Abstract
From a preliminary experiment on 98 Chinese soybean varieties, 12 varieties with somatic embryogenesis frequency ranging from 0.0% to 85.7% were selected for further study in order to enhance the efficiency of somatic embryogenesis and plant regeneration. The effects of different mannitol concentrations, abscisic acid (ABA) concentrations, and embryo explant ages (sizes) were investigated. Significant differences in somatic embryogenesis were found among the 12 soybean varieties, with initiation frequencies varying from 22.1% to 89.0% under suitable mannitol concentration, and with N25281, N25263, and N06499 having the highest somatic embryogenic capacity. The results showed that all three factors were relevant for raising rates of callus initiation and somatic embryogenesis, but with differential responses among the genotypes. The treatment of 3.0% (w/v) mannitol, 5 mg l−1 ABA, and a 4- to 5-mm-sized explant was found to be optimal for somatic embryogenesis, generating the highest explant-based regeneration rate at 83.0%. The greatest average number of plantlets regenerated per explant (1.35) was observed in N25281. The above results provide a basis for efficient regeneration of soybean and are informative for the development of genetic transformation systems in Chinese soybean germplasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated soybean [Glycine max (L.) Merr.] originated from wild soybean [Glycine soja Sieb. et Zucc.] in China and has been a major source of protein and oil nutrition for the Chinese people for several thousand years. The current cultivated acreage in China is approximately 9.68 million hectares with a total production of about 15 million tons annually (Ding 2006). Soybean oil, soymilk, tofu, bean curd, Sufu (Doufuru, Chinese cheese), soy sauce and other derivative products are used as cooking oil and food in daily Chinese life, just as butter, milk, and cheese are used in western countries (Liu 1999). During the past decade, modern oil and protein processing industries have developed rapidly in China, promoting the importation of up to 30.82 million tons of soybeans from North and South America in 2007 (Han et al. 2008). In addition, soybean has also become one of the major sources of edible oil and protein all around the world since the 1970s (Meng et al. 2007). The importance of the crop has led efforts to improve its production through conventional breeding and, more recently, through modern genetic engineering technologies (Amarasinghe and Yang 2005). The release and broad acceptance of herbicide-resistant soybean is a major example of the successful utilization of traditional breeding combined with genetic engineering (Trigo and Cap 2006).
Plant tissue culture systems for soybean have been developed through exploiting either shoot morphogenesis (Barwale et al. 1986; Wright et al. 1986, 1987) or somatic embryogenesis (Christianson et al. 1983; Ranch et al. 1985) as the route for plant regeneration. For shoot morphogenesis, both cotyledonary nodes (Barwale et al. 1986; Wright et al. 1986), and primary leaf tissue (Wright et al. 1987) have been used to obtain cultures that form shoots when placed on a medium-containing benzyladenine. In a protocol relying on somatic embryogenesis, the starting material has been the immature zygotic embryo, i.e., the intact zygotic embryo (Ranch et al. 1985), the excised embryo axis (Christianson et al. 1983), or the excised cotyledons (Lazzeri et al. 1985). Christianson et al. (1983) were the first to report regeneration of soybean. Since their first work on somatic embryogenesis, significant progress has been made, all of which indicates that the most important step in the regeneration process is the initiation of the somatic embryo (Santarem et al. 1997; Simmonds and Donaldson 2000; Meurer et al. 2001; Walker and Parrott 2001). Even though improvements have been made, regeneration capacity remains relatively low in comparison with other crops (Walker and Parrott 2001), such that soybean is still recognized as one of the recalcitrant crops for genetic engineering (Hofmann et al. 2004).
