Abstract
Introduction
Hepatic adenomas (HAs) are a benign and relatively rare type of liver neoplasms. We review the diagnosis, evaluation, and potential therapeutic management options for patients with HA.
Methods
A comprehensive review of the English literature was performed utilizing MEDLINE/PubMed and Web of Science databases with end of search date the 30th April of 2018. In PubMed, the terms “hepatocellular,” “hepatic,” “liver,” and “adenoma,” “adenomatosis” were searched in the title and/or abstract.
Results
Recent advances in molecular classification of HA have determined distinct subtypes with specific clinical, pathological, and imaging characteristics. In general, cessation of exogenous hormonal administration or weight loss may lead to HA regression. Surgical resection, either open or laparoscopic, should be considered in patients with symptoms and risk factors for hemorrhage or malignant transformation. These risk factors include tumor diameter greater than 5 cm, β-catenin activated subtype, and/or male gender. The management of acute hemorrhage should primarily aim at achieving hemodynamic stability via angioembolization followed by elective resection, whereas malignant transformation is treated according to oncologic resection principles. Although pregnancy is one of the known risk factors for tumor growth and associated complications, the presence of an HA per se should not be considered a contradiction to pregnancy.
Conclusion
Future genomic-based multicenter studies are required to provide a strong basis for formulating an evidence-based risk-adapted model that guides individualized management strategies for patients with HA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread use of imaging techniques has led to an increased incidence of detecting benign asymptomatic liver lesions, which are generally divided into two major categories: cystic and solid lesions.1 Benign solid lesions are further subdivided into regenerative lesions, including hemangiomas, focal nodular hyperplasia (FNH) and inflammatory pseudotumors, as well as neoplastic masses encompassing hepatocellular adenomas and angiomyolipomas.1 According to autopsy studies, the true prevalence of hepatic adenoma (HA) may be as high as 30–50% in the general population.2 Although HAs may be less frequently encountered in clinical practice compared with other lesions, their presence may be complicated by hemorrhage and/or malignant transformation.3
The two most common approaches to the management of HA include close surveillance and elective surgical resection.3,4 In general, symptomatic and large HAs (> 5 cm) often necessitate surgical excision, while criteria for treating smaller asymptomatic tumors are somewhat vague. Moreover, factors associated with increased hormone receipt, such as pregnancy, consuming estrogen-containing medications or anabolic androgen supplements, may influence treatment strategy.3 Recently, novel insights into the molecular pathogenesis of HAs have led to improved stratification of patients that are at high risk for developing tumor-associated complications and hence could benefit most from surgical resection. In this article, we review the diagnosis, evaluation, and potential therapeutic management options for patients with HA, with a particular focus on personalized therapeutics.
Methods
A comprehensive review of the English literature was performed utilizing MEDLINE/PubMed and Web of Science databases with end of search date the 30th April of 2018. In PubMed, the terms “hepatocellular,” “hepatic,” “liver,” and “adenoma,” “adenomatosis” were searched in the title and/or abstract. The references of relevant articles were reviewed to identify additional eligible publications. Articles were assessed according to the above eligibility criteria. An expert review of the eligible literature was performed and the most relevant and informative citations were identified for inclusion.
Definition: Adenoma vs. Adenomatosis
While HA has traditionally been defined as a solitary liver lesion, the term hepatic adenomatosis was first introduced in 1985 to describe a distinct entity characterized by the presence of ten or more liver adenomas.5 Other studies have described the clinical and histologic distinction between these two entities.6,7 However, recent molecular classification of HA has suggested that HA is a unified term that encompasses both solitary and multiple adenomas.8 Of note, the number of identified adenomas might significantly vary depending on the diagnostic method, which was utilized including type of imaging technique, macroscopic intraoperative observation, and quality of the pathology of the resected specimen.8
Risk Factors and Pathogenesis
In addition to metabolic and hormonal disturbances, there are several genetic and environmental factors that are known to contribute to the development and growth of HAs. The use of estrogen-containing contraceptive pills is a well-established predisposing factor among women of reproductive age that has been described since 1970s.9,10 In the era of modern contraceptives with substantially reduced estrogen dosage, there has been a remarkable decrease in the incidence of HAs.9 Interestingly, the epidemiologic gender differences in HA incidence has been described more commonly in western studies compared to eastern investigations, potentially due to a lower utilization of oral contraceptives among Asian populations.11 Furthermore, exogenous administration of steroids among patients with Fanconi or aplastic anemia, hereditary angioedema, high-performance athletes, and transsexuals has been associated with an increased risk of HA.12,13,14 Similarly, increased levels of endogenous sex hormones among pregnant women and patients with polycystic ovarian syndrome, or Klinefelter’s syndrome have been described as HA risk factors.3,15,16
Overweight and obese patients are known to be at an increased risk for the development of HA as well.17,18 The relation of obesity and HA might be partially mediated by the IL-6 molecular pathway.19 Considering its increasing prevalence, obesity is likely to increasingly become a major risk factor for HA among the general population. Other syndromes such as glycogen storage diseases type I and III have also been described as risk factors for HA with multifocal adenomas reported in up to 51 and 25% of patients with these diseases, respectively.20,21 The presence of extra- or intrahepatic portosystemic shunts have also been reported as risk factors for HA.22,23
Molecular Characteristics
Recent genomic studies have led to a better fundamental understanding of HA pathogenesis. Several studies using direct sequencing, quantitative reverse-transcription polymerase chain reaction (RT-PCR), and immunohistochemistry have categorized HAs into four groups with specific phenotypic and clinical characteristics (Table 1).24,25,26,27,28 The first HA subtype is characterized by inactivated hepatocyte nuclear factor 1α (HNF-1α) with somatic mutations of TCF1 in the majority of cases. This subtype presents the lowest risk for malignant transformation. The HNF-1α has also been implicated in familial hepatic adenomatosis associated with maturity onset diabetes of the young type 3 (MODY 3).29 The second subtype is β-catenin-activated HA, which has been shown to be associated with the highest risk of malignant transformation. The third and most common subgroup, inflammatory HA, is characterized by the deregulated Janus kinase—signal transducer of activation (JAK-STAT) pathway. This subtype has been associated with high BMI, alcohol consumption, and disturbances in the glycogen metabolism. The final subtype is an unclassified HA, a category of exclusion for HAs in the absence of other aforementioned features.
