Abstract
The present study aimed to determine the foliar micro-morpho-anatomical features of in vitro cultured Gardenia jasminoides J. Ellis (Rubiaceae) to compare the effect of exogenous supplementation of growth regulators (cytokinins; 6-benzylaminopurine and Kinetin), in order to attenuate heterotrophic nutrition (in vitro) induced structural disorders in the proliferating shoots. Murashige and Skoog’s (MS) medium supplemented with 2.0 mg L−1 6-benzylaminopurine (BAP) in combination with 0.15 mg L−1 indole-3-acetic acid (IAA) was detected optimal for axillary bud induction. Nutrient medium containing 0.5 mg L−1 BAP and 0.25 mg L−1 IAA was found appropriate combination for proliferation of multiple shoots, and yielded 37.2 shoots (per explant) with 7.45 cm average length after 2nd subculture (8 weeks). Supplementation of NAA with cytokinins resulted in callus formation. The proliferation of shoots on kinetin (Kn) and IAA combination resulted in the formation of fragile shoots and the leaves with increased structural impairments like underdeveloped photosynthetic, vascular, and ground tissue systems, non-functional stomata, and reduced vein density. Comparatively, BAP and IAA treatment favoured healthy shoot proliferation and development of stable tissue systems with reduced structural abnormalities. Half strength MS medium augmented with 3.0 mg L−1 indole-3-butytric acid (IBA) was the optimal medium for root induction (32.0 roots per shoot with 3.5 cm in length). The regenerated plantlets showed 97% survival success during acclimatization and exhibited normal growth characteristics and morphology. The light microscopic evaluation of foliages provided technical support to in vitro regeneration techniques to understand the structural adaptational mechanism of in vitro raised plantlets, thereby substantially contributed in the reduction of the rate of mortality of regenerated plants of G. jasminoides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gardenia jasminoides J. Ellis belongs to the madder family Rubiaceae, and originally found in India, Vietnam, Southern China, Taiwan, Japan, Myanmar etc. (Khare 2007; Chen et al. 2008). It is commonly known as Anant and Gandharaj in India, Zhi Zi in China, and Cape jasmine in English. Gardenia jasminoides is an evergreen flowering plant and grown in gardens for its heavily fragrant flowers with glossy green foliage. The flowers are borne singly or in small clusters with white or yellow color; and are often strongly scented. The large berry fruits contain a sticky orange colored pulp (Keswick 2003; Chen et al. 2020). A yellow silk dye, ‘crocetin’ has been extracted from its flowers and fruits (Hong et al. 2015; Zhou et al. 2015) and used for dying of cloths, food items, and as an adulterant of Crocus sativus (Moras et al. 2018). This plant species has been recognized as an ancient medicinal herb and mentioned in the Indian, Chinese, and Korean pharmacopoeias (Phatak 2015). Traditionally, the fruits are used to treat inflammation, headache, oedema, fever, hepatic disorders, and hypertension (Koo et al. 2006). The topical application of plant extracts on dermatophagoides reduces the symptoms of atopic dermatitis and lowered the serum level of IgE (Sung et al. 2014). In modern system of medicines, the plant is used for the treatment of ankle sprain (Chen et al. 2009), osteoporosis, and melanogenesis (Kwak et al. 2013).
Cape jasmine is well known for its antiapoptotic, antiangiogenic, antiarthritis, antiatherosclerotic, anticancer, antidepressant, antihyperglycemic, anti-inflammatory, antioxidant, antiprotozoal, antithrombotic, antiviral, neuroprotective, retinoprotective activities, etc. and in the treatment of Alzheimer’s disease (Wang et al. 2012; Li et al. 2013; Qin et al. 2015). Apart from crocetin, crocin, geniposide, genipin, and geniposidic acid are the major phytoconstituents of G. jasminoides, which are responsible for wide spectrum pharmacological activities (Luo et al. 2014; Wu et al. 2014). Approximately, 19 patented reports (Chinese) on pharmacological, taxonomical, and cosmetic actions of G. jasminoides are available in literature (Phatak 2015).
The propagation of G. jasminoides is typically done using vegetative stem cuttings, which is a slow method and takes time to establish the plants in the field and to achieve flowering. Root-knot nematodes, premature flower wilting, and chlorosis even at mild stress are the other constraints. Moreover, vegetative propagation is not a reliable technique and it is labour intensive for cultivation of this plant (Kobayashi and Kaufman 2006). Therefore, successful micropropagation would be a suitable technique to satisfy the farmers, and pharmaceutical demands and to ensure supply of standard planting material within stipulate time.
Several studies were carried out to optimize the micropropagation system for G. jasminoides (Pontikis 1983; Dumanois et al. 1984; Scaramuzzi and D’Elia 1984; Economou and Spanoudaki 1985; Mizukami 1989; Al-Juboory et al. 1998; Serret and Trillas 2000; Serret et al. 2001; Chuenboonngarm et al. 2001; Duhoky and Rasheed 2009; Salim and Hamza 2017; Amer et al. 2019; Gaber and Barakat 2019; Kadhim et al. 2020; Ahmed et al. 2021). But, the rate of shoot proliferation was not effective and consequently, the negative impact of in vitro culture environments on shootlet’s morpho-anatomy and ultra-structures was rarely studied (Serret and Trillas 2000).