It is known that the efficiency of regeneration and transformation of soybean is genotype-dependent and remains effective mainly for the variety “Jack” and a few of other genotypes in the U.S. (Samoylov et al. 1998a,b, Walker and Parrott 2001, Schmidt et al. 2005). Some reports exist on screening for varieties with relatively efficient somatic embryogenesis which are relevant to Northeast China, the number one soybean-producing area (Wang et al. 2002), but no such capacity has been reported for soybean varieties relevant to the Lower and Middle Changjiang Valleys, which represent the third largest soybean producing area. Therefore, our goal was to develop somatic embryo initiation and plant regeneration systems for varieties relevant to the Changjiang region so that transgenic technologies can be developed for the integration of gene(s) imparting herbicide tolerance, disease and insect resistance, improved oil qualities, and abiotic stress tolerance into local germplasms. In a preliminary study, the role of sucrose, maltose, and mannitol as the carbohydrate source, the effect of dichlorophenoxyacetic acid (2, 4-D), thidiazuron (TDZ), and abscisic acid (ABA) as exogenous growth regulator, and the explant type, explant age, and culture temperature were evaluated. From this study, three major factors, the concentration of mannitol, the concentration of ABA, and the zygotic embryo explant age (size) were identified as significant and chosen for the further determination of their optimal level(s) and for the selection of the best treatment combinations for effective somatic embryo initiation and plant regeneration in Chinese soybean varieties.
Materials and Methods
Plant materials.
Seeds of all varieties used in the experiments were provided by the National Center for Soybean Improvement, Nanjing Agricultural University and sown at Jiangpu Experiment Station in Nanjing, China during 2005–2007. About 15 d after flowering, immature pods containing embryos with cotyledons of 4–5 mm in length and other sizes required for various experiments were collected, surface-sterilized in 3% NaClO solution containing two to three drops of Tween-20 for 20 min, and then rinsed five times with sterilized water. Immature cotyledon explants were excised and embryonic axes removed according to Lazzeri et al. (1985).
Basal medium and culture conditions.
Cotyledon halves were placed with the adaxial surface in contact with the inducing medium. All media were based on Murashige and Skoog (Murashige and Skoog 1962) basal salts, B5 vitamins (Gamborg et al. 1968), 40 mg l−1 2,4-D, and 1 g l−1 l-asparagine (MSD). Phytagel at 0.2% (w/v) was used as the solidifying agent. The pH was adjusted to 5.8 with 1 N HCl before autoclaving. Cultures were incubated under an 18-h photoperiod and a light intensity of 10 μmol photons m−2 s−1 provided by cool-white fluorescent lights and a temperature of 26 ± 1°C.
Preliminary experiment.
To concentrate our study on a limited number of varieties, 98 soybean varieties sampled from across China were screened for those with potential capacity for somatic embryogenesis. The explants of 4–5 mm in length of the tested varieties were incubated on the MSD basal medium supplemented with 3% (w/v) sucrose. The experiment was performed twice, and at least one hundred explants per replication per genotype were cultured.
Mannitol concentration and experimental design.
To evaluate the effect of the carbohydrate source on soybean somatic embryo initiation, explants of 4–5 mm in length of the 12 varieties screened out from the preliminary experiment were incubated on MSD basal medium supplemented with mannitol at five concentrations (0%, 1.5%, 3.0%, 4.5%, and 5.5%). Media lacking mannitol was supplemented with 3% (w/v) sucrose as a carbohydrate source. The experiment was performed twice (on two different dates), both in a randomized complete block design with two replications. Twenty cotyledon halves (explants) were placed in each 9-cm Petri dish containing 25 ml medium. For each treatment, at least five Petri dishes per replication per genotype were cultured.
ABA concentration and experimental design.
To determine the effect of ABA concentration, explants of 4–5 mm in length were incubated on the MSD medium supplemented with ABA at five different concentrations (0, 1, 5, 10, and 20 mg l−1). The three varieties with the best somatic embryogenic capacity from the above mannitol concentration experiment were used. The experiment was carried out in a randomized complete blocks design with three replications and the same plot setting as in the mannitol concentration test.
Explant age and endogenous ABA determination.
To investigate the effect of explant age, explants of <3, 4–5, 6–8, and >8 mm in length were collected in the field and incubated on the basal medium MSD40 [MS macro-salts, MS micro-salts, B5 vitamins, 40 mg l−1 2,4-D, 1 g l−1 l-asparagine, and 3% (w/v) sucrose]. The content of endogenous hormone (ABA) in the explants was determined by enzyme-linked immunosorbent assay (ELISA) according to Yang et al. (2001). The ELISA hormone kit was obtained from the Center of Crop Chemical Control, China Agricultural University. The same set of varieties as in the ABA concentration experiment was tested. The experiment was also carried out in a randomized complete block design with three replications and the same plot (Petri dish) settings as in the mannitol concentration test.
