Abstract
An efficient in vitro plant regeneration method through somatic embryogenesis has been established in Haworthia retusa. Somatic embryos were induced from leaf explants on Murashige and Skoog (MS) medium supplemented with different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and α-naphthaleneacetic acid (NAA) either alone or in combination with 4 μM thidiazuron (TDZ). Of the four auxins studied, IBA was found to be the most promising in terms of somatic embryo induction, followed in decreasing frequency by 2,4-D, IAA, and NAA. The highest somatic embryo induction (60.7%), with a mean of 20.7 embryos per leaf explant, was observed on MS medium amended with 20 μM IBA. The inclusion of 4 μM TDZ to the auxin-containing medium significantly (p < 0.0001) increased the somatic embryo induction frequency as well as the number of somatic embryos. The best combination for somatic embryogenesis was IBA + TDZ. The highest incidence of somatic embryo induction (100%), with a mean of 55.8 somatic embryos, was obtained on a culture medium containing 16 μM IBA + 4 μM TDZ. Somatic embryos germinated best on MS medium supplemented with 2 μM gibberellic acid. Morphological variations were observed among the regenerated plantlets. Well-developed plantlets obtained from germination media were acclimatized in the greenhouse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Haworthia (Asphodelaceae) includes approximately 151 species of desert succulents native to Southern Africa that are widely grown as ornamental plants. Haworthia retusa Duval is known as the star cactus and is endemic to Riversdale, Western Cape, South Africa. It is widely cultivated both indoors and outdoors for its attractive foliage and low maintenance. H. retusa is propagated through offshoots and seeds. In general, it is a tedious process to reproduce such slow-growing plants asexually because they produce only a few branches. On the other hand, H. retusa plants of the same genotype from the same location cannot be crossed to produce seeds because all Haworthia species are self-sterile (Rogers 1993a). Thus, an efficient alternative method for the large-scale propagation of H. retusa is required to meet the growing market demand for this species. In vitro propagation has become an attractive biotechnique for the mass production of various ornamentals (Hatzilazarou et al. 2017; Kim et al. 2017a; Wang et al. 2017), with the added advantage that plantlets can be produced on a large scale throughout the year within a short period.
In vitro plantlet regeneration methods for Haworthia species have been developed using different explants, such as gynoecia, inflorescences, flower buds, floral scapes, and leaves (Wessels et al. 1976; Ogihara and Tsunewaki 1978; Pandey et al. 1979; Beyl and Sharma 1983; Standifer et al. 1984; Sun et al. 1987; Rogers 1993a, b; Richwine et al. 1995; Mycock et al. 1997; Liu et al. 2017; Kim et al. 2017b). The composition of the growth medium and of the various plant growth regulators (PGRs) supplemented to the medium, as well as the levels of the PGRs, can influence the morphogenetic response (callus induction, shoot regeneration, or somatic embryogenesis) of explants from various Haworthia species. Kaul and Sabharwal (1972) reported that the addition of coconut milk was required for callus induction and shoot differentiation from inflorescence explants of Haworthia variegata, H. chloracantha, H. truncata x H. setata, H. atrofusca, H. angustifolia var. albensis, H. maughanii, H. turgida var. pallidifolia, and H. retusa cultured on White basal medium without or with benzyladenine (BA), kinetin (KN) + indole-3-acetic acid (IAA), and KN + α-naphthaleneacetic acid (NAA). However, Kaul and Sabharwal (1975) subsequently reported that the inclusion of inositol and a high concentration of nitrate are required for shoot differentiation from callus derived from inflorescence axes of Haworthia species cultured on White basal medium (. Wessels et al. (1976) reported callus initiation and subsequent shoot and root differentiation from leaf explants of Haworthia planifolia cultured on Linsmaier and Skoog medium containing 2.0 g l−1 polyvinylpyrrolidone and 4.0 mg l−1 kinetin.
