ABSTRACT
BACKGROUND
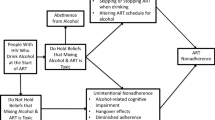
Antiretroviral therapy (ART) adherence is key to successful treatment of HIV infection and alcohol is a known barrier to adherence. Beyond intoxication, ART adherence is impacted by beliefs that mixing alcohol and medications is toxic.
PURPOSE
To examine prospective relationships of factors contributing to intentional medication non-adherence when drinking.
METHODS
People who both receive ART and drink alcohol (N = 178) were enrolled in a 12-month prospective cohort study that monitored beliefs about the hazards of mixing ART with alcohol (interactive toxicity beliefs), alcohol consumption using electronic daily diaries, ART adherence assessed by both unannounced pill counts and self-report, and chart-abstracted HIV viral load.
RESULTS
Participants who reported skipping or stopping their ART when drinking (N = 90, 51 %) demonstrated significantly poorer ART adherence, were less likely to be viral suppressed, and more likely to have CD4 counts under 200/cc3. Day-level analyses showed that participants who endorsed interactive toxicity beliefs were significantly more likely to miss medications on drinking days.
CONCLUSIONS
Confirming earlier cross-sectional studies, the current findings from a prospective cohort show that a substantial number of people intentionally skip or stop their medications when drinking. Interventions are needed to correct alcohol-related interactive toxicity misinformation and promote adherence among alcohol drinkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Antiretroviral therapies (ART) significantly suppress HIV replication and can improve the health of people living with HIV infection. Optimal health benefits for people receiving ART depend closely on treatment adherence, with all ART regimens requiring at least 85 % adherence.1,2 Among the known impediments to medication adherence is alcohol use.3 Studies demonstrate that individuals who take ART and drink alcohol experience more missed doses, medication lapses, and HIV treatment failure.3,4 Most studies of alcohol’s effects on medication adherence have focused on alcohol-induced cognitive impairments.3,5,6 More recently, studies have suggested that as many as one in four patients who take ART and drink alcohol intentionally stop taking their medications when they are drinking.7,8 Although alcohol use can contribute to liver damage in people co-infected with HIV and Hepatitis C virus,5 there is no evidence that the hepatotoxicity of alcohol is amplified in combination with ART.9–11 Beliefs that mixing medications with alcohol results in a toxic blend (i.e., alcohol–ART interactive toxicity beliefs) are associated with poor treatment adherence, even to a greater extent than alcohol use itself.8
The current study was conducted to further examine alcohol–ART interactive toxicity beliefs and behaviors among drinkers who are currently treated with ART. We used a prospective study design to simultaneously examine adherence and drinking among persons who hold alcohol–ART interactive toxicity beliefs. Our aim was to test the following three hypotheses: (a) alcohol–ART interaction beliefs and behaviors will predict HIV treatment non-adherence over and above alcohol and other drug use; (b) persons who hold alcohol–ART interaction beliefs will be significantly more likely to miss their medications on days when they drink alcohol compared to other drinkers who do not hold such beliefs; and (c) interaction beliefs will be associated with provider’s advice to avoid mixing alcohol and ART.
METHODS
Participants
People living with HIV/AIDS (N = 178) were recruited through targeted community sampling. We used both venue recruitment and snowball sampling techniques. Recruitment relied on responses to brochures placed in waiting rooms of HIV service providers and infectious disease clinics throughout Atlanta, GA. We also adapted techniques from respondent-driven sampling as a means of word-of-mouth chain recruitment.12 Specifically, participants were given brochures that described the study opportunity with a phone number to our research offices. Participants were encouraged to use the brochures to refer their HIV-positive friends to the study. The study entry criteria were (a) 18 years of age or older, (b) HIV positive and prescribed ART, and (c) drank alcohol in the past week.
Measures
Computerized Interviews
Assessments were administered using audio-computerized self-interviewing (ACASI) to reduce demand characteristics and limit socially evoked response biases.13,14 The interview required approximately 30 min.
Demographic and Health Characteristics
Participants reported basic demographic information, whether they had ever been diagnosed with any liver disease, and whether they had ever received substance abuse treatment. We also assessed 14 HIV-related symptoms of 2-weeks duration using a measure reported in previous research;15 symptoms included shortness of breath; dry cough; oral or throat sores; Thrush, Candida, or oral white patches; fatigue; unintentional weight loss; recurring fever; and night sweats.