Data analysis.
The number of explants that produced callus and somatic embryos was recorded after 1 mo incubation in all the above experiments. The analysis of variance and significant test among means were carried out for the obtained data using PC-SAS, version 9.0.
Verification of the optimal combination of mannitol concentration, ABA concentration, and explant age in somatic embryogenesis and its utilization in plant regeneration.
To verify the utility and effectiveness of the optimal combination of the three factors (mannitol concentration, ABA concentration, and explant age) on somatic embryogenesis and plant regeneration of soybean, explants of 4–5 mm in length of three high responding varieties, N25281, N25263, and N06499, were incubated on MSD medium supplemented with 5 mg l−1 ABA and 3.0% (w/v) mannitol. Globular-stage embryo clusters were obtained after 1 mo, and the frequency of somatic embryo initiation was calculated.
These globular-stage embryos were used in a proliferation experiment to develop capacity for better development and plant regeneration from the somatic embryos. Some globular-stage embryo clusters were directly transferred to the differentiation medium MS0M6AC [MS basal salts, B5 vitamins, 6% maltose, 0.5% AC, 0.3% (w/v) phytagel], while others were cultured first on a solidified proliferation medium, MSD20 [MS macro salts, MS micro salts, B5 Vitamins, 20 mg l−1 2,4-D, 1 g l−1 l-asparagine, 3% (w/v) sucrose, and 0.2% (w/v) phytagel) for 1 mo before being transferred to MS0M6AC. The experiment was conducted in triplicate, with four to five Petri dishes for each treatment and with each Petri dish containing eight to ten globular-stage embryo clusters. After 3 wk on MSD20, the resulting cotyledon-stage embryos were transferred to MS0M6 (MS basal salts, B5 vitamins, 6% (w/v) maltose, 0.3% (w/v) phytagel) for maturation. After 1 mo on MS0M6, mature embryos were placed in a sealed dish with a small piece of MS0M6 medium to allow gradual desiccation of the embryos for 1 wk. Desiccated mature embryos were then transferred to fresh B5 medium (Gamborg et al. 1968) for plantlet initiation. Regenerated plantlets with well-established roots were washed carefully with tap water to remove gellan gum and transferred to pots containing moist vermiculite. Finally, the acclimatized plants were moved to the greenhouse for flowering and seed set.
Results and Discussion
In a preliminary experiment, 98 soybean varieties sampled from across China were tested for their capacity for somatic embryogenesis (Table 1). MS macro-salts, MS micro-salts, and B5 vitamins, supplemented with 40 mg l−1 2,4-D, 1 g l−1 l-asparagine and 3% (w/v) sucrose was used as the medium for this screening process. It was found that different varieties showed a significant variation in response to somatic embryogenesis when cultured on this medium. Indeed, the embryogenic responses varied from 0.0% in N06296 (Sichuan Province) to as high as 85.7% in N25281 (Jiangsu Province). From the tested materials, 12 varieties, which included both landraces and released cultivars, were chosen for the present experiments based on their agronomic traits, potential as parental materials in breeding programs, and relatively good performance with respect to somatic embryogenesis. These varieties are adapted to the Lower and Middle Changjiang River Valleys in China and thought to be potential agronomic targets for genetic transformation.
Effect of mannitol concentration.
Immature embryo explants from the 12 selected varieties were cultured on MSD medium supplemented with various concentrations of mannitol up to 5.5% (w/v). Figure 1 a,b illustrates that somatic embryogenesis occurred on the immature embryo explant and shows multiple somatic embryos forming from the same explant. Significant differences existed among varieties, mannitol concentrations, and variety by mannitol concentration interactions for both callus initiation and somatic embryo initiation (Tables 2 and 3). Inclusion of mannitol in the induction medium was detrimental to callus formation, with frequencies of callus induction from explants cultured on medium containing mannitol decreasing with increasing concentrations of this osmoticum. The highest frequency of callus induction averaged 97.0% across the 12 varieties studied when explants were cultured on MSD medium devoid of mannitol (Tables 2 and 3).