The optimization of various physiochemical factors to influence in vitro response in plants often displays certain adverse abnormalities in morphology and micro-morpho-anatomy of the regenerated plants (Isah 2015; Manokari et al. 2021a). The characteristic in vitro heterotrophic environmental parameters such as high relative humidity, lower irradiance, increased minerals and salts contents, plant growth regulators, readymade source of carbon, growth regulators, and accumulation of ethylene and other gases in culture vessel promote anatomical, physiological, and biochemical disorders in plants (Chirinea et al. 2012; Martins et al. 2018; Manokari et al. 2021b). The microscopic assessment of in vitro regenerated plantlets revealed the presence of underdeveloped cuticular wax deposition, undifferentiated and non-functional epidermal, hypodermal, mesophyll, ground tissues, fewer vascular elements, and altered stomatal complexes (Barupal et al. 2018; Jogam et al. 2020; Manokari et al. 2020, 2021a). These abnormalities led to the loss of plantlets during the rooting and acclimatization stages and hindered the success of in vitro propagation techniques (Bidabadi and Jain 2020).
The study on the influence of plant growth regulators (PGRs) on foliar micro-morpho-anatomical characters under in vitro conditions could help in the development of effective in vitro propagation system, as structural developments are directly related to the physiological and biochemical adaptations of the tissue culture raised plantlets. Hence, an effective regeneration system and a detailed microscopic analysis of in vitro leaves of G. jasminoides, with respect to different growth regulators (cytokinins; 6-benzylaminopurine and Kinetin) at multiplication stage of plant development have been investigated in this study.
Materials and methods
Plant material and disinfection treatments
The nodal segments from freshly emerged shoots were procured from phenotypically superior plants grown in the experimental garden of the institute. The explants (2–3 cm long) were disinfected using 0.5% (w/v) aqueous solution of systemic fungicide (Bavistin, BASF India Ltd., India) for 10 min. Afterwards, the explants were surface-sterilized with 0.1% (w/v) aqueous solution of mercuric chloride (HiMedia®, Mumbai, India) under aseptic conditions for 5 min and washed (6–7 times) with autoclaved distilled water prior to inoculation.
Optimization of growth regulators for in vitro morphogenesis and high-frequency shoot proliferation
The sterilized explants were inoculated aseptically onto MS medium (Murashige and Skoog 1962) gelled with 0.2% (w/v) plant-gel and fortified with various concentrations (1.0 – 3.0 mg L−1) of cytokinins namely 6-benzylaminopurine (BAP) and 6-furfurylaminopurine (Kinetin/ Kn) alone and combined with auxins [0.05–0.3 mg L−1 indole-3 acetic acid (IAA) or α-Naphthalene acetic acid (NAA)]. After 4 weeks of incubation, the shoots of standardized length (approx. 3.0 cm) were sub-cultured for proliferation on MS medium fortified with combinations of cytokinins (0.25–1.0 mg L−1 BAP or Kn) and auxins (0.1–0.5 mg L−1 IAA or NAA). The cultures were maintained under in vitro controlled conditions (24 ± 2 °C temperature, 16 h/8 h photoperiod, 35–40 μmol m−2 s−1 irradiance, and 50–60% relative humidity). These cultures were transferred to fresh medium after every 4 weeks of incubation and the data on response, shoot numbers, and shoot length were recorded.
Effect of plant growth regulators on foliar micro-morpho-anatomy
Micro-morpho-anatomical characterization of the leaves was performed with the samples collected from the optimized treatments (BAP + IAA and Kn + IAA derived shoots). The samples were randomly collected after 2nd subculture (8 weeks of growth), and fixed in formaldehyde, acetic acid, and ethanol (FAA solution—1:1:3, v/v) for 24 h (Johansen 1940). Paradermal and transverse sections of the leaves were made using a double-edge razor. The sections were cleared using 10% sodium hypochlorite (v/v) solution, stained with safranin, and assembled on slides using 75% (v/v) glycerine. The specimens were examined using bright field light microscope (Leica microscope, model number DM750, Leica Microsystems, Heidelberg, Germany) under suitable magnification and the structural descriptions were studied. Magnifications of the figures were indicated using scale-bars. The photomicrographs were used to study the comparative foliar micro-morpho-anatomical differentiations.
Rooting and acclimatization of plantlets
The shoots proliferated on optimized medium were selected and cultured on half strength of MS medium supplemented with various concentrations (1.0–5.0 mg L−1) of indole-3 butyric acid (IBA) or NAA. After 4 weeks, the comparative rooting efficacy was noted and the plantlets were shifted to sterilized soilrite® (KelPerlite, Bangalore, India), and moistened with quarter strength of MS macro-salts solution twice a day for 4–5 weeks under the greenhouse conditions. The well hardened plantlets were subsequently shifted to nursery polybags containing garden soil and soilrite® (1:1, w/w), and maintained under shadenet, finally the completely acclimatized plantlets were exposed for field trials.
Experimental design and data analysis
The experiments were performed in a completely randomized design consisted of 20 replications for each treatment and repeated thrice. The results obtained were subjected to analysis of variance (ANOVA) and the significance of differences among mean values was compared using Duncan multiple range test at P < 0.05. All the statistical analyses were performed using the SPSS Statistics for Windows, version 17.0 (SPSS Inc., Chicago, USA).