Recently, the results of a large-scale genomic analysis of 607 HA specimens led to an updated classification that included eight categories (Table 2).30 This classification was reported to be a better predictor of HA-associated complication risk. Distribution of the β-catenin subtype into two new groups according to the level of β-catenin activation, as well as introduction of a novel subtype reflecting the activation of the Sonic Hedgehog gene were key changes of this new classification. Interestingly, the Sonic Hedgehog subtype has been associated with obesity and higher risk of bleeding.30 More recent genomic analyses have described the role of additional genes such as NF-κB/RelA, Nrf2, SLC22A1, annexin A2, fibroblast growth factor receptor 4, chitinase 3-like 1, plasmalemma vesicle-associated protein, palladin, T-cell differentiation protein like, and cytoskeletal-associated protein in the development of HA; in the future, these genes may serve as potential candidates for targeted therapeutic intervention.31,32 Advances in genomic studies may help with the identification of molecular aberrations, which play a key role in HA pathogenesis and hence serve as a basis for targeted therapies. For example, the PPAR agonist, Fenofibrate, has been demonstrated to result in regression of multiple inflammatory HAs by disrupting IL-6-induced inflammation.33
Although genomic analyses have largely been performed in the resected specimens,27 more recent data have suggested that molecular characterization may be possible with core biopsies if adequate sample are available.24,30,34 In cases with only a few available specimens, the presence of β-catenin mutations should be the priority due to the implications in subsequent patient management. While biopsy for molecular profiling may be useful and informative, further data in this field are necessary before it can be considered the standard of care.
Diagnosis
Imaging Modalities
Imaging studies play a critical role in distinguishing between benign lesions such as FNH and lesions with potentially malignant behavior such as HAs. MRI has a sensitivity and specificity of 70 and 98%, respectively, and is therefore the modality of choice for the diagnosis of FNH.34 In addition, the sensitivity and specificity of MRI for HA may be as high as 88 and 100%, respectively. Although HA characteristics on MRI are highly variable, most adenomas are hyperintense on T1-weighted images and T2 images. HA typically appear as a hyperdense signal on T2-weighted series with persistent enhancement on delayed phase gadolinium-enhanced T1-weighted images (Fig. 1).35,36 Compared with conventional MRI, three-phase hepatobiliary MRI with delayed images has a specificity of 100% as well as high sensitivity and accuracy in the diagnosis of HA and hence is particularly valuable for HA smaller than 3 cm lesions.37 In addition, MRI may be able to differentiate among HA molecular subtypes (Table 1).34,38 For example, the HNF-1α inactivated subtype typically presents with arterial enhancement and intralesional fat that is diffusely distributed. Inflammatory HA also often present with arterial enhancement that sustains on portal and delayed phases and is hyperintense on T2-weighted series. These characteristic imaging findings are attributed to diffuse repartition of fat and dilatation of sinusoids in HNF-1α inactivated and inflammatory HA, respectively.39 On the other hand, β-catenin HA has similar features to hepatocellular carcinoma, whereas the unclassified subtype has no distinct characteristics on imaging.38 Low signal intensity in the hepatobiliary phase MRI may also not provide distinct characteristics for HA sub-categorization.40 The ability of MRI to accurately differentiate HA subtypes has been questioned due to the lack of data to provide direct comparisons of MRI with histology as the gold standard of diagnosis, although a retrospective study of 47 HA patients revealed a high agreement between MRI and pathology in diagnosing HA subtype.36,41 Radiological correlations with the recently updated genomic classification remain to be evaluated in the literature.
