Abstract
Background
Outcomes of rectal adenocarcinoma vary considerably. Composite “textbook oncologic outcome” (TOO) is a single metric that estimates optimal clinical performance for cancer surgery.
Methods
Patients with stage II/III rectal adenocarcinoma who underwent single-agent neoadjuvant chemoradiation and proctectomy within 5–12 weeks were identified in the National Cancer Database (NCDB). TOO was defined as achievement of negative distal and circumferential resection margin (CRM), retrieval of ≥ 12 nodes, no 90-day mortality, and length of stay (LOS) < 75th percentile of corresponding year’s range. Multivariable logistic regression was used to identify predictors of TOO.
Results
Among 318,225 patients, 8869 met selection criteria. Median age was 62 years (IQR 54–71), and 5550 (62.6%) were males. Low anterior resection was the most common procedure (LAR, 6,037 (68.1%) and 3084 (34.8%) were treated at a high-volume center (≥ 20 rectal resections/year). TOO was achieved in 3967 patients (44.7%). Several components of TOO were achieved commonly, including negative CRM (87.4%), no 90-day mortality (98.0%), no readmission (93.0%), and no prolonged hospitalization (78.8%). Logistic regression identified increasing age, non-private insurance, low-volume centers, open approach, Black race, Charlson score ≥ 3, and abdominoperineal resection (APR) as predictors of failure to achieve TOO. Over time, TOOs were attained more commonly which correlated with increased minimally invasive surgery (MIS) adoption. TOO achievement was associated with improved survival.
Conclusions
Rectal adenocarcinoma patients achieve TOO uncommonly. Treatment at high-volume centers and MIS approach were among modifiable factors associated with TOO in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidence of rectal adenocarcinoma has increased in the USA, with > 43,000 cases diagnosed annually.1 A majority of patients with non-metastatic rectal adenocarcinoma present with invasive tumors which are not amenable to local excision and require transabdominal approaches, such as low anterior resection (LAR) or abdominoperineal resection (APR). Despite surgery remaining the cornerstone of curative-intent treatment, management has extensively evolved over the past few decades through incorporation of multimodal therapy and improvements in surgical technique.2,34.
Despite significant advances, variation across practices remains in the US, and factors affecting postoperative and oncological outcomes such as center volume and specialization have been studied.5,6,78 Conventionally, assessment of surgical quality for rectal adenocarcinoma has focused on unique outcomes such as perioperative morbidity and mortality, length of stay (LOS), and readmissions.9,10,11 Similarly, adherence to oncological standards is typically estimated by reporting on unique metrics such as margin status, lymph node (LN) numbers, and long-term survival.12,13 Solitary metrics may be imperfect when studying overall quality variation and are less relevant to stakeholders who may not fully grasp cancer care delivery nuances, including referring providers, administrators, and patients. The composite textbook outcome was introduced as a valuable encompassing measurement which reflects average “best” surgical quality.14 Indeed, textbook outcomes are particularly suited for gauging oncological quality as performance is typically affected by numerous variables. More recently, textbook oncologic outcomes (TOO) have been studied in the context of colonic, esophagogastric, and pancreatic malignancies.15,16,17 Importantly, attaining TOO has been shown to be associated with improved long-term outcomes.
The Commission on Cancer (CoC) National Accreditation Program for Rectal Cancer (NAPRC) was developed by the American College of Surgeons (ACS) to optimize RCA outcomes.18,19 However, to date, TOO’s have not been studied in rectal adenocarcinoma at CoC centers. We aimed in this study to describe TOO’s among patients undergoing proctectomy for rectal adenocarcinoma and to identify further factors associated with failure to achieve a TOO in this study.
Methods
Data Source
The National Cancer Database (NCDB) is a joint project of the American Cancer Society and the American College of Surgeons CoC. NCDB collects data from over 1500 CoC-accredited hospitals and includes over 70% of new cancer diagnoses in the USA.20,21 This study was granted institutional review board exemption. The Rectal Participant User File was utilized to identify all patients presenting with localized rectal adenocarcinoma (defined as nonmetastatic stage T3/T4 N0 or T-any N +) who underwent proctectomy (APR, LAR or pelvic exenteration) between 2010 and 2017. To reflect existing standards in management of stage II/III rectal adenocarcinoma, only patients who received single-agent neoadjuvant chemoradiation followed by proctectomy within 5–12 weeks of radiotherapy conclusion were included. Patients who received alternative doses, fractions, or radiation to areas other than the pelvis/rectum were excluded. Additional exclusion criteria were patients with different histologies, metastatic disease, multiple malignancies, those who underwent procedures other abdominal proctectomy (such as transanal excision, pull-through proctectomy, or unknown/unclear procedures), and receipt of multi-agent neoadjuvant chemotherapy. Patients with missing information on surgical approach (i.e., open, laparoscopic and robotic-assisted) were not included. Finally, patients with missing pathologic data on resection margin status, postoperative LOS, receipt of systemic chemotherapy, readmission rates, and 30- and 90- day mortality were excluded.