Substance Use
To assess global alcohol use, we administered the Alcohol Use Disorders Identification Test (AUDIT), a 10-item scale designed to measure alcohol consumption and identify risks for alcohol abuse and dependence.16 Scores on the AUDIT range from 0–40, and scores greater than 8 indicate high-risk for hazardous and harmful alcohol use;17 specificities were between 0.80 and 0.9018 (alpha = 0.90). Participants also indicated whether they had used cannabis, cocaine, amphetamine, or other drugs in the previous 4 months (yes/no).
Alcohol–ART Interaction Beliefs and Behaviors
Participants completed two items to assess beliefs and nine items to assess behaviors related to avoiding mixing alcohol and ART.7,8,19 Participants reported whether they have engaged in behaviors to avoid mixing ART with alcohol. One behavioral item asked participants about skipping their medications when they are drinking (I skip taking my medicine if I have been drinking), and another asked about stopping their medications when they know they will be drinking (I stop taking my HIV medications if I will be drinking alcohol). These two behavioral items were used to form groups of participants who do not and those who do skip/stop their medications when drinking. The remaining seven behavioral items concerned other actions associated with not taking medications in relation to drinking alcohol. The interactive belief and behavior items are shown in the Results section. These items were responded to as whether participants endorsed the belief or had performed each behavior.
Provider Communications
We assessed experiences with provider communications regarding alcohol use and medications. Three items asked whether providers had talked with participants in the past year about their use of alcohol (see Results for items). Responses indicated whether each communication occurred or not, with yes or no responses.
Medication Adherence
Participants consented to monthly unannounced telephone-based pill counts for the duration of the study, constituting a prospective measure of adherence. Unannounced pill counts are reliable and valid in assessing medication adherence when conducted in homes20 and on the telephone.21,22 In this study, we conducted unannounced cell-phone-based pill counts. Participants were provided with a free cell phone that restricted service for project contacts and emergency use. Following office-based training in the pill counting procedure, participants were called every 21 to 35 days at unscheduled times by a phone assessor. Adherence data represents the percentage of pills taken as prescribed over 12 consecutive months.
We also assessed self-reported adherence at each monthly unannounced phone assessment using a standard measure of 3-day retrospective adherence recall.23,24 This format for assessing self-reported 3-day adherence has been found reliable and valid.25 The phone interviewer asked participants to think back about what they did over the past 3 days and recall the times they had taken each of their ART medications. Three-day treatment recall formed the basis for our day-level analysis of adherence.
Electronic Drinking Diary
We used a cell-phone delivered interactive text-diary assessment to collect day-level alcohol use. Brief daily assessments were delivered using interactive short message system (SMS) response. Electronic diaries have provided reliable data collection of socially sensitive behavioral data.26,27 Assessments occurred every other month during the study. Participants received a text-prompt to initiate and answer questions about their alcohol use during the previous day. The questions specifically asked about whether participants drank alcohol yesterday and if so, how much alcohol they drank that day. Daily drinking was recorded by entering numerical responses using the cell phone keypad. The data were stored on a central secured server. Drinking assessments were administered daily for 10 consecutive days, six times during the 12-month study, resulting in 60 days of drinking data. For the current study, we focused on the 3 days of drinking diary entries that coincided with the 3-day retrospective adherence data collected in the independently administered monthly cell-phone behavioral interviews.
Chart-Abstracted Viral Load and CD4 Cell Count
We used a participant-assisted method for collecting chart-abstracted viral load and CD4 cell counts from participants’ medical records. Participants were given a form that requested their doctor’s office to provide results and dates of their most recent viral load and CD4 cell counts. The form included a place for the provider’s office stamp or signature to assure authenticity. Participants collected their chart data at the end of the study, proximal to the final assessment.
