Abstract
An effective micropropagation technique via somatic embryogenesis has been developed using tissue from serially grafted shoots generated from a mature Kalopanax septemlobus tree (~40 y old). Callus was induced from leaf segments obtained from the grafts by culturing the explants in Murashige and Skoog (MS) medium supplemented with 2,4-D and 3% w/v sucrose under darkness. The effects of sucrose, coconut water, and polyethylene glycol (PEG-3350) were evaluated as factors to promote development of somatic embryos (SEs) from embryogenic callus. More than 90% of explants formed callus; however, only 2.5%, or 20 leaf segments out of 800 explants, formed embryogenic callus after 8 wk of culture. High sucrose concentrations (3% and 5% w/v) were effective in inducing SEs. Treatment with 2–10% v/v coconut water also had a positive effect on embryo induction. A synergistic effect on SE induction was obtained using sucrose and PEG, with presence of the latter compound resulting in smaller, more uniform SEs. Embryo germination and conversion to plantlets were significantly influenced by the gelling agents. In general, gelrite-gelled medium was superior to agar-gelled medium. In gelrite-gelled medium, gibberillic acid (GA3) enhanced embryo germination. Converted plantlets in an artificial soil mixture showed a 91% survival rate and displayed no distinct morphological variations. Our results indicate that reliable somatic embryogenesis and plant production can be achieved with rejuvenated tissues after repeated grafting of shoots derived from a mature Kalopanax septemlobus tree.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kalopanax septemlobus Koizi (Syn. K. pictus) is a woody perennial tree belonging to the family Araliaceae that reaches up to 25 m in height. It is found mainly in northeast Asia and is used for timber and important medicines. The main pharmacological effects of K. septemlobus are anti-rheumatic (Choi et al. 2002b), anti-diabetic (Park et al. 1998a, b), and anti-inflammatory (Lee et al. 2001). In the spring, sprouting buds are also used as fresh vegetables, and many farmers cultivate the tree as a cash crop. Most K. septemlobus trees have a thorny trunk or branches, making the species difficult to handle for cultivation. Therefore, many farmers wishing to cultivate the species have requested the development of a cultivar lacking thorns.
The Korea Forest Research Institute (KFRI) searched for thornless individual trees of K. septemlobus among wild populations during the years 1994 to 1999. A total of 200 candidate trees were selected and several thornless candidates were identified, including one named ‘Cheongsong’ (KFRI unpublished data). The Cheongsong cultivar was found in Cheongsong Province, GyeongSangbuk-do, Korea. The cultivar was growing at 400 m above sea level, facing northeast. At the time of selection, the tree had a height of 8.5 m, 31 cm DBH (diamter at breast height) and a 4 m crown width. Cheongsong is characterized by a thin outer bark and soft, wide leaves and in addition to its thornless stems. It also shows good survival rates when propagated by either grafting or root-cutting (Kim et al. 2002). Nevertheless, propagation remained a challenge, and workers at KFRI have only been able to produce a few hundred plantlets of the cultivar since 1999. These challenges have spurred efforts to develop an efficient micropropagation technique for the cultivar.
Conventional propagation of K. septemlobus is achieved via seeds or stem cuttings but with very low efficiency (Yeoung et al. 2001). The species usually produces abundant seed; however, the seeds require 2 y to germinate under natural conditions and are in a state of extreme dormancy. Moreover, zygotic embryos at harvest time are in an immature stage, and ectothermic treatment (changes of warm and cold temperatures) is necessary to overcome the dormancy (Sato 1998; Yeoung et al. 2001).
For vegetative propagation of recalcitrant, woody plants tissue culture has been suggested as an alternative to conventional techniques, and a variety of applicable methods, including organogenesis and somatic embryogenesis, have been developed (Bonga and Von Aderkas 1992; Steinmacher et al. 2007; Zhu et al. 2007). Somatic embryogenesis presents a potentially powerful tool for improvement of forest trees. Compared to conventional propagation, this in vitro propagation system offers high multiplication rates and the production of materials suitable for cryopreservation (Park et al. 1998a, b), artificial seed production (Kim et al. 2007b) and transgenic manipulation (Merkle et al. 1997; Poupin and Arce-Johnson 2005). However, most somatic embryogenesis studies in forest tree species have been conducted using immature tissue sources as the explant. This most often includes seed or seedling-derived materials that have unproven genetic value due to being juvenile in nature and the result of sexual crossing.
Few studies exist of in vitro propagation using mature tree tissues as the source of explant material. This is especially true for those involving somatic embryogenesis of material from mature trees possessing proven elite qualities. Those reported include studies on somatic embryogenesis derived from tree species such as Larix decidua (Bonga 2004), Quercus robur (Chalupa 2000), Ceratozamia euryphyllidia (Chavez et al. 1998), Terminalia arjuna (Kumari et al. 1998), Liquidamber styraciflua (Merkle et al. 1997, 1998, 2003; Merkle and Battle 2000), and Ulmus minor (Conde et al. 2004). Among the various forest trees studied, the most intensive work has also been done on sweetgum and oak (Hernandez et al. 2003a, b; Merkle et al. 1997; Pinto et al. 2002).