Adventitious shoots have been reported to be best obtained from inflorescence or leaf explants of Haworthia species cultured on MS (Murashige and Skoog 1962) medium containing 1.0 mg l−1 KN (Rogers 1993a), 0.5 mg l−1 BA (Rogers 1993b), or 5.4 μM zeatin riboside (Richwine et al. 1995). In H. retusa, Liu et al. (2017) achieved the highest number of shoots (25.7) when the leaf-derived callus was cultured on MS medium fortified with 1.0 mg l−1 BA and 0.2 mg l−1 2,4-D, while Kim et al. (2017b) obtained the highest number of shoots (38.7) from leaf explants of H. retusa cultured on MS medium fortified with 4 μM thidiazuron [1-phenyl-3-(1,2,3,-thiadiazol-5-yl)urea (TDZ)] and 2 μM NAA. Somatic embryogenesis is considered to be an important technique for cryopreservation, mass clonal propagation, genetic manipulation, and synthetic seed production. However, only two published reports on somatic embryogenesis in Haworthia species are currently available (Beyl and Sharma 1983; Mycock et al. 1997). Beyl and Sharma (1983) induced the maximum number of somatic embryos from leaf explants of H. fasciata cultured on MS medium supplemented with 2.0 or 3.0 mg l−1 picloram + KN. However, the explants developed only a few embryos on medium containing 2,4-D + KN. In contrast, somatic embryos were significantly induced from leaf explants of H. limifolia cultured on MS medium supplemented with 2.0 mg l−1 2,4-D as compared to picloram (Mycock et al. 1997). Thus, the requirement of PGRs for somatic embryo induction in Haworthia varies according to species. To date, in vitro regeneration of H. retusa through somatic embryogenesis has not been documented. The aim of this study was to establish a consistent system for the regeneration of plantlets via somatic embryogenesis from H. retusa leaf explants.
Materials and methods
Leaves of H. retusa collected from 3-year-old greenhouse-grown plants were thoroughly washed under running water and rinsed with sterile distilled water. The leaves were surface sterilized with 70% (v/v) ethanol for 60 s, followed by 2.0% (v/v) sodium hypochlorite containing a few drops of Tween 20 for 10 min, then rinsed by five washes with sterile distilled water, and finally blotted dry using sterile filter paper to remove any traces of water. The cut ends were exposed to sterilants, and the tip of each leaf was removed with a surgical blade and cultured on MS medium supplemented with 0, 4, 8, 12, 16, or 20 μM 2,4-D, IAA, indole-3-butyric acid (IBA), and NAA either alone or in combination with 4 μM TDZ for somatic embryo induction. The experiment was conducted in triplicate with 25 explants included in each treatment. The frequency of somatic embryo induction and the number of somatic embryos were recorded after a culture period of 12 weeks. The somatic embryo induction percentage was calculated as the number of explants with embryos divided by the total number of explants cultured × 100.
Globular embryos (8 weeks old) obtained from the induction medium containing 16 μM IBA and 4 μM TDZ were cultured on MS medium supplemented with 0, 1, 2, 4, or 8 μM gibberellic acid (GA3) for maturation and germination. The experiment was conducted in triplicate, with 50 embryos included in each treatment. The frequency of embryo germination was recorded after a culture period of 5 weeks. Embryo germination percentage was calculated as the (number of germinated somatic embryos/total number of somatic embryos) × 100. Only embryos showing both elongation of the root and development of a green shoot were considered to have germinated. The globular embryos obtained from the induction medium containing 16 μM 2,4-D + 4 μM TDZ, 16 μM IAA + 4 μM TDZ, 16 μM IBA + 4 μM TDZ, and 16 μM NAA + 4 μM TDZ were cultured on MS medium supplemented with 2 μM GA3 for plantlet conversion. The experiment was conducted in triplicate, with 100 embryos included in each treatment. The embryo-derived plantlets were then transferred to MS basal medium devoid of PGRs for further growth and development. Morphological changes in regenerated plantlets were recorded after 8 weeks. Pale-green, red/green, and yellow-striped leaf explants were isolated from the embryo-derived plantlets cultured on MS medium containing 4 μM TDZ + 2 μM NAA for adventitious shoot induction. The experiment was conducted in triplicate, with 15 explants included in each treatment. The frequency of somaclonal variation (SV) was recorded after a culture period of 12 weeks. The well-developed 8-week-old plantlets (obtained from somatic embryos) were washed gently with tap water, dried under a low light intensity condition (photosynthetic photon flux density [PPFD] 15 μmol m−2 s−1) for 2 weeks, transplanted into plastic trays containing a layer (2 cm) of orchid stone (Dxnon, Japan) (bottom layer) and filled with a mixture of river sand, peat, and perlite (2:1:1), and maintained in the greenhouse under 60% shading at 22 ± 3 °C. The top of the growth medium was sprayed with water at 7-day intervals, and the plant survival rate was recorded after 6 weeks. For each treatment, 100 plantlets were used, and the experiment was repeated three times.
