui S, Chebchoub L, Lachaâl M, Karray BN (2015) Effects of 2,4-Dichlorophenoxyacetic acid combined to 6-Benzylaminopurine on callus induction, total phenolic and ascorbic acid production, and antioxidant activities in leaf tissue cultures of Crataegus azarolus L. var. Aronia. Acta Physiol Plant 37:16
Christin PA, Osborne CP (2014) The evolutionary ecology of C4 plants. New Phytol 204:765–781
Christin PA, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF (2011) Complex evolutionary transitions and the significance of C3–C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65:643–660
Compton ME, Mize CW (1999) Statistical considerations for in vitro research: I- birth of an idea to collecting data. In Vitro Cell Dev Biol-Plant 35:115–121
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Gomez KA, Gomez AA (1984) Single factor experiments. In: Statistical analysis procedure of agricultural research. John Wiley and sons, New York, pp 7–29
Gowik U, Westhoff P (2011) The path from C3 to C4 photosynthesis. Plant Physiol 155:56–63
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Huang LC, Kohashi C, Vangundy R, Murashige T (1995) Effects of common components on hardness of culture media prepared with gelrite™. In Vitro Cell Dev Biol-Plant 31:84–89
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Huh YS, Lee JK, Nam SY (2017) Effect of plant growth regulators and antioxidants on in vitro plant regeneration and callus induction from leaf explants of purple passion fruit (Passiflora edulis Sims). J Plant Biotech 44:335–342
Ignacimuthu S, Ayyanar M, Sankarasivaraman K (2008) Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia 79:562–568
Jauhar PP (2006) Modern biotechnology as an integral supplement to conventional plant breeding: the prospects and challenges. Crop Sci 46:1841–1859
Kaur RP (2015) Photoautotrophic micropropagation an emerging new vista in micropropagation- a review. Agri Rev 36:198–207
Kennedy RA, Eastburn JL, Jensen KG (1980) C3-C4 photosynthesis in the genus Mollugo: structure, physiology and evolution of intermediate characteristics. Am J Bot 67:1207–1217
Khan EU, Wang FXJ, Fan QJ, Huang XS, Zhang GN, Shi J, Liu JH (2009) Regeneration and characterization of plants derived from leaf in vitro culture of two sweet orange (Citrus sinensis (L.) Osbeck) cultivars. Sci Hortic 120:70–76
Kumar A, Aggarwal D, Gupta P, Reddy MS (2010) Factors affecting in vitro propagation and field establishment of Chlorophytum borivilianum. Biol Plant 54:601–606
Ludwig-Müller J (2000) Indole-3-butyric acid in plant growth and development. Plant Growth Regul 32:219–230
Mehandru P, Shekhawat NS, Rai MK, Kataria V, Gehlot HS (2014) Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica–three threatened medicinal asclepiads. Physiol Mol Biol Plants 20:365–373
Mendiburu F (2016) Agricolae: statistical procedures for agricultural research. R package version 1:2–4 https://CRAN.R-project.org/package=agricolae
Mohlakola EM, Cheng C, Lin Y, Guo R, Min KT, Chen Y, Lai Z (2017) Effects of 2, 4-Dichlorophenoxy acetic acid and light on growth of Gerbera (Gerbera jamesonii cv. Daxueju) callus. J Agr Sci Tech 18:385–393
Morini S, D'onofrio C, Bellocchi G, Fisichella M (2000) Effect of 2, 4-D and light quality on callus production and differentiation from in vitro cultured quince leaves. Plant Cell Tissue Organ Cult 63:47–55
Mosaleeyanon K, Chan-Um S, Kirmane C (2004) Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci Hortic 103:51–63
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nagesh KS, Shanthamma C (2011) Micropropagation and antioxidant activity of Mollugo nudicaulis lam. J Med Plants Res 5:895–902
Nicholson SE (2011) Dryland climatology. Cambridge University Press, Cambridge
Patel AK, Lodha D, Ram K, Shekhawat S, Shekhawat NS (2016) Evaluation of physiochemical factors affecting high frequency plant regeneration of Blyttia spiralis (Forssk.) D.V. Field & J.R.I. wood [synonym: Pentatropis spiralis (Forssk.) Decne.], a threatened climber of medicinal values. In Vitro Cell Dev Biol-Plant 52:10–19
Rameshkumar A, Sivasudha T (2012) In vitro antioxidant and antibacterial activity of aqueous and methanolic extract of Mollugo nudicaulis lam. Leaves. Asian Pac J Trop Biomed 2:895–900