Textbook Oncologic Outcome
TOO definition was agreed upon a priori by all coauthors and was made to be consistent with previous studies which focused on malignancies other than rectal adenocarcinoma, and after considering relevant NAPRC standards.16,22,23,24 Ultimately, included elements were ones associated with optimal surgical and oncological outcomes for rectal cancer. A TOO was achieved when all components were met and was expressed as a percentage. Those were resection to negative margins (proximal, distal (DRM) and circumferential, CRM), American Joint Committee on Cancer (AJCC) compliant LN evaluation (≥ 12 LN), LOS less than or equal to the 75th percentile by treatment year and operative approach, no unplanned 30-day readmission, and no 90-day all-cause mortality.
Statistical Analysis
Conditional logistic regression was used to compare categorical variables and mixed effect modeling to compare continuous variables between groups. Kaplan–Meier method was utilized to study overall survival (OS), which was calculated from the date of diagnosis to the date of last contact or death. Cox proportional hazards model was utilized to determine the association between clinicopathologic factors, surgical approach, center volume and TOO, and overall survival. High volume centers were those that performed 20 or more proctectomies per year.25,26,27,28,29 A log-rank test was applied to compare OS between groups. Lastly, a backward stepwise multivariable logistic regression was performed to identify significant predictors of TOO. Conditional entry was set at p < 0.05 and exclusion at p ≥ 0.05. All demographic and clinical variables that are not components of TOO were included in the regression model’s first step. Variables which remained in the final model were reported and a Bonferroni correction was applied in order to reduce inflated likelihood of type I error.
SPSS v25 (Armonk, NY) with R essentials plug-in (V3.3.3) was used for statistical analysis. Adjusted odds ratios (ORs) and 95% confidence interval (CIs) were reported, with statistical significance set at p < 0.05 throughout the study.
Results
Textbook Oncologic Outcomes
NCDB included 189,849 cases of diagnosed rectal malignancies between 2010 and 2017. After application of inclusion/exclusion criteria, 8869 patients remained (Fig. 1). Median age was 62 years (IQR 54–71), and 5550 (62.6%) were males. Cases were divided evenly among clinical stage II and stage III (46.6% vs. 53.4%, respectively). The majority of patients had clinical T3 lesions (7763, 86.4%), and 4135 (46.6%) were clinically node-negative (N0). Two-thirds of patients underwent LAR (6037, 68.1%) and 1448 patients (16.3%) had a reported complete pathologic response (pCR). Only one-third of patients (N = 3084, 34.8%) received their procedure at a high-volume center (defined as ≥ 20 rectal resections per year). Of note, only 2279 patients (25.7%) of the selected population received adjuvant chemotherapy (AC) postoperatively. Table 1 summarizes demographic and perioperative characteristics of selected patients.
Of 8869 patients, 3967 (44.7%) achieved a TOO. Retrieval of ≥ 12 lymph nodes was the least commonly attained component (6257, 70.5%), whereas negative DRM occurred commonly (8583, 96.8%). A negative CRM (defined in NCDB as > 1 mm from tumor) was reported in 7751 patients (87.4%) and overall 90-day mortality was 2.0%. Table 2 summarizes details of TOO in the selected cohort. Over the study period, an uptrend was observed in incidence of TOO from 36% in 2010 to 51% in 2017.