Results and discussion
Establishment of cultures
The type and dosage of plant growth regulators (PGRs) control the in vitro morphogenetic responses in plants. In this study, axillary bud break occurred after 2 weeks of inoculation, and significant differences were observed in the percentage of induction response and shoot numbers among the treatments applied (Table 1). To optimize the best effective concentration and type of PGRs on shoot-bud induction, node explants were placed on the MS medium containing different concentrations of cytokinins (BAP/Kn) alone or together with auxins (IAA/NAA). The highest average shoot establishment (98%) occurred on 2.0 mg L−1 BAP combined with 0.15 mg L−1 IAA and resulted in the formation of 6.0 shoots with 4.6 cm average length (Fig. 1a). The lowest bud break percentage (65%) was observed with Kn and NAA combination (Table 1). The higher concentrations of PGRs caused stunted growth of shoots during culture establishment experiments. Earlier studies on in vitro propagation of G. Jasminoides found that B5 medium supplemented with 10.0 mg L−1 BAP was better than 2iP for shoot induction (Chuenboonngarm et al. 2001). Kadhim et al. (2020) induced shoots from the nodes on MS medium containing 1.0 mg L−1 BA with adenine sulphate. Salim and Hamza (2017) regenerated shoots on MS medium supplemented with 3.0 mg L−1 TDZ and 0.3 mg L−1 IAA. The cytokinins (BAP/ Kn) treatment with IAA was more effective than NAA, and this was in agreement with Duhoky and Rasheed (2009) as they attained maximum of 1.6 shoots per explant on MS medium supplemented with 2.0 mg L−1 BA and 0.4 mg L−1 IAA. 6-benzylaminopurine metabolism has been reported to possess positive interaction with plant systems as it induces cell division and improves developmental metabolism for effective shoot organogenesis (Auer et al. 1992; Glocke et al. 2006).
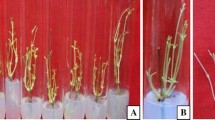
In vitro propagation of G. jasminoides via axillary bud break. a Shoot bud induction from node explants on MS medium containing 2.0 mg L−1 BAP and 0.15 mg L−1 IAA. b Shoots proliferated using 0.5 mg L−1 Kn and 0.25 mg L−1 IAA after 2nd subculture. c Shoots proliferated on 0.5 mg L−1 BAP and 0.25 mg L−1 IAA showing improved quantitative and qualitative morphological traits. d Shoot rooted in vitro on half strength MS medium supplemented with 3.0 mg L−1 IBA. e Hardening of rooted shoots using soilrite® under the greenhouse conditions. f Completely acclimatized plantlets of in vitro regenerated G. jasminoides in nursery polybags containing garden soil and soilrite®
Synergistic effect of cytokinins and auxins on shoot proliferation
The maximum shoot proliferation efficiency was achieved when the shoots were inoculated on cytokinins in combination with IAA. Incorporation of NAA did not seem to enhance the rate of shoot proliferation, instead promoted the development of callus, hence avoided. The highest proliferation rate (37.2 ± 0.30 shoots with 7.45 cm length per node after 8 weeks) was achieved on MS medium augmented with 0.5 mg L−1 BAP and 0.25 mg L−1 IAA (Table 2; Fig. 1b). This combination has also improved the number and size of foliage in cultures. On the contrary, a decreased rate of shoot multiplication was observed on MS medium containing 0.5 mg L−1 Kn and 0.25 mg L−1 IAA (25.9 ± 0.44 shoots with 6.0 cm average length). The shoots derived on Kn and IAA were weak and with reduced foliages (Fig. 1c).