The medium consisted of MS nutrients and vitamins amended with 3% (w/v) sucrose and 0.8% (w/v) plant agar (Duchefa Biochemie, Haarlem, The Netherlands). Plant hormones (GA3 and TDZ) were filter sterilized and added to the autoclaved medium. Auxins were added to MS medium before pH adjustment (5.8) and sterilization (121 °C; 1.06 kg/cm2; 20 min). The cultures were incubated at 25 ± 2 °C under a 16-h photoperiod with light provided by cool white fluorescent lamps (40 W tubes; Philips N.V., Amsterdam, The Netherlands) at a PPFD of 45 μmol m−2 s−1. The experimental results were subjected to analysis of variance (ANOVA) using a SAS software program (Release 9.2; SAS Institute, Cary, NC, USA) and expressed as the mean ± standard error. The differences between the mean values were assessed by Duncan’s multiple range test, with significance set at p < 0.05. The percentage values were transformed using arcsine square root (√P) to normalize error distribution prior to ANOVA.
Results
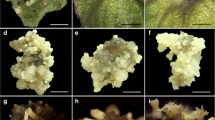
Leaf explants failed to produce somatic embryos on MS basal medium without PGR supplementation (control). Globular embryos were observed from the explants grown on medium containing auxin within 5 weeks of culture (Fig. 1a). Embryos at the scutellar and coleoptilar stages were observed after 12 weeks of culture (Fig. 1b, c). However, somatic embryos did not develop when leaf segments were cultured on media containing low concentrations of auxin (4 or 8 μM). The frequency of somatic embryo induction and number of embryos initiated per leaf explant were significantly (p < 0.0001) affected by auxin type and concentration and the interactions between auxins (Table 1). Leaf segments did not develop somatic embryos when cultured in MS medium supplemented with 2,4-D (4 μM), but somatic embyros were produced from leaf segments cultured in medium containing 8–20 μM 2,4-D. Both the frequency of somatic embryo induction and number of somatic embryos per leaf segment improved as the level of 2,4-D in MS medium increased from 8 to 16 μM, and then dropped with further increases in the 2,4-D level. The maximum frequency of somatic embryo induction (58.8%), with a mean of 12.8 embryos per leaf segment, was observed on MS medium containing 16 μM 2,4-D (Table 1). The explants did not develop somatic embryos when cultured on MS medium supplemented with 4 or 8 μM IAA, whereas somatic embryos were produced from explants cultured on MS medium containing high levels (12–20 μM) of IAA. The frequency of somatic embryo induction and the mean number of somatic embryos per leaf explant increased with increasing IAA level in the MS medium from 12 to 20 μM. The maximum frequency of somatic embryo induction (48.7%), with a mean of 7.2 embryos per explant, was observed on culture medium containing 20 μM IAA (Table 1).
Somatic embryogenesis in Haworthia retusa. a Induction of globular embryo on Murashige and Skoog (MS) medium supplemented with 16 μM indole-3-butyric acid (IBA) after 5 weeks, b induction of scutellar and coleoptilar embryos on MS medium supplemented with 16 μM IBA after 12 weeks, c formation of coleoptilar embryo on MS medium supplemented with 16 μM IBA after 12 weeks, d induction of somatic embryos on MS medium supplemented with with 16 μM IBA + 4 μM thidiazuron (TDZ) after 8 weeks, e–g different stages of embryo germination on MS medium supplemented with 2 μM gibberellic acid (GA3), h embryo-derived plantlets grown on MS medium devoid of plant growth regulators (PGRs) after 8 weeks. Scale bar: 2 cm
Leaf segments failed to produce somatic embryos when cultured on MS medium containing a low level of IBA (4 μM), but somatic embryos were produced when leaf segments were cultured on MS medium containing high concentrations of IBA (8–20 μM). The frequency of somatic embryo induction and the average number of somatic embryos per leaf explant improved as the level of IBA in MS medium increased from 8 to 20 μM. The highest frequency of somatic embryo induction (60.7%), with a mean of 20.7 embryos per explant, was observed on MS medium containing 20 μM IBA (Table 1). Leaf segments cultured on MS medium supplemented with a lower concentration of NAA (4 μM) did not develop somatic embryos, but somatic embryos were produced from leaf segments cultured in medium containing high concentrations of NAA (8–20 μM). The frequency of somatic embryo induction improved as the level of NAA in MS medium increased from 8 to 16 μM, and then dropped with a further increase in NAA level. However, a maximum of 9.3 somatic embryos per explant was observed on MS medium containing 12 μM NAA (Table 1). Based on these results, among the auxins studied, IBA was optimized for ideal induction of somatic embryos from leaf explants of H. retusa followed by 2,4-D, IAA, and NAA.