Embryogenesis and plant regeneration via somatic embryogenesis of soybean. (a) Somatic embryos (arrow) arising from the median section of the explant; (b) Somatic embryos (arrow) arising from the marginal section of the explant; (c) Globular somatic embryos proliferating on MSD20; (d) Cotyledon-stage embryos forming on MS0M6AC; (e) A single cotyledon-stage embryo cluster; (f) The rooting plantlet grown on B5 medium; (g) The regenerated plant grown in soil; (h) the flower bud (arrow) of a regenerated plant.
Conversely, inclusion of mannitol in the culture medium did have a beneficial effect on induction of somatic embryogenesis from callus tissues. In the majority of varieties, supplementation with 3.0% (w/v) mannitol increased the frequency of embryogenesis compared to the controls. Levels of this osmoticum above 3% were supraoptimal, leading to suppression of somatic embryogenesis. The results in Table 2 show that among the 12 varieties, N25281 performed the best, with a somatic embryo initiation frequency of 89.0% with 3.0% mannitol included in the MSD medium, N25263 the second most responsive at 82.0% in the presence of 1.5% mannitol, and N06227 the worst responsive at 22.1% under 3.0% mannitol concentration. In addition to increasing induction frequencies, inclusion of mannitol resulted in somatic embryos morphologically larger and stronger than those induced on the basal medium without this supplement (results not shown). Therefore, in spite of the negative relationship between callus initiation and somatic embryogenesis in the presence of mannitol, it is beneficial to include a moderate amount of mannitol (1.5–3.0% in the present study) as a compromise between callus initiation and the induction of somatic embryogenesis.
Samoylov et al. (1998a) reported that the carbohydrate source served both as a nutrient and as a source of osmotic pressure in soybean tissue culture. Thompson et al. (1986), on the other hand, considered mannitol to act as a non-plasmolyzing osmoticum with plant cells having a limited capacity to metabolize it. Here our data indicated that mannitol does perform as a carbohydrate source for the initiation of somatic embryos, since this was the only carbohydrate source included in the MSD medium. As high concentrations of mannitol were found to be detrimental to somatic embryogenesis, and since from a nutritional point of view excess carbohydrate availability should not be harmful, we postulate that the effect of mannitol on osmotic pressure in the embryo is likely the cause of the observed decrease in somatic embryogenesis.
All the somatic embryos obtained from the different mannitol concentrations in Table 2 were further cultured for plant regeneration. Of around 300 somatic embryo clusters (200 cultured with mannitol and 100 without), approximately 80 plants regenerated. The cluster-based regeneration rate of “with mannitol” was approximately 32.5% (65 out of 200) and that of “without mannitol” was 15.0% (15 out of 100), suggesting that mannitol promotes the development of somatic embryos with better capacity of plant regeneration due to its function on some morphological and physiological properties of the induced somatic embryos.
Effect of ABA concentration.
The role of exogenously applied ABA has been studied on somatic embryo initiation and development in several plant species (Tian and Brown 2000). According to the results in Table 2, N25281, N25263, and N06499 exhibited the best somatic embryogenesis frequencies and therefore were used in the ABA concentration experiment. The results show that there were significant differences among genotypes, ABA concentrations, and genotype by ABA concentration interactions in soybean (Tables 4 and 6). All three genotypes in Table 4 showed increasing formation of somatic embryos as the ABA concentration was increased to 5 mg l−1 (80.1–82.1%), while ABA concentrations at and above 10 mg l−1 suppressed embryo formation compared to that at 5 mg l−1. Our result is consistent with some of the studies on other plant species, such as Nakagawa et al. (2001) in melon and Gawronska et al. (2000) in cucumber.
Effect of explant age.
