Abstract
Background
The treatment of pancreatic stump is a critical step of pancreatoduodenectomy (PD) because leaks from this anastomosis incur major morbidity and mortality. We describe the technical details of a modified end-to-side pancreatojejunostomy (mPJ), and report on the outcome of the first 100 patients.
Methods
From October 2008 to June 2017, 424 pancreatic resections were performed, of which 203 were PD. The mPJ was introduced in November 2010 and used in 100 consecutive patients, by a single surgeon. Data were retrieved from a prospectively collected Institutional database, and used for the present retrospective evaluation. Post-operative pancreatic fistulas (POPF) were stratified with the Fistula Risk Score (FRS), based on the 2005-International Study Group of Pancreatic Fistula classification (ISGPFc) and on the subsequent 2016-revised version (ISGPSc).
Results
ISGPFc POPF occurred in 17/100 (17%): grade A in 10/100 (10%), grade B in 6/100 (6%) and grade C in 1/100 (1%). On the ISGPSc, POPF rate averaged 7%: grade B in 6/100 (6%) and grade C in 1/100 (1%). POPF rate associated with high FRS was 18.8%/6.3% (ISGPFc/ISGPSc). With low and intermediate FRS, POPFs were 5.3%/0% (ISGPFc/ISGPSc) and 21.3%/9.8% (ISGPFc/ISGPSc) respectively. Re-operation rate was 3%. In-hospital mortality rate was 2% and specific mortality rate for POPF was 1%.
Conclusions
The mPJ technique is associated with a POPF rate which was less than expected, especially for "difficult" pancreas with high FRS (soft gland texture and small duct). A larger prospective series is needed in addition to comparative studies with other techniques for robust assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most critical step of the pancreatoduodenectomy (PD) is the pancreatic reconstruction, since postoperative leakage from this anastomosis accounts for most major complications after this operation. Although during the past two decades the operative mortality has decreased dramatically, the overall morbidity rate remains high even in tertiary care centers with 30–50% of patients experiencing one or more problems, with the most serious being associated with failure of the pancreatic anastomosis, the incidence of which has remained relatively unchanged.1,2 To date, there is no universally accepted technique for management of the pancreatic stump, despite the many anastomotic techniques and methods of reconstruction, which have been proposed and investigated in the quest to reduce the incidence of postoperative pancreatic fistula (POPF).3 The most used fistula grading system is the International Study Group of Pancreatic Fistula classification (ISPGFc) proposed in 2005,4 revised in 2016 as International Study Group in Pancreatic Surgery classification (ISGPSc).5 The site of anastomosis (jejunal limb or stomach) and the anastomotic technique itself have been the most investigated in the recent published literature,3,6,7,8,9,10,11 pancreatojejunostomy (PJ) being the most common form of reconstruction practiced by 89% of surgeons.12 In the quest for decreasing the incidence of pancreatic leakage, various PJ methods have been described as alternatives to more traditional techniques with preliminary encouraging results.13,14 However, each has its intrinsic drawbacks and to date, no randomized trials have demonstrated real advantages of one technique over the others. Hence, current evidence does not support any specific technique and both ductal-direct mucosal and invagination methods are practiced widely.
In this communication, we describe the technical details of a modified end-to-side PJ (mPJ) technique, which was introduced in October 2010 and evaluated in 100 consecutive cases in terms of perioperative outcomes and the POPF (classified on 2005-ISGPFc and 2016-ISGPSc), stratified for the Fistula Risk Score (FRS).15
Materials and Methods
From October 2008 to June 2017, 424 pancreatic resections were performed at the General Surgery Unit, University of Pisa, of which 203 were PD, the mPJ being introduced in November 2010. Data of patients with periampullary lesions undergoing PD were retrieved from a prospectively collected dedicated database, and a retrospective analysis was performed on the 100 consecutive patients undergoing PD with mPJ, by a single surgeon (LM).