Univariable and Multivariable Analysis of Factors Predictive of TOO
Table 3 summarizes final step of multivariable regression analysis of factors associated with TOO achievement. Notably, younger age, female gender, Caucasian race, lower T stage, undergoing LAR, MIS approach, private insurance, and treatment at high volume centers were all among factors associated with achieving a TOO (all P < 0.05). To better understand the impact of surgical approach on TOO incidence, chronological trends were plotted over time (Fig. 2). Interestingly, as utilization of robotic surgery increased from 2% in 2010 to 24% in 2017, so did TOO rates in patients approached in that manner. In contrast, incidence of TOO in patients who underwent open resection remained somewhat stable during the study period (range: 11–13%), an effect which mirrored rates of open approach. Finally, while percentage of laparoscopic surgery contribution to TOO increased from 8% in 2010 to 16% by 2014, it plateaued afterwards. Figure 3 demonstrates temporal and fractional relationships between TOO achievement and approach.
To better understand variables associated with attainment of individual TOO components, separate regression analyses were conducted in a similar manner (Supplemental Table 1). Several trends emerged. First, increasing T stage was associated with increased positive CRM and distal margin rates. Second, patients who underwent LAR were less likely to have incomplete lymphadenectomy, have positive margins, increased LOS, or be readmitted. Interestingly, while MIS was associated with higher rates of complete lymphadenectomy, it was linked to higher 30-day readmissions. Finally, White race/ethnicity was among factors protective of prolonged LOS, and 90-day mortality, however did not correlate with readmission rates.
Survival Analysis
Cox proportional hazards models were then performed to determine impact of TOO on OS (Table 4). Failure to achieve a TOO was associated with decreased OS independent of age, race, Charlson score, clinicopathologic variables, center volume, type of resection, and surgical approach (hazard ratio (HR): 0.590, CI: 0.530–0.656, p < 0.001). Conversely, characteristics independently associated improved OS included White race, lower Charlson scores, lower N and T stages, female gender, and receipt of AC (all P < 0.05).
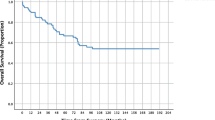
To better understand the interplay between receipt of AC and TOO incidence, unadjusted Kaplan–Meier survival functions were computed among various subgroups. As expected, patients who both achieved a TOO and received AC had the highest median survival (94.8 months ± 1.6 months) whereas patients who neither achieved a TOO nor received AC had the lowest (median 76.2 ± 0.9 months, Fig. 4). Interestingly, in patients who did not have a TOO, receipt of AC led to comparable survival to those who had a TOO but did not receive AC (89.8 ± 0.9 vs. 86.3 ± 1.3 months).
Discussion
In this study, which utilized a contemporary national dataset and included over 8000 patients, TOO was achieved in a minority of patients with nonmetastatic rectal adenocarcinoma undergoing proctectomy. TOO was defined as negative proximal, distal, and circumferential margins, regional lymph node counts consistent with national guidelines, no prolonged LOS, no 90-day mortality, and no unplanned 30-day readmission. Notably, increasing age, male gender, Black race, higher Charlson score, public insurance, cT4 lesions, undergoing APR or exenteration as opposed low anterior resection, open approach and care at low-volume centers were among factors predictive of inability to achieve TOO. Importantly, while a majority of TOO components occurred in most patients, increased adoption of robotic-assisted approach over the study period coincided with increased TOO rates. While a causal relationship between robotic surgery and TOO attainment was not established, it is possible that allowing a larger proportion of patients to undergo MIS translated to improved TOO rates in this study. As expected, achieving a TOO was associated with improved OS in this cohort, even after controlling for potential confounding factors. Finally, optimal OS occurred in patients who both achieved a TOO and had AC, whereas OS was comparable among patients who had AC but failed to achieve TOO and those who did not have AC but did attain a TOO.
In this report, multivariable analysis identified increasing age, male gender, Black race, higher Charlson score, public insurance, cT4 lesions, undergoing APR or exenteration as opposed to LAR, open approach and care at low-volume centers as factors associated with diminished odds of TOO attainment. Those results are consistent with similar studies which examined TOO in other relevant cancers. For example, in a study on colon cancer TOO by Swigert et al., logistic regression identified open cases, older age, Black race, non-private insurance, increased T stage, low volume centers, and the presence of lymphovascular invasion to be associated with decreased odds of achieving TOO.24 Undergoing APR or exenteration has been linked to inferior short-term outcomes and has been linked to positive CRM and may have further contributed to failure to achieve TOO in this cohort.30 Collectively, those findings reiterate the importance of establishing standards for rectal adenocarcinoma treatment centers as supported by the ACS and further highlight important disparities.