The PGRs are the fundamental compounds that regulate essential biochemical and physiological metabolism to support plant developmental processes responsible for cell division, photomorphogenesis and proliferation of shoots in vitro (Aremu et al. 2014). Benzylaminopurine is the universally used cytokinin as it can metabolize immediately in plant tissues and enhance the rate of shoot regeneration and proliferation (Auer et al. 1992). However, differential responses were reported by several researchers on the types and optimal concentrations of growth regulators used with regard to shoot proliferation in G. jasminoides. According to Dumanois et al. (1984), 0.3 mg L−1 BAP with 1.0 mg L−1 IAA in the medium was effective in multiple shoot production. Economou and Spanoudaki (1985) reported maximum of 6.1 shoots using 10 mg L−1 2iP after 6 weeks of incubation. Mizukami (1989) produced 5 shoots per culture on 25 µM 2iP and 12.5 µM gibberellic acid, and induced callus for the production of secondary metabolites. A maximum number of 19.9 adventitious shoots were regenerated from the leaf derived callus using TDZ and IAA by Al-Juboory et al. (1998) and Chuenboonngarm et al. (2001) who were able to proliferate 7.3 shoots on 10 mg L−1 Benzyladenine after 120 days. The synergistic effect of cytokinin-auxin combination has been reported beneficial in amplification of shoots in G. jasminoides, but the shoot proliferation frequency was comparatively low in previous studies to be used for commercial propagation. El-Ashry et al. (2018) regenerated a maximum of 2.66 shoots per explant using 1.0 mg L−1 BA and 0.2 mg L−1 IAA. In contrast to the present findings, Duhoky and Rasheed (2009) reported that MS medium containing a combination of 2.0 mg L−1 BAP and 0.4 mg L−1 IAA could develop 1.6 shoots only, whereas 2.0 mg L−1 BAP alone regenerated 2.2 shoots (1.8 cm length). Nutrient medium with 5.0 mg L−1 BAP alone proliferated 17.67 shoots/explants (Nower and Hamza 2013). Salim and Hamza (2017) were able to induce 3.8 shoots with 3.28 cm length on MS medium containing 3.0 mg L−1 TDZ and 0.3 mg L−1 IAA. Amer et al. (2019) recorded a maximum of 4.25 shoots/explants using 1.0 mg L−1 BAP, and the addition of IAA in the medium increased shoot length. According to Gaber and Barakat (2019), 2.0 mg L−1 BA combined with 0.25 mg L−1 NAA could develop 4.0 shoots at proliferation stage. In a recent attempt by Ahmed et al. (2021), a combination of 2.0 mg L−1 BAP with 0.1 mg L−1 IBA yielded 4.8 shoots per explants after 8 weeks of culture. Chen et al. (2021) germinated seeds of G. jasminoides and the in vitro grown stem pieces were used for further multiplication of shoots. These used MS medium with 1.0 mg L−1 BAP and 0.1 mg L−1 NAA to produce 9.43 shoots with more amount of calli whereas, MS medium fortified with 0.5 mg L−1 BAP and 0.1 mg L−1 NAA induced 6.25 shoots with less calli. The present findings demonstrate many folds proliferation of shoots of G. jasminoides as compared to the existing reports.
Effect of PGRs on foliar micro-morpho-anatomical developments
The optimized growth regulators and physiochemical parameters implemented certain inherent micro-morpho-anatomical disorders in leaves under in vitro conditions and significant differences were observed in the foliages developed on BAP + IAA and Kn + IAA media combinations after 2nd subculture (incubated under same culture conditions).
Analysis of foliar anatomical characteristics
The leaves derived from Kn and IAA treatment composed of thin midrib, fragile lamina with two layered palisade, and 2–3 layered spongy parenchyma tissues (Fig. 2a). The midrib possessed thin walled epidermal cells, followed by 2–3 layered collenchymas tissues, and thin walled globular ground tissues (Fig. 2a–c). The vascular bundle was concave in arrangement with underdeveloped and few xylem elements facing adaxial surface and thin phloem ring around the xylem elements (Fig. 2b). Whereas, the leaves from BAP and IAA combination possessed thick walled epidermal cells, dense deposition of cuticle on lamina, well organized palisade and spongy parenchyma, ground tissues, and vascular elements (Fig. 2d–f). The transverse sections of leaf revealed thick midrib and lamina. The midrib consisted of thick walled epidermal cells, 4–5 layered collechymatous hypodermis, and several layered well developed parenchymatic ground tissues without intercellular spaces (Fig. 2d). The mesophyll was made of 3 layered palisade and 4–6 layered spongy parenchyma tissues (Fig. 2f). The vascular tissue system presented comparatively advanced structural features such as thick walled xylem and clear phloem tissues (Fig. 2e). More calcium oxalate druses (Fig. 2d) and improved vascular tissues were detected in lateral vascular bundles of BAP and IAA derived leaves (Fig. 2f), whereas, few and underdeveloped druses and less number of vascular elements were observed on Kn and IAA derived leaves (Fig. 2c).
Effect of cytokinins and IAA on foliar anatomy in G. jasminoides at multiplication stage (ABS abaxial surface, ADS adaxial surface, black arrow stomata, CO collenchyma, CU cuticle (red arrow), EP epidermis, GT ground tissue, LVB lateral vascular bundle, MX metaxylem, PH phloem, PL palisade parenchyma, PX protoxylem, SP spongy parenchyma, VB vascular bundle, (*) calcium oxalate druses). a Transverse section (T.S.) of the in vitro derived leaf on Kn and IAA containing medium representing underdeveloped structural features. b T.S. of midrib showing underdeveloped vascular elements and ground tissues. c Cross section of the lamina cultured in Kn and IAA medium showing reduced mesophyll tissues. d T.S. of leaf derived from BAP and IAA combination with comparatively improved structural parameters. e Effect of BAP and IAA on the development of collenchymas, ground tissues, and vascular elements in midrib portion. f T.S. of lamina with increased density of mesophyll tissues
Comparative evaluation of stomatal apparatus
Leaves of G. jasminoides were amphistomatic, characterized by paracytic stomata. The comparative study of the structure, development, and distribution of stomatal apparatus from the leaves derived from BAP + IAA and Kn + IAA revealed that the foliar developments in both treatments have undergone significant changes. The leaves developed on Kn and IAA combination possessed undifferentiated stomata with higher stomatal index (53.0 ± 1.70) and number of contiguous stomata (Fig. 3a). The stomata of BAP and IAA treatment were responsive to culture environment, uniform in size, and distributed with reduced stomatal density and index (22.8 ± 1.13). The contiguous stomata were rarely detected in these leaf samples (Fig. 3b).