Preoperative data included age, gender, body mass index (BMI), American Society of Anaesthesiologists (ASA) score, and comorbidities. The preoperative workup included abdominal ultrasonography, chest radiography, abdomen CT scan, and/or magnetic resonance imaging. Perioperative data included operative findings, pancreatic texture (firm or soft) and pancreatic duct size, the FRS,15,16 together with operative time, estimated blood loss, need for blood transfusions, and the presence and the type of vascular resection. Based on the gland texture, pancreatic duct size, diagnosis, and estimated intraoperative blood loss, we calculated the FRS (Table 1), and patients were stratified accordingly.16
Postoperative data included histology of the resected specimen, time to first oral liquid intake, length of hospital stay, morbidity, and postoperative (in hospital) mortality. Morbidity included intra-abdominal fluid collection, wound infection, POPF, intestinal obstruction, pulmonary or urinary tract infections, and 90-day hospital re-admissions. Postoperative complications were graded using the Clavien-Dindo classification.17 POPF was defined and classified using the ISGPFc to compare the POPF rate with data reported in literature.4 However, the new revised 2016-ISGPSc of POPF was also used.5 This new classification renames the former “grade A POPF” into “biochemical leak” (BL), to exclude it from “category of fistula” as these cases do not have clinical relevance, defines a grade B POPF each fistula that requires a change in the management of the expected postoperative pathway and, whenever a grade B POPF leads to organ failure or to clinical instability such that a re-operation is needed, the POPF becomes a grade C.5
Surgical mortality was defined as perioperative death within the first 30 days following surgery or during the original hospital stay if longer than 30 days. The study was approved by the Institutional review board. All patients received an extensive explanation of the procedure and provided informed consent.
Technical Details of mPJ
General Principles
We used the term “modified technique,” as it merges the two most popular methods of creating PJ reported in literature10,18,19: combined end-to-side duct-to-mucosa and invagination technique, together with personal technical details. The main key points are as follows:
-
1.
Careful selection of the widest segment of the jejunum for transection, and for anastomosis, which ensures an easier termino-lateral invagination of the pancreatic stump, generally obtained by section of the jejunum very near to the Treiz ligament.
-
2.
Limited mobilization of the pancreatic stump.
-
3.
Tightening the knot over the jejunum both for the interrupted posterior and anterior sutures, and for the continuous running sutures.
-
4.
Placement of the small enterotomy, exactly opposite to the location of the pancreatic duct, without placing any sutures into the pancreatic ductal epithelium, but opposing and stenting them.
-
5.
Use of a 5/0 monofilament Prolene sutures which is atraumatic and less prone to infection than a braided suture.
Anastomotic Technique
Transection of the pancreas is performed vertically, with careful hemostasis. The pancreatic stump is freed by only about 1–2 cm from the splenic artery and vein. The jejunum is transected just distal to the Treitz angle or 2–3 cm distal the Treitz ligament, to obtain the jejunum with the largest diameter. The end of the selected jejunal loop is then closed and the jejunal limb brought in a retro-mesenteric or trans-mesocolic fashion to the supra-mesocolic compartment.
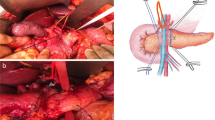
The initial step after transporting the jejunal limb to the supra-mesocolic area is to lay it posterior to the 1–2-cm freed pancreatic stump, and determine the best position for the invagination. The anastomosis is commenced by placement of interrupted atraumatic transverse 5-0 polypropylene sutures (Prolene; Ethicon, Inc., NJ), starting at the posterior surface of the pancreas. The dorsal capsule/parenchyma of the pancreas is sutured to the seromuscular layer of the jejunum about 10 mm from the transected surface of the pancreas. The u-sutures are placed starting from the jejunum, after which, the needle is passed transversally through the pancreas and finally, passed again through the jejunum. This ensures that the knots are tied over the jejunum, thereby reducing the risk of pancreas laceration during knot tying (Fig. 1). Then, the pancreas and the jejunum are approximated and the sutures are tied. A needle tip cautery is used to create a small enterotomy in the jejunum of equivalent size to the pancreatic duct after ascertaining the enterotomy location opposite to the pancreatic duct. A posterior single continuous running 5-0 polypropylene non-absorbable suture is placed between the posterior cut surface of the pancreatic duct, passing the needle from the capsule and parenchyma and the seromuscular layer of the jejunum, keeping the little enterotomy in the center of the elliptical line of internal layer (Fig. 2). The suture on the posterior wall is progressively tightened but left untied with the needle having passed through the jejunum. Care must be taken not to place any of these suture bites through the