For conventional vegetative propagation of mature forest trees, the choice of more juvenile tissues, such as the epicormic shoot or materials generated through rejuvenation techniques such as serial grafting onto a root stock, can be the key to successful propagation (Chalupa 2000). This is also true for in vitro propagation. In some species, successful conventional vegetative propagation of mature trees has been accomplished through rejuvenation techniques (Beck et al. 1998; Moon and Yi 1993; Danthu et al. 2002; Onay et al. 2004); however, somatic embryogenesis using rejuvenated tissues from mature trees, to our knowledge, has yet to be reported.
Tissue culture techniques for the purpose of plant production have been applied to several Araliaceae species, suggesting that the techniques could also be used for large-scale production of K. septemlobus (Choi et al. 1999, 2002a; Moon and Youn 1999). Recently, we developed a plant propagation system for Kalopanax pictus by means of either organogenesis or somatic embryogenesis (Moon et al. 2002, 2005). We successfully induced embryogenic callus from mature zygotic embryos of K. pictus (Moon et al. 2005); however, we did not induce embryogenic callus from the mature tree. Since it was difficult to induce callus or embryogenic callus from the mature tree directly, as shown in oak tree (Hernandez et al. 2003a, b), we applied a rejuvenation technique by means of grafting. There are several reports that grafting onto younger stock can restore juvenility or rooting competence (Danthu et al. 2002; Onay et al. 2004). To study the potential for somatic embryogenesis in K. septemlobus, we attempted to rejuvenate mature tissue by means of grafting. We conducted first and second successive grafting onto 2-y-old younger rootstocks using scions taken from the Cheongsong cultivar, however, we could produce only non-embryogenic calli. Therefore, we tried a third round of grafting in an attempt to obtain embryogenic calli capable of normal embryogenesis. In the present study, we have focused on improving the efficiency of somatic embryo induction from serially grafted rejuvenated tissues of Cheongsong cultivar of K. septemlobus.
Materials and Methods
Grafting and mature tree rejuvenation. Several branches 20–40 cm in length with terminal buds were taken from the tree ‘Cheongsong’ in February 2001. The branches were wrapped with moistened tissue and polyvinyl, put into an icebox, and transferred to the laboratory. The materials were stored in a refrigerator at 4°C until used. Splice grafting was conducted onto rootstocks of the same tree species in a greenhouse at Korea Forest Research Institute (KFRI) located in Suwon, Gyeonggido, Korea, in mid-March. Grafting was carried out using a procedure modified for this purpose in avocado (Raharjo and Litz 2005) and pistachio (Onay et al. 2004). Two-year-old rootstocks of the K. septemlobus tree were decapitated by a single slice through the stem 10–15 cm above the ground, and a vertical slit was made. Excised scions taken from the Cheongsong cultivar were 3–5 cm in length and possessed one or two terminal buds. These were cut with a matching V-shaped base, inserted into the rootstock, and secured with parafilm. Grafted plants were raised in the greenhouse at temperatures of 20 to 30° C, under 30% shading. The scions of the grafts grew to 20–30 cm in length after one growing season. Each spring, the same grafting procedure was conducted by taking tissue from the scions of the grafts such that three serial graftings were accomplished from 2001 to 2003. Young leaves 3–5 cm long from the current year's shoot growth were collected from the grafts in May and used as explants for the present study.
Surface disinfection and inoculation. About ten young, expanding leaves 3–5 cm in length were excised from each graft and used as explants. For surface disinfection, five to ten leaves were placed into a 500 ml flask and washed by vigorous shaking in tap water containing a few drops of Tween 20® (Sigma, St. Louis, USA). Thereafter, in aseptic conditions, the leaves were disinfected in 70% w/v ethanol for 1 min, in 2% w/v NaClO for 5 min, and rinsed five times with sterile distilled water. Explants were finally immersed in sterilized distilled water for about 30 min. Leaf segments of about 5 mm2 were prepared with a surgical blade (no. 11), with each explant segment possessing some leaf vein tissue. The explants were inoculated onto Murashige and Skoog (MS) (Murashige and Skoog 1962) basal medium supplemented with 4.5 μM 2,4-dichlorophenoxy acetic acid (2,4-D) and 3% w/v sucrose (Sigma). The pH of the culture medium was adjusted to 5.7 before the addition of 0.3% w/v gellan gum (Phytagel, Sigma). The culture media were autoclaved at 120° C, 1 kg cm2 for 20 min and dispensed to plastic 87 × 15 mm Petri dishes at 25 ml each. Ten explants per Petri-dish were inoculated with the abaxial side face down on the medium. There were a total of five petri dishes per replication. The cultures were maintained at 25 ± 1° C with a 16-h photoperiod provided by cool-white fluorescent lamps (40 μmol m−2 s−1).