Guidelines from the National Comprehensive Cancer Network endorse AC for rectal adenocarcinoma patients who qualify for neoadjuvant chemoradiation; however, there remains debate on its value in low-risk tumors.31,32,33 For example, in a recent analysis of the NCDB, adjuvant chemotherapy did not confer a survival advantage in patients with T3N0M0 rectal adenocarcinoma patients who did not receive neoadjuvant chemoradiation, perhaps indicating a risk of overtreatment in stage II rectal adenocarcinoma. Conversely, another recent analysis of the NCDB found the contrary.34 While the benefit of postoperative chemotherapy following preoperative chemoradiotherapy in rectal adenocarcinoma has not been consistently demonstrated in randomized controlled trials,35 poor compliance to adjuvant systemic treatment is believed to be an essential limitation of those data.36 In the seminal EORTC trial, only 43% of patients received adjuvant treatment owing to postoperative complications, drug toxicity, disease progression, and patient refusal.37 This effect has been observed elsewhere.38 In this study, 2279 patients (25.7%) received AC after surgical resection and an incremental OS advantage was observed in patient subgroups who did. Interestingly, OS was comparable among patients who did not have a TOO and had AC and those who had a TOO but did not receive AC, which supports considering AC whenever possible.
Over the study period, a modest but statistically significant increase in rates of TOO attainment was noted which likely reflects improvements in surgical technique and perioperative care. To this extent, a TOO was more commonly achieved in patients who underwent an MIS approach, which also was generally utilized more frequently over time. More specifically, while rates of laparoscopic assisted resections plateaued after 2014, robotic-assisted utilization increased steadily. Those findings suggest that robotic-assisted techniques are more readily adoptable by surgeons, possibly due to a less steep learning curve compared to laparoscopy.39 MIS’s short-term benefits for rectal adenocarcinoma, including decreased pain, LOS, and overall complications, have been established,40,41 and large retrospective studies have reported incremental oncological advantages with MIS. For example, in a study that utilized NCBD, 6313 patients with nonmetastatic locally advanced rectal adenocarcinoma were included, and approaches (open, laparoscopic, and robotic) were compared.42 Compared with open surgery, the authors found that MIS was associated with lower positive circumferential margins and improved survival. Indeed, data from randomized trials supports equivalence of MIS and open approaches with respect to perioperative and oncological outcomes. Specifically, COLOR II found similar rates of CRM and comparable survival among MIS and open groups.43 Similarly, ACOSOG Z6051 found improved pathologic outcomes (including CRM and completeness of total mesorectal excision) with open surgery compared to MIS, whereas OS was similar.44 Finally, in AlaCaRT, laparoscopic approach failed to achieve non-inferiority with respect to similar pathologic outcomes.45 While this was not the case in the present study, it is possible that patients recruited to clinical trials are less susceptible to selection bias and that patients with more favorable clinicopathologic profiles are selected for MIS.
Treatment at high-volume centers (defined in this study as those that perform ≥ 20 proctectomies/year) emerged as a critical modifiable independent predictor of achieving a TOO. Low-volume centers were defined as those performing less than 20 proctectomies per year as this cutoff is associated with improved short- and long-term outcomes.46 Rectal adenocarcinoma’s center volume-outcome relationship has been long recognized as outcomes clearly and consistently are superior when rectal adenocarcinoma is treated at experienced institutions.26,46,47 Certainly, that high-volume centers more commonly achieved a TOO in this study is not unexpected and lends additional evidence regarding the value of center expertise, and is further consistent with the ACS CoC NAPRC’s mission.18,19.
In this report, Black race was among factors predictive of failure to achieve a TOO. Disparities in outcomes for black patients with rectal adenocarcinoma are well established and are consistently less favorable, even when treatment and stage are accounted for.48,49 Racial disparities in cancer care delivery are often a consequence of the interaction of a complex set of factors such as access to healthcare, conscious and unconscious bias, and lack of healthcare literacy.50,51 Findings from the present study are consistent with existing literature on this topic and lends additional evidence on how this group remains disadvantaged. Certainly, targeted interventions on the local and national scale are necessary in order to overcome those barriers.