Stomatal and vein density of G. jasminoides as in vitro response of different hormonal treatments (CS contiguous stomata, EP epidermal cells, PS paracytic stomata, VI vein-islets, VT veinlet terminations). a Impact of Kn and IAA on increased stomatal density and structural abnormalities in leaves. b Underdeveloped vein density of leaves derived from Kn and IAA medium. c Structural stability and stomatal density of leaves developed on Kn and IAA containing medium. d Increased venation pattern and vein density of leaves derived from BAP and IAA medium
Influence of growth regulators on vein architecture
The foliages of G. jasminoides possessed reticulate venation with vein-islets and veinlet terminations. The combinations of growth regulators considerably affected the second and third order veins development, but did not affect the distribution pattern of the first order of veins. The number of vein-islets and veinlet termination formation were few in the leaves developed from Kn and IAA treatment as there were more spaces between the veins (Fig. 3b). Whereas, BAP and IAA derived leaves possessed relatively organized order of veins, reduced spacing, resulted in the increased vein density (Fig. 3d). In general, vein-islets were rectangular shaped and the veinlet terminations were simple. The higher vein-islet density (23.6) and veinlet terminations (12.0) were observed with the leaves derived from BAP and IAA, whereas, Kn and IAA derived leaves possessed comparatively reduced vein-islets (14.0) and veinlet terminations (5.0).
Though the heterotrophic culture conditions implemented common in vitro induced structural aberrations, the leaves of shoots grown in BAP + IAA containing medium found to posses superior features which can withstand rooting and acclimatization stress factors than the Kn and IAA derived leaves. The micro-morpho-anatomical studies of the foliar apparatus of BAP + IAA and Kn + IAA derived shoots revealed developmental changes in stomatal complex, vein architecture and internal anatomy, which took place as the leaves developed in different hormonal conditions. These changes in structure are responsible for establishing proper physiological and biochemical metabolisms during shoot development (Shekhawat and Manokari 2017; Revathi et al. 2018; Rajput et al. 2020).
Besides culture proliferation, the type of growth regulators may also affect the structural development of in vitro cultured plants (Eburneo et al. 2017; Martins et al. 2018). The combination of benzyladenine and IAA stimulate axillary growth by promoting increased upward movement of nutrients (Black and Osborne 1965). The abnormal stomatal functionality and higher density of stomata on cytokinin enriched medium in vitro has been well discussed in various reports (Martins et al. 2014; Shekhawat and Manokari 2016; Pereira et al. 2016; Wafa et al. 2016).
Taken together, the results suggest that shoots proliferated using BAP and IAA combination showed the most promising structural stability. This could be of pivotal importance to optimize the appropriate growth regulators for effective plantlet production with functional photosynthetic organs.
Rooting of proliferated shoots
A good number of roots were successfully induced from the cut ends of the in vitro produced shoots using various concentrations of IBA, IAA and NAA in almost all the previous tissue culture/micropropagation studies in G. jasminoides (Pontikis 1983; Dumanois et al. 1984; Scaramuzzi and D’Elia 1984; Economou and Spanoudaki 1985; Mizukami 1989; Al-Juboori et al. 1998; Serret and Trillas 2000; Serret et al. 2001; Chuenboonngarm et al. 2001; Duhoky and Rasheed 2009; Salim and Hamza 2017; Amer et al. 2019; Gaber and Barakat 2019; Kadhim et al. 2020; Ahmed et al. 2021). In the present study, roots were induced from the shoots on all types and concentrations of auxins tested, but significant differences in the mean number and length were observed (Table 3). In general, NAA and IBA play crucial roles in root induction and development (Cui et al. 2019; Kannan et al. 2021; Manokari et al. 2021b). Half strength MS medium augmented with 3.0 mg L−1 IBA resulted in 100% rooting of shoots, and 32.0 average number of roots with 3.5 cm length of roots after 4 weeks of incubation (Fig. 1d). In contrast, implementation of NAA (3.0 mg L−1) had a low rate (about 83%) of induction of adventitious roots from shoots (24.8 roots). These results were distinctly corroborated with the optimal auxin type for effective rooting in other woody plants of the family Rubiaceae such as Morinda citrifolia and M. coreia (Shekhawat et al. 2015a, b). Whereas, a maximum of 20.66 roots/shoot was reported by El-Ashry et al. (2018) on 2.0 mg L−1 each of IBA and NAA in G. jasminoides. Maintaining proper levels of auxins and the establishment of an auxin gradient in the tissues is essential to establish root patterning and meristem formation (Iyer-Pascuzzi and Benfey 2009). Indole-3 butyric acid holds significant role in various aspects of root development, including regulation of root apical meristem size, root hair elongation, lateral root development, and formation of adventitious roots (Frick and Strader 2018). Although, IBA may be predominantly transported in the form of conjugates, the uptake of IBA molecule itself is a saturable process (Rashotte et al. 2003).