Embryogenic callus induction and maintenance. Four weeks after culture initiation, the explants were transferred onto half-strength MS (1/2MS) medium supplemented with 2% w/v sucrose and 0.3% w/v gellan gum without phytohormones to induce embryogenic callus. White, pale yellow, and friable embryogenic callus was induced after 8 wk of culture. To stimulate proliferation, these calli were carefully selected under a microscope and subcultured onto MS medium supplemented with 4.5 μM 2,4-D, 1.0 g/l L-glutamine, 5% w/v sucrose and 0.5% w/v gellan gum, and then further subcultured onto fresh medium of the same type at 3-wk intervals. All steps of induction and maintenance of the embryogenic callus were undertaken in the dark in a culture room at 25 ± 1° C.
Somatic embryo induction. To obtain somatic embryos (SEs), approximately 0.5 g of embryogenic callius was placed into a 100 ml Erlenmeyer flask containing 30 ml of 1/2 MS liquid medium supplemented with 2% w/v sucrose but lacking phytohormones. The Erlenmeyer flasks with the embryogenic calli were placed on a shaker and agitated as 120 rpm for 24 h. Tissues were then removed and passed through using a 40-mesh stainless steel sieve (pore size 380 μm, Sigma). Subsequently, 1.5 ml of the culture containing 7.5 mg fresh weight (FW) of cells was poured over a 5.5 cm diameter sterile filter paper (Whatman no. 2) in a Buchner funnel. A short vacuum pulse of 5 s at 45.7 cmHg was applied to drain the liquid and the whole filter paper then transferred onto the embryo induction medium (EIM) in a Petri dish (87 × 10 mm). EIM medium consisted of MS basal salts supplemented with 0.41 μM abscisic acid (ABA), 0.02% w/v activated charcoal (AC), and solidified with 0.5% w/v gellan gum. In this EIM, different levels of sucrose, from 0 to 5% w/v were tested. Subsequently, coconut water (CW; Sigma) at 0, 2, 5, 7 and 10% v/v and PEG at 0, 1, 3, 7, and 10% were tested in the presence of 3% w/v sucrose. The pH of each medium was adjusted to 5.7 before the addition of gellan gum. An appropriate aliquot of filter-sterilized stock solution of L-glutamine (also pH adjusted to 5.7), PEG, and ABA were added to the medium after autoclaving. All cultures were kept at 25 ± 1° C under a 16-h photoperiod at 40 μmol m−2 s−1 provided by cool-white fluorescent lamps for 4 wk.
Periodically, developing somatic embryos were examined under a dissecting microscope and the total number of torpedo and early cotyledonary stage somatic embryos were counted.
Embryo germination and conversion to plants. Somatic embryos showing early cotyledonary stages were transferred individually to 1/2MS medium containing one of two different gelling agents, either 0.3% w/v gellan gum or 0.8% w/v agar (Sigma, A 1296) to induce embryo germination and plantlet conversion. Also, to promote embryo germination, various levels of GA3 (2.9, 8.7, 14.4 or 28.9 μM) were added to the medium (Table 1). There were 20 to 25 SEs cultured in each plate with ten or more such replicates per experiment.
All cultures were carried out under the conditions described above. Three weeks later, the germinated embryos were transferred to larger culture vessels (SPL Life Science Co. Korea, 11.7 × 5.0 cm) containing 1/2MS basal medium with 2% w/v sucrose and 0.02% w/v activated charcoal. After 6 wk of culture, the germination and conversion rates were determined. Embryos with elongated hypocotyls and root growth, but without new epicotyl growth, were counted as having germinated, whereas the plantlets with new epicotyl growth and root growth were counted as having undergone conversion.
Soil transfer and acclimatization. Normally converted plantlets (5–10 cm in height) that possessed an apical shoot bud with expanded leaves and a primary root with many hairy, secondary roots were washed with tap water to remove adhering agar and then transferred to plastic rectangular boxes (40 × 70 × 20 cm, Fig. 1 H) containing an artificial soil mixture comprised of peatmoss, vermiculite, and perlite at (1:1:1, v/v/v). The boxes were covered with polyvinyl, placed in a greenhouse, and kept at more than 90% relative humidity and 20–30° C. Seventy plantlets were planted in each plastic box and replicated more than five times. To maintain the appropriate moisture conditions, the plants in the box were sprayed with water one or two times everyday for 2 wk. Gradual opening of the polyvinyl cover started after this 2-wk period and the polyvinyl cover was completely removed by the end of the third week after transplanting to soil. Surviving plants were recorded after growing in the soil medium for a total of 2 mo.
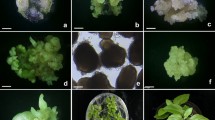
Somatic embryogenesis, somatic embryo maturation, plantlet formation, and acclimatization to the soil environment of Kalopanax septemlobus. A Somatic embryos maturing from embryogenic callus on MS medium containing 5% v/v coconut water (bar = 1.2 mm); B somatic embryos maturing from embryogenic callus on MS medium containing 7% PEG. Embryos are relatively small and uniform in size (bar = 1.5 mm); C somatic embryos at mature cotyledon stage; D and E SEM photographs showing different developmental stages of embryos. Arrows show secondary somatic embryos formed at the root tip of a somatic embryo (bar = 0.2 mm); F germinating somatic embryos with expended cotyledons and elongated roots when cultured on 1/2 MS medium containing 2.9 μM GA3 for 10 d (bar = 1.7 mm); G converted plantlets with leaves growing on 1/2 MS medium containing 0.02% activated charcoal (bar = 17 mm); H acclimatized plantlets in the plastic rectangular boxes containing a mixture of artificial soil; I normally growing plantlets exhibiting different growth heights under greenhouse conditions.