The addition of 4 μM TDZ to the culture medium in combination with auxin significantly improved the number of explants forming somatic embryos and the number of somatic embryos induced per leaf segment (Table 2). The inclusion of TDZ to MS medium containing lower concentrations of auxin also promoted somatic embryo induction. The frequency of somatic embryo induction ranged from 56.2 to 100% when the medium was fortified with 4–20 μM 2,4-D + 4 μM TDZ. The highest rate of somatic embryo induction (100%), with a mean of 46.5 somatic embryos per leaf explant, was obtained on a culture medium containing 16 μM 2,4-D + 4 μM TDZ. The frequency of somatic embryo induction ranged from 33.7 to 75.5% when the medium was fortified with 4–20 μM IAA + 4 μM TDZ. The highest rate of somatic embryo induction (75.5%) was observed on MS medium containing 20 μM IAA + 4 μM TDZ. However, a maximum of 23.7 somatic embryos per explant was obtained on MS medium containing 16 μM IAA + 4 μM TDZ (Table 2). The frequency of somatic embryo induction ranged from 27.3 to 100% when the medium was fortified with 4–20 μM IBA + 4 μM TDZ. The highest incidence of somatic embryo induction (100%), with a mean of 55.8 somatic embryos per leaf explant was obtained on a culture medium containing 16 μM IBA + 4 μM TDZ. The frequency of somatic embryo induction ranged from 33.8 to 98.4% when the medium was fortified with 4–20 μM NAA + 4 μM TDZ. The highest rate of somatic embryo induction (98.4%) was observed on MS medium containing 20 μM IAA + 4 μM TDZ. However, the maximum of 31.7 somatic embryos per leaf explant was obtained on MS medium containing 16 μM NAA + 4 μM TDZ (Table 2). Although 100% somatic embryo induction was achieved when both auxins (2,4-D and IBA) were supplemented to the culture medium, statistically different results were obtained when the production of somatic embryos/explant was considered, with 55.8 somatic embryos per explant obtained on IBA-supplemented medium and 46.5 somatic embryos per explant obtained on the 2,4-D-supplemented medium.
Somatic embryo maturation and instantaneous conversion to plantlets is a phase of somatic embryogenesis that depends solely on somatic embryo quality (Sivanesan et al. 2012). The globular embryos (Fig. 1d) obtained from the explants cultured on MS medium supplemented with 16 μM IBA + 4 μM TDZ were matured and germinated on MS basal medium without or with GA3 after 5 weeks of culture. The frequency of germination was 44.8% when the embryos were cultured on MS medium devoid of GA3. The addition of low levels of GA3 (1 and 2 μM) to the MS medium significantly enhanced the conversion of globular embryos (Fig. 1e–g); however, the germination of somatic embryos was strongly inhibited in culture medium containing a higher concentration of GA3 (8 μM), as compared with the control and other treatments. The maximum frequency of germination (87.8%) was observed on MS medium containing 2 μM GA3 (Fig. 2). The plantlets obtained from the germinated embryos were cultured on MS medium without GA3 for further growth and development for 8 weeks (Fig. 1h).
A few pale-green, red/green, and variegated (yellow-striped) plantlets were observed among the converted plantlets (Fig. 3a–c). The incidence of these variegated plantlets was significantly affected by the type of auxin supplemented to the culture medium. The highest frequency of SV was observed when the somatic embryos were obtained from medium containing 16 μM 2,4-D + 4 μM TDZ, followed in order of decreasing frequency of SV by 16 μM IAA + 4 μM TDZ, 16 μM IBA + 4 μM TDZ, and 16 μM NAA + 4 μM TDZ (Fig. 4). Of the three variants, more pale-green plantlets were produced than variegated yellow-striped and red/green plantlets. The variants were cultured on PGR-free MS medium for further growth and development. Leaf explants isolated from each variant developed adventitious shoots on MS medium supplemented with 4 μM TDZ + 2 μM NAA (shoot induction medium). Green (63.2%), pale-yellow (23.2%), and variegated yellow-striped (13.7%) adventitious shoots were obtained when the pale-green leaf explants were cultured on shoot induction medium (Fig. 3d). Red/green leaf explants cultured on shoot induction medium developed green, pale-yellow, yellow-striped, green-striped, and purple-striped variegated shoots (Fig. 3e) at a shoot induction frequency of 53.3, 28.3, 11.5, 3.7, and 3.2%, respectively (Fig. 5). Green, pale-green, and variegated yellow-striped adventitious shoots were obtained when the yellow-striped variegated leaf explants were cultured on shoot induction medium (Fig. 3f), and the frequency of shoot induction was 28, 31.2, and 40.8%, respectively (Fig. 5). The variants were separated and subjected to multiplication for further study. Well-developed plantlets obtained from the germination medium were acclimatized in the greenhouse; the survival rate was 100% (Fig. 6).