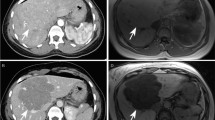
Hepatic adenomatosis in an asymptomatic 39-year-old female. Axial postgadolinium MRI demonstrated the hypervascular nature of all detected lesions with intense enhancement in the arterial phase (a) that persisted in the delayed phase (b). Images taken from De Kock I et al. 201435
In addition to MRI, US and CT scan may provide additional diagnostic value under certain circumstances.41 Contrast-enhanced US (CEUS) has particularly been described as an effective modality in the evaluation of hepatic lesions. In a recently published series of 324 patients, FNH and HA had distinct features in terms of homogeneity, echogenicity, arterial enhancement pattern, presence of a central scar, central artery, steatosis, necrosis or thrombus, and enhancement in the late venous phase on CEUS.42 Interestingly, the HNF-1α inactivated HA subtype has also been reported to present as a false-positive finding on 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) scan.43 The reason for this finding may relate to increased cell metabolism, spatial cell density, or presence of inflammatory cells related to high fat concentration.43 Future studies are required to better delineate the role of various imaging modalities in assessing HA.41 The same imaging modality, ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI), should be ideally performed for surveillance of HA in consecutive assessments. Figure 2 describes a case illustrating the value of MRI examination in the diagnosis and surveillance of HA.34
An illustrative case of a HA discovered incidentally in a 42-year-old female. Axial T2-weighted MRI (a) revealed an ill-defined lesion with vague hyperintense areas. A signal drop on the periphery of the lesion seen in axial T1 in-phase (b) and out-of-phase (c) MRI is attributable to the presence of perilesional steatosis. Following the administration of contrast agent, axial MRI showed moderate enhancement in the arterial phase (d) and mildly hypointense in the delayed phase (e). At that time, US-guided biopsy confirmed the diagnosis of HA with no molecular characterization. At the 2-year follow up MRI examination (f), T2-weighted sequence showed HA growth (short arrow) and vague hyperintense areas (long arrow). After the administration of contrast agent, moderate heterogeneous enhancement was seen in the arterial phase (g) that became mildly hypointense (short arrow) in the delayed phase (h) with mild delayed enhancement of a vague central scar (long arrow). Based on these findings, the patient underwent surgical resection of the lesion. The histo-immunopathology revealed the HA along with multifocal, well-differentiated HCC. Further genetic analysis demonstrated the presence of β-catenin mutation. Case and images were taken from Khanna M et al. 201534
Biopsy
Although tissue biopsy is the diagnostic gold standard, it should be performed only when imaging studies are inconclusive and surgical resection is not being considered or not feasible.3,44 Immunohistochemistry assessment of tissue specimens can significantly enhance the diagnostic accuracy of distinguishing among different HA molecular subtypes.36,45 Evaluation of surrounding non-tumoral hepatic tissue is also required to be sampled as well.44,46
Major Complications of HA
Bleeding
Acute hemorrhage is one of the potential complications of HA that might be associated with tumor rupture and subsequent hemoperitoneum. Among patients presenting with spontaneous liver hemorrhage, HA should always be considered in the differential diagnosis.47,48 In a systematic review of 1176 patients with HA, Van Aalten et al. reported an incidence rate of 27.2% for hemorrhage and 17.5% for rupture and intraperitoneal bleeding.49 Tumor diameter greater than 3.5 cm, history of hormone usage within the past 6 months, presence of prominent tumoral arteries, exophytic growth pattern, sub-capsular location, and localization in the left lateral section of the liver were identified as significant risk factors for HA bleeding.50,51 Both the HNF-1α and inflammatory HA subtypes may have an elevated risk of bleeding compared with the β-catenin subtype.34 Of note, the recently identified Sonic Hedgehog subtype has also been associated with a substantial risk of bleeding.30
Early diagnosis plays a critical role in the management of a bleeding HA. Following establishment of hemodynamic stability, imaging studies with intravenous contrast agents should be performed to identify the source of bleeding (Fig. 3).52 In the presence of active bleeding, hemostasis can typically be achieved with angiographic embolization. Definitive treatment includes surgical resection, ablation, or surveillance.47,48 Since the risk of re-bleeding can be as high as 5–10%, surgical resection should strongly be considered in patients with previous history of HA hemorrhage, especially among patients with tumor diameter greater than 5 cm.53
Bleeding HA in a 39-year-old female. A hyperintense mass with low peripheral signal was shown in axial T1WI in-phase (a) and T2WI with fat suppression (b). These findings were consistent with intralesional hemorrhage and haemosiderin rim deposits. Images taken from Shao N et al. 201852
Malignant Transformation