Scanning electron microscope (SEM) analysis. In order to observe embryo development, samples showing various stages of embryogenic development were fixed in 4% v/v glutaraldehyde in 1.25% w/v piperazine-N, N-bis-(2-ethanesulfonic acid) buffer (pH 7.6). After dehydration, samples were critical-point dried with CO2, sputter-coated with gold, and examined using a Jeol JSM-5400 scanning electron microscope (Pinto et al. 2002).
Experimental design and data analysis. Experiments were carried out in randomized design and each treatment was repeated twice with five replicates. Data were subjected to Duncans' multiple range test using the SAS program (SAS Institute, Cary, NC, USA). Mean values with different letters differ significantly at P ≤ 0.05.
Results
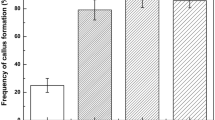
Embryogenic callus induction. Expanding leaves 3–5 cm in length were excised from the growing grafted shoots and used as explants. Surface disinfection of explants was highly effective with only about 5% of explants developing contamination. Calli were formed from slight swellings of the explants at wound areas after 2 wk of culture on MS basal medium supplemented with 4.5 μM 2,4-D and 3% w/v sucrose. The calli formed were pale green in color, relatively compact, and grew slowly with callus induction rates reaching 91% after 4 wk of culture (results not shown). The remaining explants did not form callus and died gradually. Explants derived from first and second successive generations of graftings onto younger root-stocks were able to induce callus from leaves. However, all of these calli were non-embryogenic (results not shown). Explants prepared from third generation grafts also produced callus that also appeared to be non-embryogenic in nature. As was observed in first- and second-generation grafted explants, the callus growth was slow and relatively compact with a pale green color. However, when these calli were transferred onto 1/2MS basal medium containing 2% w/v sucrose, they began to grow quickly. After a further 8 wk in culture, white and pale yellow embryogenic calli were observed developing sporadically among the other, browning callus. A total of 20 segments from 800 explants (2.5%) formed embryogenic callus after 8 wk of culture on this hormone-free medium. The embryogenic calli were pale yellow or white and milky in color and had a soft, friable composition, whereas non-embryogenic calli were pale green and compact. Embryogenic calli were proliferated by transfer onto MS medium supplemented with 4.5 μM 2,4-D, 1.0 g/l glutamine, 5% w/v sucrose, 0.5% w/v gellan gum and repeated subculture on fresh medium of the same type at 3-wk intervals in the dark. The cultures were maintained for more than 3 y in this manner without any loss of embryogenic competence.
Somatic embryo induction. Embryogenic calli were dispensed into 1/2MS liquid medium supplemented with 2% w/v sucrose in flasks and cultured overnight. The following day, the tissues were filtered to remove the medium and the embryogenic calli plated onto embryo induction medium (EIM) consisting of MS basal salts supplemented with 0.41 μM ABA, 0.02% w/v activated charcoal, and solidified with 0.5% w/v gellan gum. The effect of sucrose concentration was studied (Fig. 2). Globular-stage somatic embryos began to form 2 wk later. Most somatic embryos were produced within 3 wk irrespective of the treatment; however, the numbers of somatic embryos differed significantly depending on the sucrose level. The average number of SEs per plate was 19 on 1% sucrose, 85 on 3% sucrose, and 82 on 5% sucrose (Fig. 2). Culture on medium without sucrose did not result in somatic embryo production, while supplementation of 1% w/v sucrose caused somatic embryos to form slowly over a 3-wk culture period. When 3% and 5% w/v sucrose concentrations were incorporated into the medium, embryos developed more rapidly, with many observed within 2 wk and more advanced developmental stages present after 3 wk in culture (Fig. 1 D).
Inclusion of coconut water (CW) was found to have a beneficial effect on the average number of somatic embryos that developed on each plate (Fig. 3). Inclusion of 5% v/v CW was the most effective concentration for SE production, while increasing CW to as high as 10% had no additional benefit (Fig. 3). The SEs cultured with CW were generally larger, with an average length of 2 mm (Fig. 1 A), while those produced on 7% and 10% PEG-containing medium were less than 1 mm at the torpedo stage. The most striking results in embryo induction were achieved when PEG was added to the somatic embryo development medium. In this treatment, after 2 wk of culture, many SEs could be seen under the microscope, and they were relatively small and uniform in size. Addition of either 1% or 3% PEG induced a similar number of SEs, ranging from 220–300 produced per plate. Elevation of the PEG concentrations up to 7% resulted in a significant increase, more than doubling the average number of SEs produced to greater than 600 per plate (Fig. 4). Somatic embryos formed from embryogenic callus in the presence of PEG were milky in color and embryo differentiation reached the early cotyledonary stage (Fig. 1 B). However, addition of 10% PEG resulted in retarded growth at the torpedo to early-cotyledonary stages.