Cotyledons from immature zygotic embryos (3–4 mm in length) have been reported to be capable of somatic embryogenesis in soybean (Lazzeri et al. 1985; Finer 1988; Parrott et al. 1988). The three varieties N25281, N25263, and N06499, chosen for the results in the ABA concentration test, were also used in the explant age experiment. Here, four sizes of immature zygotic embryos from the three soybean varieties were used to investigate the effects of explant age. The results show that significant differences existed among genotypes, explant ages, and genotype by age interactions (Tables 5 and 6). Explants derived from immature zygotic embryos of <3 mm in length exhibited relatively low somatic embryo initiation on the basal medium. The highest frequency of somatic embryogenesis was obtained from the explants derived from immature embryos of 4–5 mm in length for the all three varieties (43.5% for N25281, 75.0% for N25263, and 76.2% for N06499), although not significantly different from 6–8 mm immature embryos. For explants of >8 mm in length, the frequency of somatic embryo initiation was the lowest, at an average of 1.3% over the three varieties (Table 5).
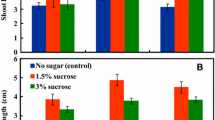
From the results of previous reports, the physiological condition and developmental stage of explants play an important role in somatic embryogenesis (Tae-Seok and Korban 2004). In the present study, the concentration of endogenous ABA in different sizes of explants was measured using ELISA. Figure 2 shows that there was a similar tendency among varieties: the endogenous ABA content increased along with the increase of explant size, with the >8 mm explant having the highest content, although the actual levels in different-sized explants varied between the varieties. Since the 4–5 mm explants provided the best somatic embryogenesis frequency, their endogenous ABA content might be at a physiologically optimal level for somatic embryogenesis. In relation to the result that the best exogenous ABA concentration was 5 mg l−1, it may be postulated that the endogenous ABA content in 4–5 mm explants and the exogenous ABA content of 5 mg l−1 might be a best combination, or in other words, the endogenous hormone might play its best role under a proper amount of exogenous hormone.
Verification of the optimal combination of treatment parameters in somatic embryogenesis and its utilization in plant regeneration.
The three varieties N25281, N25263, and N06499 were used to verify the effectiveness of the optimized treatment combination of the three factors [3.0% (w/v) mannitol, 5 mg l−1 ABA, and 4–5 mm explant] on somatic embryogenesis and plant regeneration of soybean. The mean frequencies of somatic embryo initiation for the three varieties were 86.8% for N25281, 82.1% for N25263, and 79.2% for N06499, which did demonstrate the effectiveness of the selected treatment combination on somatic embryogenesis.
In the plant regeneration experiment using three genotypes, Table 7 shows that globular-stage embryo clusters incubated first on MSD20 medium, when compared to those not incubated on MSD20, yielded higher germination frequencies (77.2–84.6% vs. 35.1–68.7%) and average numbers of plantlets per explant (1.21–1.35 vs. 0.86–0.93). The highest regeneration rate (83.0%) and highest average number of plantlets per explant (1.35) were observed on N25281 with incubation on solid MSD20 medium (Fig. 1 c). This frequency was of practical meaning for utilization of the best treatment combination in plant regeneration and was similar to that reported in soybean by Samoylov et al. (1998b). These authors showed that somatic embryos proliferated on solid MSD20 medium without liquid suspension resulted in superior conversion rates. To our understanding, the use of solidified MSD20 not only provides a means of proliferation of somatic embryos, but also for screening for potential somatic embryos. In our experiment, well-developed, cotyledon-stage embryos were obtained after histo-differentiation (Fig. 1 d and e). We noted from the observations in the plant regeneration process that the desiccation treatment with MS0M6 is possibly also important for plantlet initiation of the mature cotyledon-stage embryos.
After the plantlets reached a height of 10–15 cm, they were transferred to soil (Fig. 1 f). The regenerated plants rooted, flowered (Fig. 1 g and h) and matured, with the exception of only a few, which were weak and thus failed to reach maturity. The explant-based plant regeneration rate was about 76.2–83.0%, and varied somewhat among the varieties.