Malignant transformation is another complication of HA with a reported incidence of 4.2%.54 Histologically, hepatocellular carcinoma (HCC) develops directly within HA as a distinct nodule, suggestive of malignant transformation of adenoma cells rather than a synchronous lesion.4 Male gender, tumor size, and β-catenin subtype are known risk factors for malignancy among patients with HA. Specifically, men with HA have an eight- to tenfold increased risk of developing HCC with a 10-year cumulative risk of 60%.55,56 Malignant transformation usually occurs in large HAs and rarely occurs in tumors smaller than 5 cm.54 Among HA subtypes, the β-catenin activated subtype presents the highest risk of malignant transformation, with a reported rate as high as 50%.27 Since HCC might arise many years following diagnosis, patients with HA require lifelong surveillance.57 Patients undergoing androgen replacement therapy, such as individuals with Fanconi anemia or aplastic anemia, are at increased risk of malignant transformation as well. In contrast, consumption of oral contraceptives, underlying liver glycogen storage disease, and the number of liver adenomas have not been associated with risk of malignant transformation.55 HCC in the setting of HA usually has a better prognosis compared with non-HA-related HCC, mainly due to earlier detection and higher feasibility of complete surgical resection.4 Disease stage and patient characteristics usually dictate the locoregional as opposed to systematic treatment approach.58
Formulating a Personalized Therapeutic Approach
Considering its heterogeneous clinical course, the management of HA necessitates a multidisciplinary approach with tailored care addressing particular clinical situations (Fig. 4).59 The treatment strategy should be formulated according to the tumor characteristics such as the size and location, underlying liver disease, and patient-related variables such as the gender and general physical condition.8
Conservative Treatment
All exogenous hormone replacement therapies, including estrogens and androgens, should be discontinued upon diagnosis of HA.60 In a review of 96 HA patients who were undergoing hormonal therapy, Van Aalten et al. noted that withdrawal of oral contraceptives resulted in a regression rate of 79% with complete resolution of the tumor in some patients.61 Of note, the presence of multiple adenomas did not preclude a conservative approach.62 In obese patients, weight loss should be considered as an initial approach that may result in HA regression. In fact, bariatric surgery in obese patients has been shown to lead to tumor regression.63,64 In addition, dietary modification might also result in regression of HAs associated with glycogen storage disease type I.65
Conservative management with surveillance has been suggested for asymptomatic tumors smaller than 5 cm that typically have a benign and uncomplicated clinical course.60, 66 In the absence of worrisome characteristics, close follow-up with CT or MRI at 6-month intervals for the first 2 years and annually thereafter is a recommended approach. The reliability of serum alpha fetoprotein evaluation has not been established in the follow-up setting.12 Caution needs to be exerted, however, in cases with large HAs.63,67
While women of reproductive age historically have been advised against pregnancy, some investigators have suggested that pregnancy should not be discouraged among women with a HA smaller than 5 cm. In one study that documented close follow-up of 12 women with documented HA during a total of 17 pregnancies, 2 pregnancies (one patient) required cesarean section at 34–36 gestational week because of an assumed high risk of rupture and one patient underwent radiofrequency ablation in the first trimester.15 The clinical course of the other 14 pregnancies was uneventful with a successful maternal and fetal outcome that did not require any intervention. The Pregnancy And Liver adenoma Management (PALM) study is an ongoing European multicenter prospective study designed to investigate the clinical course of 50 pregnant patients with small HA (< 5 cm). Although pregnancy may be associated with increased risk of HA-related complications, the presence of small asymptomatic HA per se is not considered as a contraindication for pregnancy. Women with symptoms, tumor size > 5 cm, or with a previous history of complicated HA should be considered, however, for surgical resection prior to planned pregnancy.15,68
Angiographic Embolization
Transarterial embolization (TAE) is considered a first option in the management of hemodynamically stable patients with bleeding HA.3 TAE has also been performed in the elective setting as an alternative to surgical intervention. In a systematic review of 851 patients with HA, 151 patients (17.7%) underwent TAE with a reported tumor regression rate of 75%. Complete tumor disappearance was observed in 10% of patients and surgery was avoided in 45% of patients.69 The rate of surgery prevention in 49 patients who had elective TAE was as high as 84%. In a separate study, Zhao et al. proposed TAE as a safe alternative to surgical resection in the elective management of most HAs.70 Overall complete and partial response rates were 10.6 and 71.7%, respectively, with no TAE-associated mortality.
Ablative techniques such as microwave ablation, percutaneous irreversible electroporation, and thermal ablation may also be considered for patients with underlying medical comorbidities who are not candidates for surgical resection or in patients with centrally located tumors.71,72,73 While current studies have only involved a limited number of patients, ablative methods have typically demonstrated efficacy for small HAs (< 5 cm).