In conclusion, including optimized levels of sucrose at 3%, CW at 5%, and PEG at 7% to the MS-based medium led to an average somatic embryo production of more than 600 SEs per plate.
Embryo germination and plantlet conversion. Using the SEs derived from growth on EIM, early cotyledonary stage somatic embryos were produced on 1/2MS medium containing 2% w/v sucrose and 0.3% w/v gellan gum. After 3 wk in culture, these embryos were transferred to 1/2MS containing different levels of GA3 and one of two different gelling agents, either 0.3% w/v gellan gum or 0.8% w/v agar. In agar-solidified medium, germination was slow and began 1 wk later. However, in gellan-solidified medium, germination was relatively faster regardless of the GA3 concentration and SEs began to germinate after 3 d in culture. In general, more than 80% of the SEs showed radicle elongation first, while cotyledon and epicotyl formation was delayed or absent altogether.
After 2 wk of culture, embryo germination was achieved in all GA3-treated media at high to very high frequencies of 87–100% and was not influenced by the type of gelling matrix used (Table 1). The plant conversion rate, however, differed significantly depending on the gelling agents. After 3 wk in culture, converted plantlets showed conspicuous cotyledon and leaf development (Fig. 1 F) and reached a height of 3.0 to 5.0 cm after 5 wk (Fig. 1 G). Gelrite was significantly more effective, inducing more than 70% conversion, while embryos cultured on medium solidified with agar converted to plantlets at a maximum of only 45% (Table 1).
The conversion rate was not enhanced by addition of GA3 to gellan-solidified medium and indeed was diminished by GA3 when this growth regulator was added to agar-solidified medium (Table 1). GA3 levels from 2.9 to 28.9 μM in gellan-solidified medium produced no significant differences in the conversion rate. In agar-solidified medium the conversion rate gradually decreased with increasing GA3 level and the plantlets showed retarded root development. In general, smaller sized plantlets with darker green leaves and stunted, reddish hypocotyls and roots were formed in GA3-free control medium. In contrast, larger plantlets with pale green, elongated hypocotyl and roots developed in GA3-containing medium, irrespective of the type of medium and GA3 concentration. Secondary embryogenesis, in which new embryos were formed sporadically at either the lower hypocotyl or radicle of the developing somatic embryos (Fig. 1 E). However, the frequency of such events was low (less than 1%) and most SEs germinated as shown in Fig. 1 F. Therefore, these secondary SEs did not inhibit the embryo germination and conversion. Although some mature embryos showed variation in cotyledon number at the beginning of germination, they grew normally in subsequent cultures.
Plant acclimatization and transfer to soil. When somatic embryo-derived plantlets reached 3.0–5.0 cm in height they were transferred to plastic rectangular boxes containing a mixture of artificial soil. More than 92% of such plants survived after 3 wk (Fig. 1 H). Although the plantlets grew normally, they exhibited different growth height under greenhouse conditions (Fig. 1 I). The differences in height might be caused by differences in the transferring time to the acclimatization stages. At this stage they were planted in an outdoor field where they remain under study. Currently more than 2,000 plantlets have been regenerated in this manner and transferred to the field, where they have been growing without any morphological abnormalities for 2 y.
Discussion
Woody species are often difficult to micropropagate because their tissues have a reduced capacity for vegetative propagation (Chalupa 2000; Greenwood 1987). However, even mature trees possess juvenile tissues that can act as starting material for in vitro manipulation. Such juvenile tissues may occur naturally, for example as root suckers or stump and epicormic shoots, or they may be induced by various chemical treatments. Selection of suitable explants from juvenile parts of a mature tree via a rejuvenation technique can be an important step for successful in vitro propagation (Arnaud et al. 1993; Beck et al. 1998; Chalupa 2000; Ewald and Naujoks 1996; Ponsonby and Mantell 1993). In various oak trees, somatic embryogenesis and plant regeneration systems have been developed via juvenile tissue selection through epicormic shoot or sprout induction (Chalupa 2000; Pinto et al. 2002; Hernandez et al. 2003a, b; Toribio et al. 2004). In addition, successful somatic embryo induction and plant regeneration have been achieved in mature sweetgum using staminate inflorescences (Merkle et al. 1997; 1998; 2003; Merkle and Battle 2000). The above results suggest the possibility of vegetative propagation of mature trees through either juvenile tissue selection or application of rejuvenation techniques.
To address this issue in Kalopanax septemlobus, we applied a rejuvenation technique by means of grafting. We conducted first and second successive graftings onto younger rootstocks using scions taken from a mature tree and then were able to induce callus from leaf explants derived from this growth. However, all of these calli appeared to be non-embryogenic. After a third round of grafting we obtained embryogenic calli capable of normal embryogenesis, although the induction frequency, at 2.5%, was very low. These results suggested to us that a mature K. septemlobus tree might be progressively rejuvenated or invigorated through successive grafting of suitable tissue onto younger rootstocks, as was observed previously for other species (Chalupa 2000; Greenwood 1987; Moon and Yi 1993). These embryogenic calli readily proliferated under optimized culture conditions and produced normal SEs as well as formed plantlets that were very similar to those described for embryogenic tissues derived from zygotic embryos of this species (Moon et al. 2005).