Procedures
Following written informed consent, participants completed the ACASI assessment. Participants were then provided with a text-enabled study cell-phone and instructed in its use for both voice and text message functions. We trained participants in the steps required to compete the monthly unannounced pill counts as well as the bimonthly interactive text-response drinking diaries. We established a schedule for conducting the drinking diary assessments in synch with the 3-day retrospective recall adherence assessments. Specifically, the unannounced phone interviews were timed to commence after the fourth day of interactive text message drinking assessments, to allow for 3 overlapping days of self-report adherence and interactive text response drinking data. Drinking assessments were conducted every other month to reduce participant burden and potential assessment reactivity. Thus, we obtained time-linked drinking and adherence data for 18 days over the study period. Participants were provided with cash reimbursements for the baseline ACASI ($40), monthly unannounced pill counts ($20/month), electronic diary assessments ($2/day), and providing their viral load and CD4 counts from medical records ($25). Data were collected between November 30, 2009 and June 29, 2011, and the University of Connecticut Institutional Review Board approved the study.
Data Analyses
We first conducted descriptive analyses to examine the frequency of endorsed interaction beliefs and behaviors. We then formed two groups based on participants’ responses to two interactive toxicity behaviors; (a) individuals who indicated that they do not skip or stop their ART when drinking and (b) individuals who indicated that they do skip or stop their ART when drinking. The two groups were compared on demographics, health characteristics and alcohol–ART beliefs and behaviors using t-tests and contingency table X 2 tests. The adherence results stemmed from prospective analyses of unannounced pill counts conducted over the 12 months. We analyzed adherence using generalized estimating equations (GEE) with unstructured working correlation matrixes and binomial distribution for adherence defined as 85 % of prescribed pills taken. The GEE models controlled for total AUDIT scores and non-alcohol drug use.
Analyses of medication adherence in relation to alcohol use were also prospective, examining the 18 days of drinking diary data collected in synch with the same 18 days of self-reported ART adherence. For the day-level analysis, daily drinking and daily adherence were time-linked and coded for number of days on which participants both drank alcohol and did not take their ART. There were 3 days of linked adherence and drinking behaviors available for each bimonthly text assessment, yielding up to 18 daily assessments of linked adherence and drinking data. Analyses of daily adherence and drinking were performed using GEE, with unstructured working correlation matrixes and binomial distribution for daily events of non-adherence and drinking, controlling for AUDIT scores and drug use. All of the analyses were conducted in SPSS version 19.0, and used a complete case approach to missing data by including all available data for each analysis. GEE models were based on complete case estimations and report odds ratios with 95 % confidence intervals.
RESULTS
Among the 449 persons screened for the study, 344 (76 %) were currently taking ART and 320 (71 %) were currently drinking. A total of 178 (39 %) participants were both taking ART and currently drinking. All current drinkers who were taking ART agreed to participate. The study sample consisted of 129 men and 39 women, and 10 transgender persons. Participants were primarily African American (N = 165, 93 %) and were diverse with respect to their sexual orientation; 101 (56 %) identified as Gay, 49 (26 %) heterosexual, and 28 (18 %) identified as bisexual. The sample had a median of 12.5 years of education and 46 (26 %) were unemployed.
With respect to substance use, 126 (71 %) participants reported drinking once a week or less, 37 (21 %) drank two to three times a week, and 14 (8 %) drank at least four times a week. One hundred (56 %) participants reported drinking one to two drinks during typical drinking days, 50 (28 %) drank three to four drinks, and 27 (16 %) drank five or more drinks. A total of 74 (42 %) participants had received substance use treatment in their lifetime, with 22 (12 %) treated in the past year. The mean AUDIT score for the sample was 5.7 (SD = 6.0), with 44 (25 %) scoring above 8, possibly indicating hazardous drinking.
A total of 78 (43 %) participants reported that they skip taking their medications if they have been drinking alcohol, and 61 (34 %) indicated that they stop taking their medications if they know they will be drinking. In all, 90 (51 %) participants stated that they either skip their medications or stop taking them in relation to drinking. Conversely, 88 (49 %) did not report either skipping or stopping their ART when drinking. One participant reported stopping ART over the course of the study because they were drinking. Participants who reported skipping or stopping their medications indicated greater hazardous drinking, cocaine use and HIV symptoms. Participants who skipped or stopped their ART when drinking were also significantly more likely to have detectable viral loads, and more likely to have CD4 cell counts less than 200 cell/cc3. Groups did not differ on other participant characteristics (see Table 1).
Table 2 shows the prevalence of alcohol–ART interaction beliefs and behaviors. Participants who skip or stop their ART were more likely to endorse seven of the nine interaction beliefs and behaviors. It is noteworthy that interactive toxicity beliefs and behaviors were also common among participants who do not skip or stop ART when drinking.