Hardening and acclimatization of regenerated plantlets
The rooted shoots were carefully washed with water and transplanted into paper cups containing sterile soilrite® and moistened with quarter strength of MS macro-salts solution, and maintained in the greenhouse for 5 weeks (Fig. 1e). One of the usual problems of plant propagation using in vitro methods is the acclimatization to the ex vitro conditions, which adds to a higher mortality of plantlets caused by non-functional and under developed structural features (Isah 2015; Shekhawat and Manokari 2016). Thus, the evaluation of foliar micro-mopho-anatomy assists in understanding of structural developments in essential foliar constants (Moyo et al. 2015; Shekhawat and Manokari 2018). The results of hardening showed significant differences in the acclimatization of plantlets among the tested concentrations and combinations of growth regulator types (data not shown). The hardened plantlets were shifted to the perforated nursery polybags filled with garden soil and soilrite® (1:1, w/w) for 5 weeks in the greenhouse (Fig. 1F) and finally transferred to the field, where about 97% survival success was noted after 3 months. The field transferred plantlets showed no evident variations with respect to growth characteristics of the donor plant.
Conclusion
The present study reported an effective mechanism for plant regeneration in G. jasminoides via direct organogenesis using the nodal meristems. The effect of growth regulators on in vitro induced structural aberrations were determined using conventional light microscopy. The foliar micro-morpho-anatomy based selection of growth regulators for plant regeneration technique could be employed for large-scale propagation and successful field survival of plants, thereby making a valuable contribution to germplasm conservation and for further research in genetic transformation for production of valuable bioactive compounds from Cape jasmine.
Data availability statement
Data sharing not applicable to this article as no datasets were generated during the current study.
References
Ahmed ZS, Salim AM, Allawi AK (2021) Effect of growth regulators (BA, IBA) on micropropagation of Gardenia jasminoides Ellis. in vitro. Int J Agric Stat Sci 17:181–186
Al-Juboory KH, Skirvin RM, Williams DJ (1998) Callus induction and adventitious shoot regeneration of gardenia (Gardenia jasminoides Ellis) leaf explants. Sci Hortic 72:171–178
Amer EM, Fetouh MI, Rasha E-S, El-Laban HM (2019) Micropropagation and acclimatization of Gardenia jasminoides Ellis. J Biol Chem Envion Sci 14:107–120
Aremu AO, Plačková L, Bairu W, Novák O, Plíhalová L, Doležal K, Finnie JF, Van Staden J (2014) How does exogenously applied cytokinin type affect growth and endogenous cytokinins in micropropagated Merwilla plumbea? Plant Cell Tissue Organ Cult 118:245–256
Auer CA, Laloue M, Cohen JD et al (1992) Uptake and metabolism of benzyladenine during shoot organogenesis in Petunia leaf explants. Plant Growth Regul 11:105–114
Barupal M, Kataria V, Shekhawat NS (2018) In vitro growth profile and comparative leaf anatomy of the C3–C4 intermediate plant Mollugo nudicaulis Lam. In Vitro Cell Dev Biol Plant 54:689–700
Bidabadi SS, Jain SM (2020) Cellular, molecular and physiological aspects of in vitro plant regeneration. Plants 9:702
Black MK, Osborne DJ (1965) Polarity of transport of benzyladenine, adenine and indole-3-acetic acid in petiole segments of Phaseolus vulgaris. Plant Physiol 40:676–680
Chen Q, Youn UJ, Min B, Bae KH (2008) Pyronane monoterpenoids from the fruit of Gardenia jasminoides. J Nat Prod 71(6):995–999
Chen QC, Zhang WY, Youn U, Kim H, Lee I, Jung HJ, Na M, Min B, Bae K (2009) Iridoid glycosides from Gardeniae fructus for treatment of ankle sprain. Phytochemistry 70(6):779–784
Chen L, Li M, Yang Z, Tao W, Tian P, Li X, Wang W (2020) Gardenia jasminoides Ellis: ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J Ethnopharmacol. https://doi.org/10.1016/j.jep.2020.112829
Chen B, Zhang J, Wu J, Li J, Fan H (2021) Optimizing the propagation techniques for Gardenia jasminoides Ellis. Pak J Bot. https://doi.org/10.30848/PJB2023-2(29)
Chirinea CF, Pasqual M, de Araujo AG, Pereira AR, de Castro EM (2012) Acclimatization and leaf anatomy of micropropagated fig plantlets. Rev Bras Fruticult 34:1180–1188
Chuenboonngarm N, Charoonsote S, Bhamarapravi S (2001) Effect of BA and 2iP on shoot proliferation and somaclonal variation of Gardenia jasminoides Ellis in vitro culture. Sci Asta 27:137–214
Cui Y, Deng Y, Zheng K, Hu X, Zhu M, Deng X, Xi R (2019) An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci Rep 9:9634
Duhoky MMS, Rasheed KA (2009) Micropropagation of Gardenia Gardenia jasminoides by using single nodes. Mesopotamia J Agric 37:1–12