Surgical Resection
Surgical resection has traditionally been the preferred therapeutic approach for the management of symptomatic and large (< 5 cm) HAs. Although hepatectomy is considered to be a safe procedure, major complications and perioperative mortality can still occur, necessitating appropriate patient selection.74,75,76 Recent reports of liver resection for HAs, including hemorrhagic cases, have confirmed the safety of the procedure in high-volume centers with a reported perioperative mortality of approximately 0.5%.77,78,79,80 Surgical resection is generally recommended for patients who are at substantial risk of developing complications such as those with tumor size larger than 5 cm, increasing size, β-catenin activated subtype, imaging features suspicious for malignancy, concurrent dysplasia and/or inability to rule out HCC, progressively rising alpha fetoprotein levels, and male patients.26 As the number of lesions has not been associated with additional risk of complications, patients with hepatic adenomatosis can follow the same criteria for surgical resection.26
One controversial issue in the surgical management of HAs is the timing of resection after cessation of hormonal therapy. Current guidelines suggest that surgical resection should be considered in patients who have HA greater than 5 cm and whose tumor does not regress or progresses during the 6-month interval following interruption of oral contraceptives. However, Klompenhouwer et al. reported that 69 of 118 patients (58.5%) with HA had tumor size regression to 5 cm or smaller after a median follow-up of 104 weeks (95% CI 80–128).81 In addition, the time to regression was longer for patients who initially had larger tumor. Since no complications were observed during the follow-up period, the authors recommended increasing the surveillance period for assessment of regression following discontinuation of contraceptives to 12 months.
Laparoscopic surgical resection is also a feasible option with comparable efficacy and safety compared with open resection. In fact, the minimally invasive approach has been associated with less intraoperative blood loss (93 vs. 196 ml, p < 0.001), reduced need for transfusion (8 vs. 24 red blood cells units, p < 0.001), and shorter hospital stay (5 vs. 7 days, p < 0.001) versus open surgical resection, respectively.82
Liver Transplantation
Liver transplantation (LT) for HA should be restricted to very select situations.83 The presence of multiple lesions with suspicious or proven malignant transformation, not amenable to surgical resection, is considered the main indication for LT in patients with HAs. The presence of a portosystemic venous shunt has also been reported to be another indication for LT.84,85 In one of the largest reported case series, Chiche et al. proposed a guideline that included one major criteria (histologic proof of malignant transformation) and five minor criteria (more than two previous life-threatening hemorrhage, more than two previous hepatectomies, β-catenin mutated or inflammatory adenomas, underlying liver disease such as major steatosis or vascular abnormalities, and age > 30 years) as a useful tool to guide LT decision making in patients with HAs.84 Patients with either one major criteria or at least three minor criteria were considered candidates for LT.
Conclusion
HAs are benign lesions of the liver with heterogeneous clinical course and potential for developing major complications such as acute hemorrhage and malignant transformation. Recent advances in genomic profiling have contributed to a better understanding of molecular pathogenesis of HA and its potential association with long-term outcomes. A wide arrange of therapeutic options should be considered when managing HA (Table 3). Considering the heterogeneous behavior of HAs, future studies with a focus on molecular markers predicting the clinical course of adenomas are required to formulate risk-stratified management strategies.
Change history
10 October 2019
The following reference in this paper refers to a paper that has been since retracted.
10 October 2019
The following reference in this paper refers to a paper that has been since retracted.
References
Belghiti J, Cauchy F, Paradis V, Vilgrain V. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014;11(12):737–49.
Cristiano A, Dietrich A, Spina JC, Ardiles V, de Santibanes E. Focal nodular hyperplasia and hepatic adenoma: current diagnosis and management. Updates Surg. 2014;66(1):9–21.
Agrawal S, Agarwal S, Arnason T, Saini S, Belghiti J. Management of hepatocellular adenoma: recent advances. Clin Gastroenterol Hepatol. 2015;13(7):1221–30.
Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21(4):491–7.
Flejou JF, Barge J, Menu Y, Degott C, Bismuth H, Potet F, et al. Liver adenomatosis. An entity distinct from liver adenoma? Gastroenterology. 1985;89(5):1132–8.
Ribeiro A, Burgart LJ, Nagorney DM, Gores GJ. Management of liver adenomatosis: results with a conservative surgical approach. Liver Transpl Surg. 1998;4(5):388–98.
Grazioli L, Federle MP, Ichikawa T, Balzano E, Nalesnik M, Madariaga J. Liver adenomatosis: clinical, histopathologic, and imaging findings in 15 patients. Radiology. 2000;216(2):395–402.
Frulio N, Chiche L, Bioulac-Sage P, Balabaud C. Hepatocellular adenomatosis: what should the term stand for! Clin Res Hepatol Gastroenterol. 2014;38(2):132–6.
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA. 1979;242(7):644–8.
Baum JK, Bookstein JJ, Holtz F, Klein EW. Possible association between benign hepatomas and oral contraceptives. Lancet. 1973;2(7835):926–9.
Lin H, van den Esschert J, Liu C, van Gulik TM. Systematic review of hepatocellular adenoma in China and other regions. J Gastroenterol Hepatol. 2011;26(1):28–35.
Vijay A, Elaffandi A, Khalaf H. Hepatocellular adenoma: An update. World J Hepatol. 2015;7(25):2603–9.
Paustian L, Chao MM, Hanenberg H, Schindler D, Neitzel H, Kratz CP, et al. Androgen therapy in Fanconi anemia: a retrospective analysis of 30 years in Germany. Pediatr Hematol Oncol. 2016;33(1):5–12.
Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol. 2004;77(3):257–67.