Morphological variation of shoots obtained from somatic embryos and leaf explants of H. retusa. a–c Pale-green (a), red/green (b), and variegated (yellow-striped) (c) plantlets developed from somatic embryos. d Induction of green and pale-yellow shoots from pale-green leaf explants cultured on MS medium supplemented with 4 μM TDZ + 2 μM α-naphthaleneacetic acid (NAA), e induction of green, yellow, and variegated shoots from red/green leaf explants cultured on MS medium supplemented with 4 μM TDZ + 2 μM NAA, f induction of green, pale-yellow, and variegated shoots from yellow-striped leaf explants cultured on MS medium supplemented with 4 μM TDZ + 2 μM NAA. Scale bar: 2 cm
Influence of auxins on somaclonal variation in H. retusa. Bars represent the mean, whiskers represent the standard error (SE). Different lowercase letters (a–c) above bars indicate a significant difference in mean somaclonal variation frequency according to DMRT at p < 0.05. 2,4-D 2,4-Dichlorophenoxyacetic acid, IAA indole-3-acetic acid
Morphological variation of shoots obtained from pale-green, red/green, and variegated yellow-striped leaf explants of H. retusa. Bars represent the mean, whiskers represent the SE. Different lowercase letters (a–d) above bars indicate a significant difference in mean somaclonal variation frequency according to DMRT at p < 0.05
Discussion
Auxin supplementation is necessary for the induction of somatic embryos from leaf segments of H. retusa (Table 1). Auxins are a powerful plant hormone that is indispensable for the initiation of somatic embryo development in most plants, including Haworthia species (Beyl and Sharma 1983; Mycock et al. 1997). However, the ability of different types of auxins and their optimal concentration to induce somatic embryogenesis can vary among plants. In Haworthia species, MS medium supplemented with 2,4-D (H. limifolia) or picloram (H. fasciata) was reported to be the best culture medium for somatic embryo induction. Specifically, Beyl and Sharma (1983) reported that in culture media supplemented with 2.0 mg l−1 picloram, 87% of the lower half of leaf explants of H. fasciata developed a mean of 11 somatic embryos, while Mycock et al. (1997) reported that the addition of 2.0 mg l−1 2,4-D to the MS medium resulted in the induction of somatic embryos in 73.3% of leaf segments of H. limifolia. In the present study, although all tested auxins (2,4-D, IAA, IBA, and NAA) produced somatic embryos from the leaf explants of H. retusa, the best somatic embryo induction (60.7%) and a large number of somatic embryos per leaf explant (20.7) were obtained on MS medium supplemented with 20 μM IBA. The induction of somatic embryos in other monocots, such as Amorphophallus konjac, Anthurium andraeanum, Curcuma longa, Curcuma manga, Iris sibirica, Lachenalia viridiflora, Malaxis densiflora, and Urochloa species, has been achieved on culture medium containing 2,4-D, IAA, IBA, NAA, 2,3,5-triiodobenzoic acid (TIBA), dicamba, or picloram (Pinheiro et al. 2014; Stanišić et al. 2015; Takamori et al. 2015; Kumar et al. 2016; Mahendran and Narmatha Bai 2016; Pikulthong et al. 2016; Zhong et al. 2017).