A number of culture variables were studied here in efforts to develop efficient somatic embryo production and regeneration. Sucrose is one of the most important carbon sources, and it has been used frequently in plant tissue-culture work. In somatic embryogenesis, it has been used as a carbon and energy source, and at high concentrations, it enhances SE induction frequency by causing osmotic stress (Iraqi and Tremblay 2001). A similar result was observed in the present study. No SEs were recoverable on medium without sucrose treatment. Although SE induction could be induced using 1% sucrose, the optimum for SE induction was 3–5% for mature tissue from K. septemlobus. Treatment with CW produced a significant improvement in somatic embryo induction in the present study (Fig. 3). Sharma and Chowdhury (1977) also reported that addition of CW enhanced embryogenesis in Datura innoxia. They explained that because CW contains zeatin and zeatin ribosides as the major cytokinin components, it might be able to induce cell division responses such as embryogenesis. In the present study, these positive effects on embryogenesis were also observed. However, as for Datura innoxia (Sharma and Chowdhury 1977), CW also enhanced proliferation of non-embryogenic callus and further experimentation is required to evaluate this additional effect on SE induction in K. septemlobus. In any event, those SEs that were produced by treatment with CW were larger (about 2–4 mm) at the torpedo stages as compared to embryos induced in the absence of CW. The larger SEs germinated more quickly and were more likely to convert to plantlets.
PEG treatment was very effective in inducing SEs in the present study in a manner similar to our recent results with a coniferous species (Kim and Moon 2007a). It has been reported that PEG promotes SE induction by provoking osmotic stress (Iraqi and Tremblay 2001; Lipvska et al. 2000); however, the exact mechanism is still unclear. Some authors reported that PEG suppresses normal root development of SEs and hampers normal plantlet conversion (Bozhkov and von Arnold 1998). Fortunately, these problems were not evident in the present study, and we are now pursuing physiological and anatomical studies in this area.
Generally, poor germination of embryos into plantlets is a major limitation of somatic embryogenesis for many tree species (Cuenca et al. 1999). Therefore, to reach a practical, commercial-scale mass-propagation system, an efficient method of plant formation is required. In the present study, we could obtain satisfactory embryo germination and plant conversion by GA3 treatment. However, the effect was produced only on gellan-solidified medium and may reflect differences in the physical properties of the gelling agent, for example, differences in gel strength and water availability (Klimaszewska et al. 2000). Several studies have also reported the effects of GA3 on SE germination, which varied among different species (Choi et al. 1999). In somatic embryogenesis of Terminalia arjuna, GA3 application suppressed normal SE germination. In contrast, treatment at 3.0 mg/l enhanced embryo germination for fused cotyledonary embryos (Kumari et al. 1998). These results suggest that GA3 action may be related to either the concentration of GA3 or the developmental stage of the embryo.
In conclusion, the present study showed that a mature K. septemlobus tree could be effectively propagated via a somatic embryogenesis system using the technique of rejuvenation via successive grafting. Although there are some disadvantages in applying rejuvenation techniques—for example, the requirement for annual grafting onto juvenile rootstocks up to three times, which is both time-consuming and laborious—the culture system is potentially a powerful one because the embryogenic calli retain their embryogenic capacity for more than 3 y. However, we suspect that use of a repeated micro-grafting approach will shorten the time period required for rejuvenation of a mature tree.
Generally, mature trees are recalcitrant to micropropagation, and application of somatic embryogenesis to mature trees especially is still rare. Therefore, the present study suggests that propagation of mature trees may be achieved by rejuvenation. Moreover, the method would permit vegetative propagation of genetically desirable cultivars based on phenotypes observed in mature trees. Finally, these results provide basic information on rejuvenation of a woody species as well as supporting materials for genetic transformation of the K. septemlobus tree.
References
Arnaud Y.; Franclet A.; Tranvan H.; Jacques M. Micropropagation and rejuvenation of Sequoia sempervirens: a review. Annales des Sciences Forestieáres 50: 173–275; 1993.
Beck S. L.; Dunlop R.; van Staden J. Rejuvenation and micropropagation of adult Acacia mearnsii using coppice material. Plant Growth Regulation 26: 149–153; 1998.
Bonga J. M. The effect of various culture media on the formation of embryo-like structures in cultures derived from explants taken from mature Larix decidua. Plant Cell, Tissue and Organ Culture 77: 43–48; 2004.
Bonga J. M.; Von Aderkas P. In vitro culture of trees. Forestry sciences, vol. 38. Kluwer, The Netherlands, 1992 p 236.
Bozhkov P. V.; von Arnold S. Polyethylene glycol promotes maturation but inhibits further development of Picea abies somatic embryos. Physiologia Plantarum 104: 211–224; 1998.