Noels JE, van Aalten SM, van der Windt DJ, Kok NF, de Man RA, Terkivatan T, et al. Management of hepatocellular adenoma during pregnancy. J Hepatol. 2011;54(3):553–8.
van Aalten SM, Broker ME, Busschbach JJ, de Koning HJ, de Man RA, Steegers EA, et al. Pregnancy and liver adenoma management: PALM-study. BMC Gastroenterol. 2012;12:82.
Broker MEE, Gaspersz MP, Klompenhouwer AJ, Hansen BE, Terkivatan T, Taimr P, et al. Inflammatory and multiple hepatocellular adenoma are associated with a higher BMI. Eur J Gastroenterol Hepatol. 2017;29(10):1183–8.
Bunchorntavakul C, Bahirwani R, Drazek D, Soulen MC, Siegelman ES, Furth EE, et al. Clinical features and natural history of hepatocellular adenomas: the impact of obesity. Aliment Pharmacol Ther. 2011;34(6):664–74.
Bioulac-Sage P, Taouji S, Possenti L, Balabaud C. Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int. 2012;32(8):1217–21.
Oterdoom LH, Verweij KE, Biermann K, Langeveld M, van Buuren HR. Hepatocellular adenomas and carcinoma in asymptomatic, non-cirrhotic type III glycogen storage disease. J Gastrointestin Liver Dis. 2015;24(4):515–8.
Demo E, Frush D, Gottfried M, Koepke J, Boney A, Bali D, et al. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol. 2007;46(3):492–8.
Sanada Y, Mizuta K, Niki T, Tashiro M, Hirata Y, Okada N, et al. Hepatocellular nodules resulting from congenital extrahepatic portosystemic shunts can differentiate into potentially malignant hepatocellular adenomas. J Hepatobiliary Pancreat Sci. 2015;22(10):746–56.
Seyama Y, Sano K, Tang W, Kokudo N, Sakamoto Y, Imamura H, et al. Simultaneous resection of liver cell adenomas and an intrahepatic portosystemic venous shunt with elevation of serum PIVKA-II level. J Gastroenterol. 2006;41(9):909–12.
Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med. 2014;138(8):1090–7.
Raft MB, Jorgensen EN, Vainer B. Gene mutations in hepatocellular adenomas. Histopathology. 2015;66(7):910–21.
Liau SS, Qureshi MS, Praseedom R, Huguet E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg. 2013;17(10):1869–82.
Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43(3):515–24.
Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46(3):740–8.
Cantu S, Krier J, Hashemi N. Hepatocyte nuclear factor 1alpha mutation-associated MODY-3 and familial liver adenomatosis. J Clin Gastroenterol. 2016;50(2):181–2.
Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152(4):880–94 e6.
Kohler UA, Bohm F, Rolfs F, Egger M, Hornemann T, Pasparakis M, et al. NF-kappaB/RelA and Nrf2 cooperate to maintain hepatocyte integrity and to prevent development of hepatocellular adenoma. J Hepatol. 2016;64(1):94–102.
Liu D, Liu P, Cao L, Zhang Q, Chen Y. Screening the key genes of hepatocellular adenoma via microarray analysis of DNA expression and methylation profiles. Oncol Lett. 2017;14(4):3975–80.
Poupon R, Cazals-Hatem D, Arrive L. Fenofibrate-induced massive regression of mutiple inflammatory hepatocellular adenoma. Clin Res Hepatol Gastroenterol. 2016;40(1):e1–3.
Khanna M, Ramanathan S, Fasih N, Schieda N, Virmani V, McInnes MD. Current updates on the molecular genetics and magnetic resonance imaging of focal nodular hyperplasia and hepatocellular adenoma. Insights Imaging. 2015;6(3):347–62.
De Kock I, Mortele KJ, Smet B, Gillardin P, Pauwels W, De Backer AI. Hepatic adenomatosis: MR imaging features. JBR-BTR. 2014;97(2):105–8.
Ronot M, Bahrami S, Calderaro J, Valla DC, Bedossa P, Belghiti J, et al. Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology. 2011;53(4):1182–91.
Roux M, Pigneur F, Baranes L, Calderaro J, Chiaradia M, Decaens T, et al. Differentiating focal nodular hyperplasia from hepatocellular adenoma: is hepatobiliary phase MRI (HBP-MRI) using linear gadolinium chelates always useful? Abdom Radiol (NY). 2017.
Dharmana H, Saravana-Bawan S, Girgis S, Low G. Hepatocellular adenoma: imaging review of the various molecular subtypes. Clin Radiol. 2017;72(4):276–85.
Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48(3):808–18.
Guo Y, Li W, Cai W, Zhang Y, Fang Y, Hong G. Diagnostic value of gadoxetic acid-enhanced mr imaging to distinguish HCA and its subtype from FNH: a systematic review. Int J Med Sci. 2017;14(7):668–74.
van den Esschert JW, van Gulik TM, Phoa SS. Imaging modalities for focal nodular hyperplasia and hepatocellular adenoma. Dig Surg. 2010;27(1):46–55.