In conclusion, for raising the success rate in genetic transformation through somatic embryogenesis, the first step is to enhance the initiation rate of somatic embryogenesis. All three factors examined in this study—mannitol concentration, ABA concentration, and explant size—were relevant for raising initiation rates of callus and somatic embryogenesis, but with differential responses among the soybean genotypes. The three soybean varieties N25281, N25263, and N06499, all agronomically important to the Lower and Middle Changjiang Valleys, were found to have a high somatic embryogenic capacity and are therefore recommended in cultivar development through genetic engineering. The treatment parameters of 3.0% (w/v) mannitol, 5 mg l−1 ABA, and 4–5 mm explant represented the best treatment combination for somatic embryogenesis, with which the highest explant-based plant regeneration rate (83.0%) and mean number of plantlets per explant (1.35) were observed in N25281. The solidified proliferation process is important for somatic embryos to differentiate and mature, while a desiccation treatment of mature cotyledon-stage embryos is possibly also necessary for a high plantlet initiation frequency.
references
Amarasinghe A. A. Y.; Yang Y. S. Screening of high somatic embryogenic soybean varieties and innovation of culture methods. Journal of South China Agricultural University. 26: 84–88; 2005.
Barwale U. B.; Kerns H. R.; Widholm J. M. Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta. 167: 473–481; 1986. doi:10.1007/BF00391223.
Christianson M. L.; Warnick D. A.; Carlson P. S. A morphogenetically competent soybean suspension culture. Science. 222: 632–634; 1983. doi:10.1126/science.222.4624.632.
Ding S. J. Imperative need of vitalizing soybean industry of China. China Oils and Fats. 10: 7–13; 2006.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell. Res. 50: 151–158; 1968. doi:10.1016/0014-4827(68)90403-5.
Gawronska H.; Burza W.; Bolesta E.; Malepszy S. Zygotic and somatic embryos of cucumber (Cucumis sativus L.) substantially differ in their levels of abscisic acid. Plant Science. 157: 129–137; 2000. doi:10.1016/S0168-9452(00)00277-6.
Han T. F.; Hou W. S.; Wang J. M. Developing transgenic soybean to promote soybean industry in China. J. Agric. Sci. Technol. 10: 1–5; 2008.
Hofmann N.; Nelson R. L.; Korban S. S. Influence of medium components and pH on somatic embryo induction in three genotypes of soybean. Plant Cell, Tissue Organ Cult. 77: 157–163; 2004. doi:10.1023/B:TICU.0000016819.74630.59.
Lazzeri P. A.; Hildebrand D. F.; Collins G. B. A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant Mol. Biol. Rep. 3: 160–167; 1985. doi:10.1007/BF02886752.
Liu K. S. Soybeans: Chemistry, Technology and Utilization. Aspen, Gaithersbyrg, Maryland, 1999.
Meng Q.; Zhang C.; Gai J.; Yu D. Molecular cloning, sequence characterization and tissue-special expression of six NAC-like genes in soybean (Glycine max (L.) Merr.). J. Plant Physiol 164: 1002–1012; 2007. doi:10.1016/j.jplph.2006.05.019.
Meurer C. A.; Dinkins R. F.; Redmond C. T.; McAllister K. P.; Tucker D. T.; Walker D. R.; Parrott W. A.; Trick H. N.; Essig J.; Frantz H. M.; Finer J. J.; Collins G. B. Embryogenic response to multiple soybean [Glycine max (L.)Merr.] cultivars across three locations. In Vitro Cell Dev Biol. 37: 62–67; 2001. doi:10.1290/1071-2690(2001)037<0062:E>2.0.CO;2.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 15: 473–497; 1962. doi:10.1111/j.1399-3054.1962.tb08052.x.
Nakagaw H.; Saijyo T.; Yamauchi N. Effects of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L.) expanded cotyledon. Scientia Horticulturae. 90: 85–92; 2001. doi:10.1016/S0304-4238(00)00259-4.
Parrott W. A.; Dryden G.; Vogt S. Optimization of somatic embryogenesis and embryo germination in soybean. In Vitro Cell Dev Biol. 24: 817–820; 1988. doi:10.1007/BF02623653.