Cytokinins are often included in auxin-containing culture media for enhancing somatic embryo induction in several monocots. Beyl and Sharma (1983) observed that a combination of 2.0 mg l−1 picloram and 0.25 mg l−1 KN significantly improve the number of somatic embryos (22) produced per leaf segment in H. fasciata. Similarly, somatic embryos were successfully induced on medium containing picloram and KN in H. koelmaniorum (Mycock et al. 1997). However, information on the influence of TDZ on somatic embryogenesis of Haworthia species is still not available. TDZ is more efficient than other cytokinins for inducing somatic embryos in various monocots, such as Anoectochilus elatus (Sherif et al. 2018), Colocasia esculenta (Verma 2017), Crocus sativus (Devi et al. 2014), Lachenalia viridiflora (Kumar et al. 2016), and Malaxis densiflora (Mahendran and Narmatha Bai 2016). Various combinations, such as 2,4-D + TDZ (Mahendran and Narmatha Bai 2016; Verma 2017), NAA + TDZ (Sherif et al. 2018), and picloram + TDZ (Devi et al. 2014; Kumar et al. 2016), have been found to enhance somatic embryogenesis. In this study, the addition of TDZ auxin-containing media significantly improved somatic embryo induction in H. retusa. The best combination of PGRs that induced somatic embryogenesis in our study was IBA + TDZ (Table 2). Similar results have been reported in Zingiber officinale by Lincy et al. (2009) who observed a maximum of somatic embryos directly induced from 30% of stem segments cultured on medium containing 1.0 mg l−1 IBA + 0.5 mg l−1 TDZ.
Although a limited number of globular embryos matured into the scutellar and coleoptilar stages in the induction medium (Fig. 1a–c), plantlet conversion was not observed. Similarly, in H. koelmaniorum, Mycock et al. (1997) reported that somatic embryos induced on the medium containing picloram + KN failed to convert into plantlets. These authors also reported that the somatic embryoids formed in the presence of abscisic acid (ABA) developed into plantlets on regeneration medium. As the presence of PGRs (auxin and cytokinin) in the culture medium often inhibit the development of somatic embryos, somatic embryos formed in vitro are often transferred to PGR-free medium for maturation and germination. The inclusion of ABA, activated charcoal, GA3, polyethylene glycol, and sugar alcohol into the culture medium enhances somatic embryo maturation and germination. In the present study, the supplementation of GA3 to the MS basal medium significantly improved the germination of somatic embryos. It is well known that GA3 stimulates the formation and conversion of somatic embryos, and the positive effect of GA3 on embryo maturation and germination has been reported in a number of plant species, such as Colocasia esculenta (Verma 2017), Crocus vernus (Sivanesan et al. 2012), Curcuma longa (Raju et al. 2015), and gladiolus (Mujib et al. 2017).
Phenotypic changes were observed among the embryo-derived plantlets (Fig. 3). Explant source, genotype, composition of the growth medium, PGR supplementation, culture age, and culture environment can influence the occurrence of SV in plant tissue culture (Bairu et al. 2011; Sivanesan and Jeong 2012). SV is frequently associated with gene mutations, chromosome rearrangements, and changes in DNA sequence (Bairu et al. 2011). Ogihara (1981) observed SV in Haworthia setata plantlets developed from 2-year-old callus cultures, noting that SV in this species is mainly due to chromosome variation. SV has also been observed in somatic embryo-derived plantlets of banana (Moradi et al. 2017), jewel orchid (Sherif et al. 2018), Metabriggsia ovalifolia (Ouyang et al. 2016), and olive (Bradaï et al. 2016). In the present study, the occurrence of SV may have been due to the presence of both auxin and TDZ in the culture medium. Higher concentrations of auxin and TDZ disturb the cell cycle and DNA synthesis (Stanišić et al. 2015; Ouyang et al. 2016). More studies, such as biochemical, cytological, and molecular analyses of phenotypic variants in H. retusa, are required for a better understanding of the phenomena underlying the obtained results. Although the incidence of SV in embryo-derived plantlets of H. retusa was very low, the resulting variants can be utilized for new cultivar development.
Conclusion
For the first time, direct somatic embryogenesis was demonstrated in Haworthia. The auxin IBA proved to be the best auxin for the induction of somatic embryos from leaf segments of H. retusa. The inclusion of TDZ to the auxin-containing medium enhanced somatic embryo induction. The high frequency of somatic embryo induction, plantlet conversion, and less variation among the regenerated plantlets suggest that this protocol can be used for the mass propagation and improvement of this ornamental plant species.