Chalupa V. In vitro propagation of mature trees of pedunculate oak (Quercus robur L.). Journal of Forest Science 46: 537–542; 2000.
Chavez V. M.; Litz R. E.; Monroy M.; Moon P. A.; Vovides A. M. Regeneration of Ceratozamia euryphyllidia (Cycadales, Gymnospermae) plants from embryogenic leaf cultures derived from mature-phase trees. Plant Cell Reports 17: 612–616; 1998.
Choi Y. E.; Kim J. H.; Youn E. S. High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Annals of Botany 83: 309–314; 1999.
Choi Y. E.; Ko S. K.; Lee K. S.; Youn E. S. Production of plantlets of Eleutherococcus sessiliflorus via somatic embryogenesis and successful transfer to soil. Plant Cell, Tissue and Organ Culture 69: 201–204; 2002a.
Choi J. W.; Huh K.; Kim S. H.; Lee K. T.; Park H. J.; Han Y. N. Actinociceptive and anti-rhumatoidal effects of Kalopanax pictus extract and its saponin components in experimental animals. Journal of Ethnopharmacology 79: 199–204; 2002b.
Conde P.; Loureiro J.; Santos C. Somatic embryogenesis and plant regeneration from leaves of Ulmus minor Mill. Plant Cell Reports 22: 632–639; 2004.
Cuenca B.; San-Jose M. C.; Martinez M. T.; Ballester A.; Vieitez A. M. Somatic embryogenesis from stem and leaf explants of Quercus robur L. Plant Cell Reports 18: 538–543; 1999.
Danthu P.; Hane B.; Sagna P.; Gassama Y. K. Restoration of rooting competence in mature Faidherbia albida, a Sahelian leguminous tree, through serial root sucker midrografting. New Forests 24: 239–244; 2002.
Ewald, D., & Naujoks, G. (1996). Attempts to get and to improve tissue cultures of adult oak. In F. O' Riodain (Ed.), Development of integrated systems for large scale propagation of elite plants using in vitro techniques (pp 156–160). Report of Activities, COST 822, European Connission, Directorate General for Science, Research and Development, Brussels.
Greenwood M. S. Rejuvenation of forest trees. Plant Growth Regulation 6: 1–12; 1987.
Hernandez L.; Celestino C.; Alegre J.; Toribio M. Vegetative propagation of Quercus suber L. by somatic embryogenesis. II. Plant regeneration from selected cork oak trees. Plant Cell Reports 21: 765–770; 2003b.
Hernandez L.; Celestino C.; Toribio M. Vegetative propagation of Quercus suber L. by somatic embryogenesis. I. Factors affecting the induction in leaves from mature cork oak trees. Plant Cell Reports 21: 759–764; 2003a.
Iraqi D.; Tremblay F. M. The role of sucrose during maturation of black spruce (Picea mariana (Mill.) BSP) and white spruce (Picea glauca (Moench) Voss) somatic embryos. Physiologia Plantarum 111: 381–388; 2001.
Kim Y. W.; Choi Y. E.; Yi J. S.; Moon H. K. Germination of artificial seeds by encapsulation of somatic embryos of Kalopanax septemlobus with alginic acid. Korean Journal of Plant Biotechnology 34: 229–235; 2007b.
Kim S. H.; Chung H. G.; Jang Y. S. A new thornless Castor aralia cultivar, “Cheongsong”. Korean Journal of Breeding 34: 383–384; 2002.
Kim Y. W.; Moon H. K. Enhancement of somatic embryogenesis and plant regeneration in Japanese larch (Larix leptolepis). Plant Cell, Tissue and Organ Culture 88: 241–245; 2007a.
Klimaszewska K.; Bernier-Cardou M.; Cyr D. R.; Sutton B. S. C. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cellular & Developmental Biology-Plant 36: 279–286; 2000.
Kumari N.; Jaiswal U.; Jaiswal V. S. Induction of somatic embryogenesis and plant regeneration from leaf callus of Terminalia arjuna Bedd. Current Science 75: 1052–1055; 1998.
Lee E. B.; Li D. W.; Hyun J. E.; Kim I. H.; Whang W. K. Anti-inflammatory activity of methanol extract of Kalopanax pictus bark and its fractions. Journal of Ethnopharmacology 77: 197–201; 2001.
Lipvska H.; Svobodova H.; Albrechtova J.; Kumstyrova L.; Vagner M.; Vondrakova Z. Carbohydrate status during somatic embryo maturation in Norway spruce. In Vitro Cellular & Developmental Biology-Plant 36: 260–267; 2000.
Merkle S. A.; Bailey R. L.; Pauley B. A.; Neu K. A.; Kim M. K.; Rugh C. L. et al. Somatic embryogenesis from tissues of mature sweetgum trees. Canadian Journal of Forest Research 27: 959–964; 1997.
Merkle S. A.; Battle P. J. Enhancement of embryogenic culture initiation from tissues of mature sweetgum trees. Plant Cell Reports 19: 268–273; 2000.