Ranch J. P.; Oglesby L.; Zielinski A. C. Plant regeneration from embryo-derived tissue cultures of soybean. In Vitro Cell Dev Biol. 21: 653–658; 1985. doi:10.1007/BF02623299.
Samoylov V. M.; Tucker D. M.; Parrott W. A. Soybean embryogenic cultures: the role of sucrose and nitrogen content on proliferation. In Vitro Cell Dev Biol. 34: 8–13; 1998a.
Samoylov V. M.; Tucker D. M.; Thibaud-Nissen F.; Parrott W. A. A liquid medium-based protocol for rapid regeneration from embryogenic soybean cultures. Plant Cell Rep. 18: 49–54; 1998b. doi:10.1007/s002990050530.
Santarem E. R.; Pelissier B.; Finer J. J. Effect of explant orientation, pH, solidifying agent and wounding on initiation of soybean somatic embryos. In Vitro Cell Dev. Biol. 33: 13–19; 1997.
Schmidt M. A.; Tucker D. M.; Cahoon E. B.; Parrott W. A. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep. 24: 383–391; 2005. doi:10.1007/s00299-005-0950-z.
Simmonds D. H.; Donaldson P. A. Genotype screening for proliferative embryogenesis and biolistic transformation of short season soybean genotypes. Plant Cell Rep. 19: 485–490; 2000. doi:10.1007/s002990050760.
Tae-Seok K. O.; Korban S. S. Enhancing the frequency of somatic embryogenesis following Agrobacterium-mediated transformation of immature cotyledons of soybean [Glycine max (L) Merrill]. In Vitro Cell Dev. Biol. 40: 552–558; 2004.
Thompson M. R.; Douglas T. J.; Obata-Sasamoto H. Mannitol metabolism in cultured plant cells. Physiol. Plant. 67: 365–369; 1986. doi:10.1111/j.1399-3054.1986.tb05749.x.
Tian L. N.; Brown D. C. W. Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Can. J. Plant Sci. 80: 721–276; 2000.
Trigo E. J.; Cap E. J. Ten Years of Genetically Modified Crops in Argentine Agriculture. ArgenBio, Buenos Aires, Argentina, 2006.
Walker D. R.; Parrott W. A. Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell, Tissue Organ Cult. 64: 55–62; 2001. doi:10.1023/A:1010601402098.
Wang P.; Wu Y.; Yang W.; Wang G. Somatic embryogenesis from immature cotyledons of soybean and analysis of correlative factors. Chinese Journal of Oil Crop Science. 24: 29–32; 2002.
Wright M. S.; Koehler S. M.; Hinchee M. A.; Carnes M. G. Plant regeneration by organogenesis in Glycine max. Plant Cell Rep. 5: 150–154; 1986. doi:10.1007/BF00269257.
Wright M. S.; Ward D. V.; Hinchee M. A.; Carnes M. G.; Kaufman R. J. Regeneration of soybean (Glycine max L. Merr.) from cultured primary leaf tissue. Plant Cell Rep 6: 83–89; 1987.
Yang Y. M.; Xu C. N.; Wang B. M.; Jia J. Z. Effect of plant growth regulations on secondary well thickening of cotton fibers. Plant Growth Regul. 35: 233–237; 2001. doi:10.1023/A:1014442015872.
Acknowledgements
The project was supported by the National Key Basic Research Program (2006CB101708, 2009CB118404), the National “863” Program (2006AA100104), the Natural Science Foundation of China (30671266), and the Ministry of Education 111 Project (B08025). The authors wish to express their sincere thanks to Drs. N. J. Taylor, Z. Y. Zhang, and B. Vanderbeld for their valuable help in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Nigel Taylor
Rights and permissions
About this article
Cite this article
Yang, C., Zhao, T., Yu, D. et al. Somatic embryogenesis and plant regeneration in Chinese soybean (Glycine max (L.) Merr.)—impacts of mannitol, abscisic acid, and explant age. In Vitro Cell.Dev.Biol.-Plant 45, 180–188 (2009). https://doi.org/10.1007/s11627-009-9205-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9205-y