Rajamanikandan S, Sindhu T, Durgapriya D, Sophia D, Ragavendran P, Gopalakrishnan VK (2012) Protective effect of Mollugo nudicaulis lam. On acute liver injury induced by perchloroethylene in experimental rats. Asian Pac J Trop Med 5:862–867
Rathore MS, Rathore MS, Shekhawat NS (2013) Ex vivo implications of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight. & Arn.—an endangered plant. Environ Exper Bot 86:86–93
Rawsthorne S (1992) C3–C4 intermediate photosynthesis: linking physiology to gene expression. Plant J 2:267–274
Rufai S, Hanafi MM, Rafii MY, Mohidin H, Omar SRS (2016) Growth and development of Moringa (Moringa oleifera L.) stem cuttings as affected by diameter magnitude, growth media and indole-3-butyric acid. Ann For Res 59:209–218
Sage RF, Christin PA, Edwards EJ (2011) The C4 lineages of planet earth. J Exp Bot 62:3155–3169
Sage RF, Khoshravesh R, Sage TL (2014) From proto-Kranz to C4 Kranz: building the bridge to C4 photosynthesis. J Exp Bot 65:3341–3356
Schlüter U, Weber AP (2016) The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant Cell Physiol 57:881–889
Sharma U, Kataria V, Shekhawat NS (2017) In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa lam.: a leguminous tree with medicinal values. Physiol Mol Biol Plants 23:969–977
Stata M, Sage TL, Rennie TD, Khoshravesh R, Sultmanis S, Khaikin Y, Ludwig M, Sage RF (2014) Mesophyll cells of C4 plants have fewer chloroplasts than those of closely related C3 plants. Plant Cell Environ 37:2587–2600
Stobbe H, Schmitt U, Eckstein D, Dujesiefken D (2002) Developmental stages and fine structure of surface callus formed after debarking of living lime trees (Tilia sp.). Ann Bot 89:773–782
Sukhorukov AP, Kushunina M (2016) Taxonomic revision and distribution of herbaceous Paramollugo (Molluginaceae) in the eastern hemisphere. PhytoKeys 73:93–116
Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116
The International Plant Names Index (2012) http://ipni.org names: 363731–1. Cited on 15 Jan 2018
Thole V, Rawsthorne S (2003) Efficient regeneration systems for two closely related Moricandia species possessing a C3 or C3–C4 intermediate photosynthetic character. Plant Cell Rep 21:707–712
Tholen D, Boom C, Zhu XG (2012) Opinion: prospects for improving photosynthesis by altering leaf anatomy. Plant Sci 197:92–101
Thulin M, Moore AJ, El-Seedi H, Larsson A, Christin PA, Edwards EJ (2016) Phylogeny and generic delimitation in Molluginaceae, new pigment data in Caryophyllales, and the new family Corbichoniaceae. Taxon 65:775–793
Vincent MA (2003) Molluginaceae Rafinesque: Flora of North America Editorial Committee. Flora of North America, volume 4: Magnoliophyta: Caryophyllidae, Part 1, Oxford Univ. Press, Oxford, UK
Voznesenskaya EV, Koteyeva NK, Edwards GE, Ocampo G (2010) Revealing diversity in structural and biochemical forms of C4 photosynthesis and a C3–C4 intermediate in genus Portulaca L. (Portulacaceae). J Exp Bot 61:3647–3662
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158
Yancheva SD, Golubowicz S, Fisher E, Lev-Yadun S, Flaishman MA (2003) Auxin type and timing of application determine the activation of the developmental program during in vitro organogenesis in apple. Plant Sci 165:299–309
Ziv M, Altman A (2003) TISSUE CULTURE: General Principles. In: Thomas B, Murphy DJ, Murray BG (eds) Encyclopedia of applied plant sciences. Elsevier, Oxford, pp 1341–1353
Acknowledgments
Authors are thankful to the University Grant Commission (UGC), New Delhi, for providing Special Assistance Program (SAP) in the form of Centre of Advanced Study (CAS) to the Department of Botany, Jai Narain Vyas University, and Jodhpur.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Masaru Nakano
Rights and permissions
About this article
Cite this article
Barupal, M., Kataria, V. & Shekhawat, N.S. In vitro growth profile and comparative leaf anatomy of the C3–C4 intermediate plant Mollugo nudicaulis Lam.. In Vitro Cell.Dev.Biol.-Plant 54, 689–700 (2018). https://doi.org/10.1007/s11627-018-9945-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-018-9945-7