References
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173. https://doi.org/10.1007/s10725-010-9554-x
Beyl CA, Sharma GC (1983) Picloram induced somatic embryogenesis in Gasteria and Haworthia. Plant Cell Tissue Organ Cult 2:23–132. https://doi.org/10.1007/BF00043357
Bradaï F, Pliego-Alfaro F, Sánchez-Romero C (2016) Somaclonal variation in olive (Olea europaea L.) plants regenerated via somatic embryogenesis: influence of genotype and culture age on phenotypic stability. Sci Hortic 213:208–215. https://doi.org/10.1016/j.scienta.2016.10.031
Devi K, Sharma M, Ahuja PS (2014) Direct somatic embryogenesis with high frequency plantlet regeneration and successive cormlet production in saffron (Crocus sativus L.). S Afr J Bot 93:207–216. https://doi.org/10.1016/j.sajb.2014.04.006
Hatzilazarou S, Kostas S, Economou A, Scaltsoyiannes A (2017) Efficient propagation of Nerium oleander L. through tissue culture. Propag Ornam Plants 17:64–74
Kaul K, Sabharwal PS (1972) Morphogenetic studies on Haworthia: establishment of tissue culture and control of differentiation. Am J Bot 59:377–385. https://doi.org/10.2307/2441548
Kaul K, Sabharwal PS (1975) Morphogenetic studies on Haworthia: effects of inositol on growth and differentiation. Am J Bot 62:655–659. https://doi.org/10.1002/j.1537-2197.1975.tb14098.x
Kim DH, Kang KW, Sivanesan I (2017a) In vitro propagation of Cymbidium hybrid. Propag Ornam Plants 17:48–54
Kim DH, Kang KW, Sivanesan I (2017b) Micropropagation of Haworthia retusa Duval. Propag Ornam Plants 17:77–82
Kumar V, Moyo M, Van Staden J (2016) Enhancing plant regeneration of Lachenalia viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci Hortic 211:263–268. https://doi.org/10.1016/j.scienta.2016.08.024
Lincy KA, Remashree AB, Sasikumar B (2009) Indirect and direct somatic embryogenesis from aerial stem explants of ginger (Zingiber officinale Rosc.). Acta Bot Croat 68:93–103
Liu B, Fang H, Meng C, Chen M, Chai Q, Zhang K, Liu S (2017) Establishment of a rapid and efficient micropropagation system for succulent plant Haworthia turgida haw. HortScience 52:1278–1282. https://doi.org/10.21273/HORTSCI12056-17
Mahendran G, Narmatha Bai V (2016) Direct somatic embryogenesis of Malaxis densiflora (A. Rich.) Kuntze. J Genet Eng Biotechnol 14:77–81. https://doi.org/10.1016/j.jgeb.2015.11.003
Moradi Z, Farahani F, Sheidai M, Satari TN (2017) Somaclonal variation in banana (Musa acuminate colla cv. Valery) regenerated plantlets from somatic embryogenesis: histological and cytogenetic approaches. Caryologia 70:1–6. https://doi.org/10.1080/00087114.2016.1198665
Mujib A, Ali M, Tonk D, Zafar N (2017) Nuclear 2C DNA and genome size analysis in somatic embryo regenerated gladiolus plants using flow cytometry. Adv Hortic Sci 31:165–174. https://doi.org/10.13128/ahs-21956
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Mycock DJ, Watt MP, Hannweg KF, Naicker K, Makwarela M, Berjak P (1997) Somatic embryogenesis of two indigenous south African Haworthia spp. (H. limifolia and H. koelmaniorum). South Afr J Bot 63:345–350. https://doi.org/10.1016/S0254-6299(15)30784-5
Ogihara Y (1981) Tissue culture in Haworthia. Theor Appl Genet 60:353–363. https://doi.org/10.1007/BF00264330
Ogihara Y, Tsunewaki K (1978) Tissue culture in Haworthia I. effects of auxins and kinetin on callus growth. Bot Mag Tokyo 91:83–91. https://doi.org/10.1007/BF02489105
Ouyang Y, Chen Y, Lü J, Teixeira da Silva JA, Zhang X, Ma G (2016) Somatic embryogenesis and enhanced shoot organogenesis in Metabriggsia ovalifolia W. T. Wang. Sci Rep 19:6–24. https://doi.org/10.1038/srep24662
Pandey KN, Sabharwal PS, Calkins J (1979) Effects of ionizing radiation (60Co gamma rays) on growth and morphogenesis of Haworthia mirabilis, haw. callus tissues. In Vitro 15:246–251. https://doi.org/10.1007/BF02618947
Pikulthong V, Teerakathiti T, Thamchaipenet A, Peyachoknagul S (2016) Development of somatic embryos for genetic transformation in Curcuma longa L. and Curcuma manga Valeton & Zijp. Agric Nat Resour 50:276–285. https://doi.org/10.1016/j.anres.2015.08.004
Pinheiro MVM, Martins FB, da Cruz ACF, de Carvalho ACPP, Ventrella MC, Otoni WC (2014) Somatic embryogenesis in anthurium (Anthurium andraeanum cv. Eidibel) as affected by different explants. Acta Sci Agron 36:87–98. https://doi.org/10.4025/actasciagron.v36i1.16557
Raju CS, Aslam A, Shajahan A (2015) High-efficiency direct somatic embryogenesis and plant regeneration from leaf base explants of turmeric (Curcuma longa L.). Plant Cell Tissue Organ Cult 122:79–87. https://doi.org/10.1007/s11240-015-0751-1
Richwine AM, Tipton JL, Thompson GA (1995) Establishment of Aloe, Gasteria and Haworthia shoot cultures from inflorescence explants. HortScience 30:1443–1444.