ART Adherence
Analyses of ART adherence obtained from monthly unannounced pill counts, and controlling for alcohol and other drug use, showed that participants who reported skipping or stopping their medications when drinking were significantly more likely to have adherence below 85 % of pills taken (see Table 3).
Day-Level ART Adherence and Alcohol Use
Analyses showed that participants who stated that they skip or stop their medications when drinking also had poorer adherence assessed by the 3-day recall measure (see Table 3). Participants who skipped or stopped their ART when drinking did not differ in number of drinking days or the amount of alcohol they drank. Confirming our second hypothesis, we found that individuals who stated at baseline that they skip or stop their ART when drinking had significantly more days with concurrent alcohol use and missed medications at the day level.
Provider Communications
The majority of participants reported that medical care providers have discussed their alcohol use (see Table 4). Failing to confirm our third hypothesis, we did not find any differences between participants who do not skip or stop ART and those who do on any provider communications variables.
DISCUSSION
The current study observed poor treatment adherence among HIV-positive men and women who drink alcohol. Over the entire year of observation, 43 % of participants had lower than 85 % ART adherence, placing them at risk for HIV treatment failure and developing treatment resistant virus.1,28,29 Results of this prospective study also confirm previous research, finding that half of persons who drink and take ART skip or stop their medications when drinking. The associations between alcohol–ART beliefs and non-adherence were robust and observed for monthly unannounced pill counts, as well as bimonthly retrospective recall measures. Importantly, we observed an association between a propensity to skip or stop medications when drinking and the number of days that participants concurrently drank and missed medications. These data, for the first time, confirm prospective relationships of what has until now only been cross-sectional associations between alcohol-medication interactive beliefs and ART adherence. Thus, intentionally skipping or stopping ART when drinking is a source of non-adherence in people living with HIV.
Although ART and alcohol interactions can exacerbate hepatotoxicity in patients with HIV and liver disease, there are fewer known risks for alcohol interactions in patients with normal liver function. For many patients, the harms caused by ART non-adherence may outweigh those of ART and alcohol interactions. Nevertheless, the potential for adverse reactions exist in response to mixing ART with other drugs.9,10 In addition, the metabolic pathways involved in absorbing some classes of ART are shared with some classes of psychoactive drugs, such as amphetamine. In some cases, psychotropic medications can interfere with the absorption of ART, reducing antiviral potency and therefore effectiveness.11 The risks for adverse effects may therefore increase when multiple substances are used simultaneously with ART.
These findings should be interpreted in light of the study limitations. First, the sample was one of convenience and cannot be considered representative of people living with HIV infection. The study also relied on self-report instruments to assess health, beliefs, alcohol use and daily medication adherence. While each of these measures was collected using validated techniques, they may still be subject to biases. However, convergence of results with adherence assessed by unannounced pill counts, as well as medical chart-abstracted viral load and CD4 cell counts, bolsters confidence in our findings. Another limitation of our study was our definition of non-adherence applied to all medication regimens, which differ in their demand for optimal adherence. We selected 85 % adherence as a cut-off because most combination ART regimens risk resistance below this level of adherence.2,28,30 Having considered these limitations, we believe that the current study findings have implications for improving ART adherence among patients who drink.
People living with HIV who deliberately stop their medications when they are drinking are at risk for treatment failure. Although the detrimental impact of alcohol use on medication adherence has been described, only a few studies have examined medication interaction beliefs in relation to non-adherence.3 Interventions are needed to correct misinformation, dispel myths, and dispute interactive toxicity beliefs. People living with HIV who are receiving ART are by definition connected to care, affording numerous opportunities for patient education. Patients should be counseled to reduce their drinking as a matter of general health improvement, as well as to facilitate optimal medication adherence. More than 80 % of participants in this study reported that a provider has told them not to mix their ART with alcohol. However, only 65 % indicated that a provider specifically discussed their alcohol use. This discrepancy suggests that providers may offer advice about how to optimally take medications without necessarily addressing patients’ drinking. Thus, instructions to not mix medications with alcohol may be left open to patient interpretation. Providers may assume patients will stop drinking to avoid mixing their medications, whereas patients may actually stop taking their medications when they are drinking. Health care providers should therefore capitalize on routine clinical consultations to correct patient misperceptions and erroneous beliefs in relation to mixing alcohol and medications.