Limitations of this study should be noted. First, it is a retrospective review of a large national oncology dataset and is therefore susceptible to selection and omitted variable biases. For example, selection bias may plausibly account for the association between TOO and age, which may affect the interpretation of survival outcomes. Second, despite rigorous quality-standard processes, errors in data coding are conceivable. Third, while TOO’s definition in this study was both clinically relevant and largely consistent with previous similar reports, it remains subjective as standardized components have not been agreed upon through consensus. Lastly, NCDB lacks granularity on relevant variables such as severity and impact of postoperative complications and type and extent of adjuvant therapy. Moreover, details of rates of diverting ileostomy formation and reasons for readmission are not captured in NCDB. This shortcoming may, in turn, limit interpretability as details on why adjuvant therapy was not pursued are not made clear. Given considerably low compliance rates, there likely were factors other than postoperative morbidity, which may have contributed to that effect. Despite those shortcomings, this study utilizes a robust national dataset to describe TOO’s in rectal adenocarcinoma and successfully identifies modifiable factors that may be targeted for future research that aims to improve outcomes in this population.
Conclusion
A TOO for nonmetastatic rectal adenocarcinoma was outlined in this study. This composite outcome metric may inform stakeholders on the overall quality of cancer care. This data also adds evidence to the significance of delivering comprehensive cancer care as TOO affects survival and only occurs in a minority of rectal adenocarcinoma patients.
References
Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-64. doi:https://doi.org/10.3322/caac.21601.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731-40. doi:https://doi.org/10.1056/NEJMoa040694.
Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81(9):503-8.
Ma B, Gao P, Wang H, Xu Q, Song Y, Huang X et al. What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer. 2017;141(5):1052-65. doi:https://doi.org/10.1002/ijc.30805.
Dorrance HR, Docherty GM, O'Dwyer PJ. Effect of surgeon specialty interest on patient outcome after potentially curative colorectal cancer surgery. Dis Colon Rectum. 2000;43(4):492-8. doi:https://doi.org/10.1007/BF02237192.
Archampong D, Borowski D, Wille-Jorgensen P, Iversen LH. Workload and surgeon's specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. 2012(3):CD005391. doi:https://doi.org/10.1002/14651858.CD005391.pub3.
Khani MH, Smedh K. Centralization of rectal cancer surgery improves long-term survival. Colorectal Dis. 2010;12(9):874-9. doi:https://doi.org/10.1111/j.1463-1318.2009.02098.x.
Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Who performs proctectomy for rectal cancer in the United States? Dis Colon Rectum. 2011;54(10):1210-5. doi:https://doi.org/10.1097/DCR.0b013e31822867a5.
Ricciardi R, Roberts PL, Read TE, Marcello PW, Schoetz DJ, Baxter NN. Variability in reconstructive procedures following rectal cancer surgery in the United States. Dis Colon Rectum. 2010;53(6):874-80. doi:https://doi.org/10.1007/DCR.0b013e3181cf6f58.
Huebner M, Hubner M, Cima RR, Larson DW. Timing of complications and length of stay after rectal cancer surgery. J Am Coll Surg. 2014;218(5):914-9. doi:https://doi.org/10.1016/j.jamcollsurg.2013.12.042.
Monson JR, Probst CP, Wexner SD, Remzi FH, Fleshman JW, Garcia-Aguilar J et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg. 2014;260(4):625–31; discussion 31–2. doi:https://doi.org/10.1097/SLA.0000000000000928.
Klos CL, Bordeianou LG, Sylla P, Chang Y, Berger DL. The prognostic value of lymph node ratio after neoadjuvant chemoradiation and rectal cancer surgery. Dis Colon Rectum. 2011;54(2):171-5. doi:https://doi.org/10.1007/DCR.0b013e3181fd677d.
Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89(3):327-34. doi:https://doi.org/10.1046/j.0007-1323.2001.02024.x.
Kolfschoten NE, Kievit J, Gooiker GA, van Leersum NJ, Snijders HS, Eddes EH et al. Focusing on desired outcomes of care after colon cancer resections; hospital variations in 'textbook outcome'. Eur J Surg Oncol. 2013;39(2):156-63. doi:https://doi.org/10.1016/j.ejso.2012.10.007.
Kulshrestha S, Bunn C, Patel PM, Sweigert PJ, Eguia E, Pawlik TM et al. Textbook oncologic outcome is associated with increased overall survival after esophagectomy. Surgery. 2020. doi:https://doi.org/10.1016/j.surg.2020.05.038.