Rogers SMD (1993a) Optimization of plant regeneration and rooting from leaf explants of five rare Haworthia. Sci Hortic 56:157–161. https://doi.org/10.1016/0304-4238(93)90016-J
Rogers SMD (1993b) Culture phenotype affects on regeneration capacity in the monocot Haworthia comptoniana. In Vitro Cell Dev Biol Plant 26:9–12. https://doi.org/10.1007/BF02632232
Sherif NA, Benjamin JHF, Kumar TS, Rao MV (2018) Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult 132:303–316. https://doi.org/10.1007/s11240-017-1330-4
Sivanesan I, Jeong BR (2012) Identification of somaclonal variants in proliferating shoot cultures of Senecio cruentus cv. Tokyo Daruma. Plant Cell Tissue Organ Cult 111:247–253. https://doi.org/10.1007/s11240-012-0186-x
Sivanesan I, Son MS, Jana S, Jeong BR (2012) Secondary somatic embryogenesis in Crocus vernus (L.) hill. Propag Ornam Plants 12:163–170
Standifer LC, O’Rourke EN, Porche-Sorbet R (1984) Propagation of Haworthia from floral scapes. Plant Prop 30:4–6
Stanišić M, Raspor M, Ninković S, Milošević S, Ćalić D, Bohanec B, Trifunović M, Petrić M, Subotić A, Jevremović S (2015) Clonal fidelity of Iris sibirica plants regenerated by somatic embryogenesis and organogenesis in leaf-base culture-RAPD and flow cytometer analyses. South Afr J Bot 96:42–52. https://doi.org/10.1016/j.sajb.2014.10.014
Sun Y, Heil BM, Kahl G, Kohlenbach HW (1987) Plant regeneration from protoplasts of the monocotyledonous Haworthia magnifica v. Poelln. Plant Cell Tissue Organ Cult 8:91–100. https://doi.org/10.1007/BF00040736
Takamori LM, Neto NBM, Vieira LGE, Ribas AF (2015) Optimization of somatic embryogenesis and in vitro plant regeneration of Urochloa species using picloram. In Vitro Cell Dev Biol Plant 51:554–563. https://doi.org/10.1007/s11627-015-9701-1
Verma VM (2017) Direct somatic embryogenesis and organogenesis from axillary meristem in taro (Colocasia esculenta var. esculenta). Am J BioSci 5:114–122. https://doi.org/10.11648/j.ajbio.20170506.13
Wang X, Li Y, Zeng H, Cai N, Qiao Z, Wang X (2017) Micropropagation of Weigela florida ‘tango’ through in vitro shoot culture. HortScience 52:274–277. https://doi.org/10.21273/HORTSCI11413-16
Wessels DC, Groenewald EG, Koeleman A (1976) Callus formation and subsequent shoot and root development from leaf tissue of Haworthia planifolia cf. var. setulifera v. Poelln. Z Pflanzenphysiol 78:141–145. https://doi.org/10.1016/S0044-328X(78)80185-8
Zhong L, Liu E, Yang C, Jin S, Diao Y, Hu Z (2017) High embryogenic ability and regeneration from floral axis of Amorphophallus konjac (Araceae). Open Life Sci 12:34–41. https://doi.org/10.1515/biol-2017-0004
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University.
Author information
Authors and Affiliations
Contributions
DHK, KWK, and IS conceived and designed the experiments and wrote the paper. IS performed the somatic embryogenesis studies, and KWK performed the greenhouse experiments. All authors have seen and agreed to the submitted manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, D.H., Kang, K.W. & Sivanesan, I. Influence of auxins on somatic embryogenesis in Haworthia retusa Duval. Biologia 74, 25–33 (2019). https://doi.org/10.2478/s11756-018-0151-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0151-1