Taimr P, Broker MEE, Dwarkasing RS, Hansen BE, de Knegt RJ, De Man RA, et al. A model-based prediction of the probability of hepatocellular adenoma and focal nodular hyperplasia based on characteristics on contrast-enhanced ultrasound. Ultrasound Med Biol. 2017;43(10):2144–50.
Lee SY, Kingham TP, LaGratta MD, Jessurun J, Cherqui D, Jarnagin WR, et al. PET-avid hepatocellular adenomas: incidental findings associated with HNF1-alpha mutated lesions. HPB (Oxford). 2016;18(1):41–8.
Sannier A, Cazejust J, Lequoy M, Cervera P, Scatton O, Rosmorduc O, et al. Liver biopsy for diagnosis of presumed benign hepatocellular lesions lacking magnetic resonance imaging diagnostic features of focal nodular hyperplasia. Liver Int. 2016;36(11):1668–76.
Bioulac-Sage P, Cubel G, Taouji S, Scoazec JY, Leteurtre E, Paradis V, et al. Immunohistochemical markers on needle biopsies are helpful for the diagnosis of focal nodular hyperplasia and hepatocellular adenoma subtypes. Am J Surg Pathol. 2012;36(11):1691–9.
Agostini-Vulaj D, Sharma AK, Findeis-Hosey JJ, McMahon LA, Gonzalez RS. Distinction between inflammatory hepatocellular adenoma and mass effect on liver sampling. Hum Pathol. 2017;61:105–10.
Rosales A, Que FG. Spontaneous hepatic hemorrhage: a single institution's 16-year experience. Am Surg. 2016;82(11):1117–20.
Srinivasa S, Lee WG, Aldameh A, Koea JB. Spontaneous hepatic haemorrhage: a review of pathogenesis, aetiology and treatment. HPB (Oxford). 2015;17(10):872–80.
van Aalten SM, de Man RA, JN IJ, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99(7):911–6.
Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM. Risk factors for bleeding in hepatocellular adenoma. Br J Surg. 2014;101(7):847–55.
Deneve JL, Pawlik TM, Cunningham S, Clary B, Reddy S, Scoggins CR, et al. Liver cell adenoma: a multicenter analysis of risk factors for rupture and malignancy. Ann Surg Oncol. 2009;16(3):640–8.
Shao N, Pandey A, Ghasabeh MA, Khoshpouri P, Pandey P, Varzaneh FN, et al. Long-term follow-up of hepatic adenoma and adenomatosis: analysis of size change on imaging with histopathological correlation. Clin Radiol. 2018.
Klompenhouwer AJ, de Man RA, Thomeer MG, Ijzermans JN. Management and outcome of hepatocellular adenoma with massive bleeding at presentation. World J Gastroenterol. 2017;23(25):4579–86.
Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12(8):509–22.
Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig Surg. 2010;27(1):32–8.
Bossen L, Gronbaek H, Lykke Eriksen P, Jepsen P. Men with biopsy-confirmed hepatocellular adenoma have a high risk of progression to hepatocellular carcinoma: a nationwide population-based study. Liver Int. 2017;37(7):1042–6.
Sempoux C, Bisig B, Couchy G, Balabaud C, Zucman-Rossi J, Bioulac-Sage P. Malignant transformation of a beta-catenin inflammatory adenoma due to an S45 beta-catenin-activating mutation present 12 years before. Hum Pathol. 2017;62:122–5.
Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23(2):289–311.
Blanc JF, Frulio N, Chiche L, Sempoux C, Annet L, Hubert C, et al. Hepatocellular adenoma management: call for shared guidelines and multidisciplinary approach. Clin Res Hepatol Gastroenterol. 2015;39(2):180–7.
van Aalten SM, Terkivatan T, de Man RA, van der Windt DJ, Kok NF, Dwarkasing R, et al. Diagnosis and treatment of hepatocellular adenoma in the Netherlands: similarities and differences. Dig Surg. 2010;27(1):61–7.
van Aalten SM, Witjes CD, de Man RA, Ijzermans JN, Terkivatan T. Can a decision-making model be justified in the management of hepatocellular adenoma? Liver Int. 2012;32(1):28–37.
Sinclair M, Schelleman A, Sandhu D, Angus PW. Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy. Hepatology. 2017;66(3):989–91.
Dokmak S, Belghiti J. Will weight loss become a future treatment of hepatocellular adenoma in obese patients? Liver Int. 2015;35(10):2228–32.
Khaoudy I, Rebibo L, Regimbeau JM. Is bariatric surgery a potential new treatment for large inflammatory hepatocellular adenomas in obese patients? Surg Obes Relat Dis. 2018;14(4):535–8.
Beegle RD, Brown LM, Weinstein DA. Regression of hepatocellular adenomas with strict dietary therapy in patients with glycogen storage disease type I. JIMD Rep. 2015;18:23–32.
van der Windt DJ, Kok NF, Hussain SM, Zondervan PE, Alwayn IP, de Man RA, et al. Case-orientated approach to the management of hepatocellular adenoma. Br J Surg. 2006;93(12):1495–502.