Dumanois C, Godin B, Bigot C (1984) Multiplication végétative in vitro de Gardenia jasminoïdes Ellis: in vitro vegetative multiplication of Gardenia jasminoïdes Ellis. J Plant Physiol 116(5):389–407. https://doi.org/10.1016/S0176-1617(84)80131-5
Eburneo L, Ribeiro-Júnior NG, Karsburg IV, Rossi AAB, Silva IV (2017) Anatomy and micromorphometric analysis of leaf Catasetum × apolloi Benelli & Grill with addition of potassium silicate under different light sources. Braz J Biol 77:140–149
Economou AS, Spanoudaki MJ (1985) In vitro propagation of gardenia. Gortscience 20(2):213
El-Ashry AAE, Gabr AMM, Girgis ND, El-Bahr MK (2018) Influence of silver nitrate on enhancing in vitro rooting of Gardenia jasminoides Ellis. J Environ Sci Technol 11(5):238–245
Frick EM, Strader LC (2018) Roles for IBA-derived auxin in plant development. J Exp Bot 69(2):169–177
Gaber MK, Barakat AA (2019) Micropropagation and somatic embryogenesis induction of Gardenia jasminoides plants. Alex Sci Exch J 40:190–201
Glocke P, Collins G, Sedgley M (2006) 6-Benzylamino purine stimulates in vitro shoot organogenesis in Eucalyptus erythronema, E. stricklandii and their interspecific hybrids. Sci Hortic 109:339–344
Hong IK, Jeon H, Lee SB (2015) Extraction of natural dye from Gardenia and chromaticity analysis according to chi parameter. J Ind Eng Chem 24:326–332
Isah T (2015) Adjustments to in vitro culture conditions 367 and associated anomalies in plants. Acta Biol Crac Ser Bot 57(2):9–28
Iyer-Pascuzzi AS, Benfey PN (2009) Transcriptional networks in root cell fate specification. Biochim Biophys Acta 1789:315–325
Jogam P, Sandhya D, Shekhawat MS, Alok A, Manokari M, Abbagani S, Allini VR (2020) Genetic stability analysis using DNA barcoding and molecular markers and foliar micro-morphological analysis of in vitro regenerated and in vivo grown plants of Artemisia vulgaris L. Ind Crops Prod 151:112476
Johansen DA (1940) Plant microtechnique, 1st edn. McGraw Hill Book Co., New York, pp 182–197
Kadhim ZK, Nayyef MN, Awadh HAA, Jaafar HM, Abdulhussein MAA (2020) Impact of plant growth regulators and adenine sulphate on Gardenia jasminoides micropropagation. Plant Arch 20:71–75
Kannan N, Manokari M, Shekhawat MS (2021) Induction of adventitious roots from leaf explants of Morinda coreia Buch. and ham.: an important dye yielding plant. Plant Cell Tissue Organ Cult. https://doi.org/10.1007/s11240-021-02016-3
Keswick M (2003) The Chinese garden, 2nd edn. Frances Lincoln, London, p 63
Khare C (2007) Gardenia jasminoides Ellis. In: Khare C (ed) Indian medicinal plants. Springer, New York. https://doi.org/10.1007/978-0-387-70638-2_671
Kobayashi KD, Kaufman AJ (2006) Common Gardenia. College of Tropical Agriculture and Human Resources (CTAHR). University of Hawaii at Manoa, Honolulu, pp 1–7
Koo HJ, Lim KH, Jung HJ, Park EH (2006) Anti-inflammatory evaluation of Gardenia extract geniposide and genipin. J Ethnopharmacol 103(3):496–500
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee MS, Rho MC, Oh J (2013) Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol Pharm Bull 36(11):1779–1786
Li HB, Yu Y, Wang ZZ, Dai Y, Gao H, Xiao W et al (2013) Iridoid and bis-iridoid gluco sides from the fruit of Gardenia jasminoides. Fitoterapia 88:7–11
Luo YJ, Zuo YM, Zhang ZL, Cai MT, Luo GM (2014) Study on chemical constituents of Gardenia jasminoides (III). J Chin Med Mat 37(7):1196–1199
Manokari M, Cokul Raj M, Priyadharshini S, Phulwaria M, Shekhawat MS (2020) Foliar micro-morphology—a promising tool to improve survival percentage of tissue culture raised plantlets with special reference to in vitro propagation of Vitex negundo L. Vegetos 33:504–515
Manokari M, Priyadharshini S, Shekhawat MS (2021a) Micro-structural stability of micropropagated Vitex negundo L. Microsc Microanal. https://doi.org/10.1017/S1431927621000283
Manokari M, Priyadharshini S, Shekhawat MS (2021b) Influence of physio-chemical factors on high throughput plant regeneration and micro-morpho-anatomy of shoots of Ormocarpum sennoides (Willd.) DC. Acta Physiol Plant. https://doi.org/10.1007/s11738-020-03180-3
Martins JPR, Schimildt ER, Alexandre RS, Castro EM, Nani TF, Pires MF, Pasqual M (2014) Direct organogenesis and leaf anatomy modifications in vitro of Neoregelia concentric (Vellozo) L.B. Smith (Bromeliaceae). Pak J Bot 46:2179–2187
Martins JPR, Santos ER, Rodrigues LCA, Gontijo ABPL, Falqueto AR (2018) Effects of 6-benzylaminopurine on photosystem II functionality and leaf anatomy of in vitro cultivated Aechmea blanchetiana. Biol Plant 62:793–800
Mizukami H (1989) Gardenia jasminoides Ellis: in vitro propagation and the formation of iridoid glucosides. In: Bajaj YPS (ed) Medicinal and aromatic plants II. Biotechnology in agriculture and forestry, vol 7. Springer, Berlin. https://doi.org/10.1007/978-3-642-73617-9_12
Moras B, Loffredo L, Rey S (2018) Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis). Food Chem 257:325–332