Merkle S. A.; Battle P. J.; Ware G. O. Factors influencing production of inflorescence-derived somatic seedlings of sweetgum. Plant Cell, Tissue And Organ Culture 73: 95–99; 2003.
Merkle S. A.; Neu K. A.; Battle P. J.; Bailey R. L. Somatic embryogenesis and plantlet regeneration from immature and mature tissues of sweetgum (Liquidambar styraciflua). Plant Science 132: 169–178; 1998.
Moon H. K.; Kim S. H.; Kim B. K. Micropropagation of Kalopanax pictus Nakai via axillary bud culture. Journal of the Korean Forestry and Society 91: 775–780; 2002.
Moon H. K.; Kim Y. W.; Lee J. S.; Choi Y. E. Micropropagation of Kalopanax pictus tree via somatic embryogenesis. In Vitro Cellular & Developmental Biology-Plant 41: 303–306; 2005.
Moon H. K.; Yi J. S. Cutting propagation of Quercus acutissima clones after rejuvenation through serial grafting. Annales Des Sciences Forestieáres 50 Suppl 1: 314–318; 1993.
Moon, H. K., & Youn, Y. (1999). Somatic embryogenesis from winter buds of 10-year-old Aralia elata. In S. M. Jain, P. K. Gupta, & R. J. Newton (Eds.), Somatic embryogenesis in woody plants, vol. 5 (pp, 129–134). Kluwer Academic Pub.
Murashige T.; Skoog F. A. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473–479; 1962.
Onay A.; Pirinc V.; Yildirim H.; Basaran D. In vitro micrografting of mature pistachio (Pistacia vera var. Siirt). Plant Cell, Tissue and Organ Culture 77: 215–219; 2004.
Park Y. S.; Barrett J. D.; Bonga J. M. Application of somatic embryogenesis in high-value clonal forestry: deployment, genetic control, and stability of cryopreserved clones. In Vitro Cellular & Developmental Biology-Plant 34: 231–239; 1998a.
Park H. J.; Kim D. H.; Choi J. W.; Park J. H.; Han Y. N. A potent anti-diabetic agent from Kalopanax pictus. Archives of Pharmacal Research 21: 24–29; 1998b.
Pinto G.; Valentim H.; Costa A.; Castro S.; Santos C. Somatic embryogenesis in leaf callus from a mature Quercus suber L. tree. In Vitro Cellular & Developmental Biology-Plant 38: 569–572; 2002.
Ponsonby D. J.; Mantell S. H. In vitro establishment of Picea pungens f. glauca and P. sitchensis seedling rootstocks with an assessment of their suitabilities for micrografting with scions of various Picea species. Journal of Horticultural Science 68: 463–475; 1993.
Poupin M. J.; Arce-Johnson P. Transgenic trees for a new era. In Vitro Cellular & Developmental Biology-Plant 41: 91–101; 2005.
Raharjo S. H. T.; Litz R. E. Micrografting and ex vitro grafting for somatic embryo rescue and plant recovery in avocado (Persea Americana). Plant Cell, Tissue and Organ Culture 82: 1–9; 2005.
SAS Institute. (1989). SAS/STAT user's guide (4th ed.). vers. 6. SAS Inst., Cary, N. C.
Sato K. A stratification procedure to accelerate the germination of Kalopanax pictus. Journal of the Japanese Forestry Society 80: 279–282; 1998.
Sharma D. R.; Chowdhury J. B. Effects of different media on cultured anthers of Datura inoxia Mill. and comparative morphogenetic potential of haploid and diploid tissues. Indian Journal of Experimental Biology 15: 616–618; 1977.
Steinmacher D. A.; Cangahuala-Inocente G. C.; Clement C. R.; Guerra M. P. Somatic embryogenesis from peach palm zygotic embryos. In Vitro Cellular and Developmental Biology Plant 43: 124–132; 2007.
Toribio M.; Fernandez C.; Celestino C.; Martinez M. T.; San-Jose M. C.; Vieitez A. M. Somatic embryogenesis in mature Quercus robur trees. Plant Cell, Tissue and Organ Culture 76: 283–287; 2004.
Yeoung Y. R.; Lee M. H.; Kim B. S.; Kim H. K.; Kim J. H. Seed germination and softwood cutting technique of Kalopanax pictus Nakai. Korean Journal of Plant Resources 14: 53–59; 2001.
Zhu M. L.; Yang J. W.; Yu Y.; Liu S. J. Efficient organogenesis and plantlet regeneration in the timber species Cunninghamia lanceolata (Lamb.) Hook. In Vitro Cellular and Developmental Biology Plant 43: 449–455; 2007.
Acknowledgment
This work was partly supported by grant No. FG 0701-1966-01 from the Korea Forest Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Woong-Young Soh
Rights and permissions
About this article
Cite this article
Moon, H.K., Park, S.Y., Kim, Y.W. et al. Somatic embryogenesis and plantlet production using rejuvenated tissues from serial grafting of a mature Kalopanax septemlobus tree. In Vitro Cell.Dev.Biol.-Plant 44, 119–127 (2008). https://doi.org/10.1007/s11627-008-9122-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9122-5