Yamaguchi T, Kokudo T, Zingg T, Halkic N. The management of hepatocellular adenoma in obese patients: issues to consider. Liver Int. 2016;36(1):152.
Wilson CH, Manas DM, French JJ. Laparoscopic liver resection for hepatic adenoma in pregnancy. J Clin Gastroenterol. 2011;45(9):828–33.
van Rosmalen BV, Coelen RJS, Bieze M, van Delden OM, Verheij J, Dejong CHC, et al. Systematic review of transarterial embolization for hepatocellular adenomas. Br J Surg. 2017;104(7):823–35.
Zhao C, Pei SL, Cucchetti A, Tong TJ, Ma YL, Zhong JH, et al. Systematic review: benefits and harms of transarterial embolisation for treating hepatocellular adenoma. Aliment Pharmacol Ther. 2017.
Mironov O, Jaberi A, Beecroft R, Kachura JR. Retrospective single-arm cohort study of patients with hepatocellular adenomas treated with percutaneous thermal ablation. Cardiovasc Intervent Radiol. 2018;41(6):935–41.
Scheffer HJ, Melenhorst MC, van Tilborg AA, Nielsen K, van Nieuwkerk KM, de Vries RA, et al. Percutaneous irreversible electroporation of a large centrally located hepatocellular adenoma in a woman with a pregnancy wish. Cardiovasc Intervent Radiol. 2015;38(4):1031–5.
Smolock AR, Cristescu MM, Potretzke TA, Ziemlewicz TJ, Lubner MG, Hinshaw JL, et al. Microwave ablation for the treatment of hepatic adenomas. J Vasc Interv Radiol. 2016;27(2):244–9.
van Rosmalen BV, Bieze M, Besselink MG, Tanis P, Verheij J, Phoa SS, et al. Long-term outcomes of resection in patients with symptomatic benign liver tumours. HPB (Oxford). 2016;18(11):908–14.
Newhook TE, LaPar DJ, Lindberg JM, Bauer TW, Adams RB, Zaydfudim VM. Morbidity and mortality of hepatectomy for benign liver tumors. Am J Surg. 2016;211(1):102–8.
Hoffmann K, Unsinn M, Hinz U, Weiss KH, Waldburger N, Longerich T, et al. Outcome after a liver resection of benign lesions. HPB (Oxford). 2015;17(11):994–1000.
Hau HM, Kloss A, Wiltberger G, Jahn N, Krenzien F, Benzing C, et al. The challenge of liver resection in benign solid liver tumors in modern times—in which cases should surgery be done? Z Gastroenterol. 2017;55(7):639–52.
Laurent A, Dokmak S, Nault JC, Pruvot FR, Fabre JM, Letoublon C, et al. European experience of 573 liver resections for hepatocellular adenoma: a cross-sectional study by the AFC-HCA-2013 study group. HPB (Oxford). 2016;18(9):748–55.
Addeo P, Cesaretti M, Fuchshuber P, Langella S, Simone G, Oussoultzoglou E, et al. Outcomes of liver resection for haemorrhagic hepatocellular adenoma. Int J Surg. 2016;27:34–8.
Bieze M, Busch OR, Tanis PJ, Verheij J, Phoa SS, Gouma DJ, et al. Outcomes of liver resection in hepatocellular adenoma and focal nodular hyperplasia. HPB (Oxford). 2014;16(2):140–9.
Klompenhouwer AJ, Broker MEE, Thomeer MGJ, Gaspersz MP, de Man RA, JNM IJ. Retrospective study on timing of resection of hepatocellular adenoma. Br J Surg. 2017;104(12):1695–703.
Landi F, De’ Angelis N, Scatton O, Vidal X, Ayav A, Muscari F, et al. Short-term outcomes of laparoscopic vs. open liver resection for hepatocellular adenoma: a multicenter propensity score adjustment analysis by the AFC-HCA-2013 study group. Surg Endosc. 2017;31(10):4136–44.
Sundar Alagusundaramoorthy S, Vilchez V, Zanni A, Sourianarayanane A, Maynard E, Shah M, et al. Role of transplantation in the treatment of benign solid tumors of the liver: a review of the United Network of Organ Sharing data set. JAMA Surg. 2015;150(4):337–42.
Chiche L, David A, Adam R, Oliverius MM, Klempnauer J, Vibert E, et al. Liver transplantation for adenomatosis: European experience. Liver Transpl. 2016;22(4):516–26.
Thapar M, Grapp O, Fisher C. Management of hepatic adenomatosis. Curr Gastroenterol Rep. 2015;17(3):12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None declared.
Rights and permissions
About this article
Cite this article
Tsilimigras, D.I., Rahnemai-Azar, A.A., Ntanasis-Stathopoulos, I. et al. Current Approaches in the Management of Hepatic Adenomas. J Gastrointest Surg 23, 199–209 (2019). https://doi.org/10.1007/s11605-018-3917-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3917-4