Mousa GT, Abdul-Hafeez EY, Ibrahim OHM (2016) Response of Gardenia plants grown under various growth media and ferrous sulfate application. Pak J Agric Sci 52:651–658
Moyo M, Aremu AO, Van Staden J (2015) Insights into the multifaceted application of microscopic techniques in plant tissue culture systems. Planta 242(4):773–790
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nower AA (2013) Hamza EM (2013) Production of Gardenia jasminoides scions via tissue culture for grafting on Gardenia thunbergia under greenhouse conditions. J Appl Sci Res 9(4):3118–3128
Pereira MP, Rodrigues LCA, Corrêa FF, Castro EM, Ribeiro VE, Pereira FJ (2016) Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 30:807–814
Phatak RS (2015) Phytochemistry, pharmacological activities and intellectual property landscape of Gardenia jasminoides Ellis: a review. Pharmacogn J 7:254–265
Pontikis CA (1983) In vitro propagation of Gardenia jasminoides. Plant Propag 29:13–14
Qin FM, Liu BL, Zhang Y, Zhou GX (2015) A new triterpenoid from the fruits of Gardenia jasminoides var. radicans Makino. Nat Prod Res 29(7):633–637
Rajput BS, Jani M, Ramesh K, Manokari M, Jogam P, Allini VR, Kher MM, Shekhawat MS (2020) Large-scale propagation of Bambusa balcooa Roxb.: an industrially important bamboo species. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2020.112905
Rashotte AM, Poupart J, Waddell CS, Muday GK (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133:761–772
Revathi J, Manokari M, Shekhawat MS (2018) Optimization of factors affecting in vitro regeneration, flowering, ex vitro rooting and foliar micromorphological studies of Oldenlandia corymbosa L.—a multipotent herb. Plant Cell Tissue Organ Cult 134(1):1–13
Salim SAA, Hamza SY (2017) An efficient protocol for micropropagation of Gardenia jasminoides Ellis. Biosci Biotechnol Res Asia 14:757–766
Scaramuzzi F, D’Elia C (1984) Clonal multiplication of Gardenia grandiflora Lour. C R Acad Sci Paris 299:657–662
Serret M, Trillas M (2000) Effects of light and sucrose levels on the anatomy, ultrastructure, and photosynthesis of Gardenia jasminoides Ellis leaflets cultured in vitro. Int J Plant Sci 161(2):281–289
Serret M, Trillas M, Araus J (2001) The effect of in vitro culture conditions on the pattern of photoinhibition during acclimation of Gardenia plantlets to ex vitro conditions. Photosynthetica 39:67–73
Shekhawat MS, Manokari M (2016) In vitro propagation, micromorphological studies and ex vitro rooting of cannon ball tree (Couroupita guianensis aubl.): a multipurpose threatened species. Physiol Mol Biol Plants 22(1):131–142
Shekhawat MS, Manokari M (2017) Comparative foliar micromorphological studies of in vitro and field transferred plants of Morinda citrifolia L. Acta Bot Hung 59(3–4):427–438
Shekhawat MS, Manokari M (2018) Micromorpho-anatomical evaluation of in vitro and field transferred plants of Coccinia indica Wight & Arn. Agric Res 7(2):135–144
Shekhawat MS, Kannan N, Manokari M (2015a) In vitro propagation of traditional medicinal and dye yielding plant Morinda coreia Buch.-Ham. S Afr J Bot 100:43–50
Shekhawat MS, Kannan N, Manokari M, Ravindran CP (2015b) Enhanced micropropagation protocol of Morinda citrifolia L. through nodal explants. J Appl Res Med Arom Plants 2(4):174–181
Sung YY, Lee AY, Kim HK (2014) The Gardenia 455 jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J Ethnopharmacol 156:33–40
Wafa SN, Taha RM, Mohajer S, Mahmad N, Abdul BAA (2016) Organogenesis and ultrastructural features of in vitro grown Canna indica L. BioMed Res Int. https://doi.org/10.1155/2016/2820454
Wang J, Lu J, Lv C, Xu T, Jia L (2012) Three new triterpenoid saponins from root of Gardenia jasminoides Ellis. Fitoterapia 83(8):1396–1401
Wu X, Zhou Y, Yin F, Mao C, Li L, Cai B, Lu T (2014) Quality control and producing areas differentiation of Gardeniae fructus for eight bioactive constituents by HPLCDAD465 ESI/MS. Phytomedicine 21(4):551–559
Zhou Y, Zhang J, Tang R, Zhang J (2015) Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Ind Crops Prod 64:224–232
Acknowledgements
The work was financially supported by the Researchers Supporting Project (RSP-2023R86), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
MM, MSS, and AD: conceptualization, investigation, methodology. MCR and MF: data compilation and hardening of the plants. MSS, MM, AA, and AAA: writing of original draft, statistics, and revision of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
This research did not involve experiments with human or animal participants.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manokari, M., Cokul Raj, M., Dey, A. et al. Micro-morpho-anatomical changes in leaf structure of plantlets during in vitro propagation (micropropagation) of Gardenia jasminoides J. Ellis. Vegetos 37, 107–116 (2024). https://doi.org/10.1007/s42535-023-00577-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-023-00577-6