REFERENCES
Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3(7):e2783.
Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–9.
Hendershot CS, Stoner SA, Pantalone DW, et al. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202.
Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21(12):1579–89.
Braithwaite RS, McGinnis KA, Conigliaro J, et al. A temporal and dose–response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7.
Aloisi MS, Arici C, Balzano R, et al. Behavioral correlates of adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S145–8.
Sankar A, Wunderlich T, Neufeld S, et al. Sero-positive African Americans’ beliefs about alcohol and their impact on anti-retroviral adherence. AIDS Behav. 2007;11(2):195–203.
Kalichman SC, Amaral CM, White D, et al. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care STDs. 2009;23(6):449–54.
Neuman MG, Schneider M, Nanau RM, et al. HIV-antiretroviral therapy induced liver, gastrointestinal, and pancreatic injury. Int J Hepatol. 2012;2012:760706.
Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8(12):1002–12.
Szabo G, Zakhari S. Mechanisms of alcohol-mediated hepatotoxicity in human-immunodeficiency-virus-infected patients. World J Gastroenterol. 2011;17(20):2500–6.
Paquette D, Bryant J, de Wit J. Respondent-driven sampling and the recruitment of people with small injecting networks. AIDS Behav. 2012;16(4):890–9.
Gribble JN, Miller HG, Cooley PC, et al. The impact of T-ACASI interviewing on reported drug use among men who have sex with men. Subst Use Misuse. 2000;35(6–8):869–90.
Morrison-Beedy D, Carey MP, Tu X. Accuracy of audio computer-assisted self-interviewing (ACASI) and self-administered questionnaires for the assessment of sexual behavior. AIDS Behav. 2006;10(5):541–52.
Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis. 2000;188(10):662–70.
Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption II. Addictions. 1993;88(6):791–804.
Babor T, Higgins-Biddle J, Saunders J, et al. The Alcohol Use Disorders Identification Test: Guidelines for use inprimary Care. 2nd ed. Geneva: World Health Organization; 2001.
Maisto SA, Conigliaro J, McNeil M, et al. An empirical investigation of the factor structure of the AUDIT. Psychol Assess. 2000;12(3):346–53.
Altice F, Mostashari F, Friedland G. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:47–58.
Bangsberg DR, Hecht FM, Charlebois ED, et al. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic medication monitors and unannounced pill counts. AIDS Behav. 2001;5:275–81.
Kalichman SC, Amaral CM, Cherry C, et al. Monitoring Antiretroviral adherence by unannounced pill counts conducted by telephone: reliability and criterion-related validity. HIV Clin Trials. 2008;9:298–308.
Kalichman SC, Amaral CM, Stearns HL, et al. Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. J Gen Intern Med. 2007;22:1003–6.
Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12(3):255–66.
Ickovics JR, Meisler AW. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol. 1997;50(4):385–91.
Simoni J, Kurth AE, Pearson C, et al. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–331.
Bernhardt JM, Usdan S, Mays D, et al. Alcohol assessment among college students using wireless mobile technology. J Stud Alcohol Drugs. 2009;70(5):771–5.
McAuliffe TL, DiFranceisco W, Reed BR. Low numeracy predicts reduced accuracy of retrospective reports of frequency of sexual behavior. AIDS Behav. 2010;14(6):1320–9.
Bangsberg D, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72.
Li JZ, Paredes R, Ribaudo H, et al. Relationship between Minority NNRTI resistance mutations, adherence, and the risk of virologic failure. AIDS. 2011;26:185–92.
Bangsberg DR, Deeks SG. Is average adherence to HIV antiretroviral therapy enough? J Gen Intern Med. 2002;17(10):812–3.
Acknowledgements
An America Reinvestment and Recovery Act (ARRA) Challenge Grant from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) RC1AA018983 supported this research.
Conflict of Interest
The authors declare that they do not have a conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalichman, S.C., Grebler, T., Amaral, C.M. et al. Intentional Non-Adherence to Medications among HIV Positive Alcohol Drinkers: Prospective Study of Interactive Toxicity Beliefs. J GEN INTERN MED 28, 399–405 (2013). https://doi.org/10.1007/s11606-012-2231-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-012-2231-1