Sweigert PJ, Eguia E, Baker MS, Link CM, Hyer JM, Paredes AZ et al. Assessment of Cancer Center Variation in Textbook Oncologic Outcomes Following Colectomy for Adenocarcinoma. J Gastrointest Surg. 2020. doi:https://doi.org/10.1007/s11605-020-04767-4.
Sweigert PJ, Eguia E, Baker MS, Paredes AZ, Tsilimigras DI, Dillhoff M et al. Assessment of textbook oncologic outcomes following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2020;121(6):936-44. doi:https://doi.org/10.1002/jso.25861.
Wexner SD, Berho ME. The Rationale for and Reality of the New National Accreditation Program for Rectal Cancer. Dis Colon Rectum. 2017;60(6):595-602. doi:https://doi.org/10.1097/DCR.0000000000000840.
Brady JT, Xu Z, Scarberry KB, Saad A, Fleming FJ, Remzi FH et al. Evaluating the Current Status of Rectal Cancer Care in the US: Where We Stand at the Start of the Commission on Cancer's National Accreditation Program for Rectal Cancer. J Am Coll Surg. 2018;226(5):881-90. doi:https://doi.org/10.1016/j.jamcollsurg.2018.01.057.
Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110(4):738–744. doi:https://doi.org/10.1002/cncr.22852.
Merkow RP, Rademaker AW, Bilimoria KY. Practical Guide to Surgical Data Sets: National Cancer Database (NCDB). JAMA Surg. 2018. doi:https://doi.org/10.1001/jamasurg.2018.0492.
Moris D, Cerullo M, Nussbaum DP, Blazer DG, 3rd. Textbook Outcomes Among Patients Undergoing Retroperitoneal Sarcoma Resection. Anticancer Res. 2020;40(4):2107-15. doi:https://doi.org/10.21873/anticanres.14169.
Merath K, Chen Q, Bagante F, Beal E, Akgul O, Dillhoff M et al. Textbook Outcomes Among Medicare Patients Undergoing Hepatopancreatic Surgery. Ann Surg. 2020;271(6):1116-23. doi:https://doi.org/10.1097/SLA.0000000000003105.
Sweigert PJ, Eguia E, Baker MS, Link CM, Hyer JM, Paredes AZ et al. Assessment of Cancer Center Variation in Textbook Oncologic Outcomes Following Colectomy for Adenocarcinoma. J Gastrointest Surg. 2021;25(3):775-85. doi:https://doi.org/10.1007/s11605-020-04767-4.
Marusch F, Koch A, Schmidt U, Pross M, Gastinger I, Lippert H. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg. 2001;88(10):1397-402. doi:https://doi.org/10.1046/j.0007-1323.2001.01873.x.
Rogers SO, Jr., Wolf RE, Zaslavsky AM, Wright WE, Ayanian JZ. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg. 2006;244(6):1003-11. doi:https://doi.org/10.1097/01.sla.0000231759.10432.a7.
van Gijn W, Gooiker GA, Wouters MW, Post PN, Tollenaar RA, van de Velde CJ. Volume and outcome in colorectal cancer surgery. Eur J Surg Oncol. 2010;36 Suppl 1:S55-63. doi:https://doi.org/10.1016/j.ejso.2010.06.027.
Damle RN, Macomber CW, Flahive JM, Davids JS, Sweeney WB, Sturrock PR et al. Surgeon volume and elective resection for colon cancer: an analysis of outcomes and use of laparoscopy. J Am Coll Surg. 2014;218(6):1223-30. doi:https://doi.org/10.1016/j.jamcollsurg.2014.01.057.
Koeter T, de Nes LCF, Wasowicz DK, Zimmerman DDE, Verhoeven RHA, Elferink MA et al. Hospital variation in sphincter-preservation rates in rectal cancer treatment: results of a population-based study in the Netherlands. BJS Open. 2021;5(4). doi:https://doi.org/10.1093/bjsopen/zrab065.
Rickles AS, Dietz DW, Chang GJ, Wexner SD, Berho ME, Remzi FH et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg. 2015;262(6):891-8. doi:https://doi.org/10.1097/SLA.0000000000001391.
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. doi:https://doi.org/10.6004/jnccn.2018.0061.
Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol. 2010;21(9):1743-50. doi:https://doi.org/10.1093/annonc/mdq054.
Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM et al. Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol. 2009;92(2):148-63. doi:https://doi.org/10.1016/j.radonc.2009.06.027.
de Paula TR, Gorroochurn P, Kiran RP, Keller DS. Does Adjuvant Chemotherapy Improve Survival in T3N0 Rectal Cancer? An Evaluation of Use and Outcomes from the National Cancer Database (NCDB). J Gastrointest Surg. 2020;24(5):1188-91. doi:https://doi.org/10.1007/s11605-020-04541-6.
Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16(2):200-7. doi:https://doi.org/10.1016/S1470-2045(14)71199-4.
Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335-42. doi:https://doi.org/10.1001/jama.2011.749.
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184-90. doi:https://doi.org/10.1016/S1470-2045(13)70599-0.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356-62. doi:https://doi.org/10.1093/annonc/mdu147.
Jimenez-Rodriguez RM, Rubio-Dorado-Manzanares M, Diaz-Pavon JM, Reyes-Diaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis. 2016;31(12):1807-15. doi:https://doi.org/10.1007/s00384-016-2660-0.
Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212(5):844-54. doi:https://doi.org/10.1016/j.jamcollsurg.2011.01.005.
Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008(2):CD003432. doi:https://doi.org/10.1002/14651858.CD003432.pub2.
Sujatha-Bhaskar S, Jafari MD, Gahagan JV, Inaba CS, Koh CY, Mills SD et al. Defining the Role of Minimally Invasive Proctectomy for Locally Advanced Rectal Adenocarcinoma. Ann Surg. 2017;266(4):574-81. doi:https://doi.org/10.1097/SLA.0000000000002357.
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324-32. doi:https://doi.org/10.1056/NEJMoa1414882.
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269(4):589-95. doi:https://doi.org/10.1097/SLA.0000000000003002.
Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD et al. Disease-free Survival and Local Recurrence After Laparoscopic-assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial. Ann Surg. 2019;269(4):596-602. doi:https://doi.org/10.1097/SLA.0000000000003021.
Chioreso C, Del Vecchio N, Schweizer ML, Schlichting J, Gribovskaja-Rupp I, Charlton ME. Association Between Hospital and Surgeon Volume and Rectal Cancer Surgery Outcomes in Patients With Rectal Cancer Treated Since 2000: Systematic Literature Review and Meta-analysis. Dis Colon Rectum. 2018;61(11):1320-32. doi:https://doi.org/10.1097/DCR.0000000000001198.
Abbas MA, Chang GJ, Read TE, Rothenberger DA, Garcia-Aguilar J, Peters W et al. Optimizing rectal cancer management: analysis of current evidence. Dis Colon Rectum. 2014;57(2):252-9. doi:https://doi.org/10.1097/DCR.0000000000000020.
Morris AM, Wei Y, Birkmeyer NJ, Birkmeyer JD. Racial disparities in late survival after rectal cancer surgery. J Am Coll Surg. 2006;203(6):787-94. doi:https://doi.org/10.1016/j.jamcollsurg.2006.08.005.
Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol. 2018;36(1):25-33. doi:https://doi.org/10.1200/JCO.2017.74.2049.
Lee DY, Teng A, Pedersen RC, Tavangari FR, Attaluri V, McLemore EC et al. Racial and Socioeconomic Treatment Disparities in Adolescents and Young Adults with Stage II-III Rectal Cancer. Ann Surg Oncol. 2017;24(2):311-8. doi:https://doi.org/10.1245/s10434-016-5626-0.
Fields AC, Lu PW, Yoo J, Irani J, Goldberg JE, Bleday R et al. Treatment of stage I-III rectal cancer: Who is refusing surgery? J Surg Oncol. 2020;121(6):990-1000. doi:https://doi.org/10.1002/jso.25873.
Author information
Authors and Affiliations
Contributions
Study concepts: FD, SN.
Study design: SN, FD, SK.
Manuscript preparation: FD, SN.
Data acquisition: SN, FD.
Quality control of data algorithm: SK, GS, BW, MA.
Manuscript review: SN, FD, SK, GS.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Synopsis
Textbook outcomes of rectal adenocarcinoma in NCDB are achieved uncommonly. MIS and volume are modifiable factors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naffouje, S.A., Ali, M.A., Kamarajah, S.K. et al. Assessment of Textbook Oncologic Outcomes Following Proctectomy for Rectal Cancer. J Gastrointest Surg 26, 1286–1297 (2022). https://doi.org/10.1007/s11605-021-05213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05213-9