main pancreatic duct. A stylet placed into the pancreatic duct is used to facilitate identification and prevent inadvertent closure of the duct. After completing the posterior layer, a ureteral stent (usually 5 Fr) is placed with the straight end in the Wirsung duct and the pigtail end in the jejunal lumen (Fig. 2). Next, the anterior wall is sutured by continuous running suture, between the anterior pancreatic capsule and parenchyma, and the anterior seromuscular layer of the jejunum (Fig. 3). The last passage of the running suture is performed through the jejunum and both posterior and anterior running sutures come out from the jejunum, where the suture is then tightened and tied with multiple throws. As the jejunum approximates the pancreas, its seromuscular edge should roll over onto the pancreatic capsule. The outer-anterior-layer anastomosis is completed with monofilament non-absorbable horizontal mattress interrupted sutures using 5-0 polypropylene suture (Prolene; Ethicon, Inc., NJ). Each suture starts with the needle entry in the anterior seromuscular layer of the jejunum, when the jejunum is rolled over onto the pancreatic capsule to verify the correct point for the horizontal needle passage in the pancreas. The needle should exit from the pancreas 5 mm away from the initial entry point of the suture into the organ. The suture is completed with the second passage through the jejunum. In this way, as the sutures are pulled with adequate tension and tied, the jejunum rolls over onto the pancreas and the knots are tied over the jejunum. To reduce the risk of pancreas laceration, the finger used to tie the knot facilitates the rolling of the jejunum over the pancreas (Fig. 4). These sutures are placed approximately 3 to 5 mm apart such that they imbricate the anterior seromuscular jejunum over the pancreas. The sutures should be tied to allow snug apposition of the jejunum over the pancreas without causing ischemia or laceration to the pancreas. The final view of the completed anastomosis is shown Fig. 4. Two drains are placed, one above and the other below the completed pancreatic jejunal anastomosis.
Posterior interrupted suture. The dorsal capsule of the pancreas is sutured to the seromuscular layer of the jejunum with interrupted transverse u-stitches placed starting from the jejunum (a), then transversally passed through the pancreas (b), and finally again through the jejunum (c). After that each suture is passed, the pancreas and the jejunum are approximated before the sutures are tied (d). ( EL, external layer; IL, internal layer; pIS, posterior interrupted suture)
Posterior running suture. A small enterotomy is created in the jejunum of the same size and exactly opposite to the location of the pancreatic duct (a). A posterior continuous running suture is placed between the posterior pancreatic capsule and the seromuscular layer of the jejunum, keeping the little enterotomy in the center of the elliptical line of the internal layer (b). A stylet is placed in the pancreatic duct to avoid inadvertent closure. A ureteral stent is placed with the straight end in the Wirsung duct and the pigtail end in the jejunum (c-d). ( pRS posterior running suture)
Anterior running suture. The anterior suture line is effected by continuous running suture, between the anterior pancreatic capsule and parenchyma, and the anterior seromuscular layer of the jejunum (a–b). The last passage of the running suture is performed through the jejunum and both posterior and anterior running sutures come out of the jejunum (c). The knot is tied over the jejunum (d). ( aRS anterior running suture, aIS anterior interrupted suture)
Anterior interrupted suture. The outer-anterior-layer anastomosis is completed with horizontal mattress interrupted sutures. Stitches are placed again starting from the jejunum (a), then transversally passed through the pancreas (b) and finally again through the jejunum (c). The knots are tied over the jejunum (d). The final view of the anastomosis (e). ( aIS, anterior running suture; S, stent; IS, interrupted suture; RS, running suture)
Postoperative Management
Perioperative evaluation of POPF, management of peritoneal drains, and treatment of fluid collection related to POPF were standardized, with daily measurement of drain output volume and amylase content being assayed on the third and fifth postoperative days and, when positive, every 3 days until drain removal. Prophylactic somatostatin (3 mg/12 h intravenously, for 5 days) was administered if peritoneal fluid amylase activity was negative or replaced with octreotide 0.2 mg, intramuscularly, t.d.s and gabesate mesilate (1 g/24 intravenously, for 3 days) if fluid amylase activity was positive for the presence of a POPF. Amylase activity was also measured in fluid samples obtained by aspiration of intra-abdominal collections or ascites. POPF was diagnosed when the amylase concentration in the drainage fluid on or after POD 3 was more than three times the upper limit of the normal serum level, and graded in accordance with the 2005-ISGPFc and 2016-ISGPSc. The drainage tubes were removed at POD 5 in patients judged as ISGPF grade none or A, and without any signs of intra-abdominal infection and decreasing amylase concentrations from peripancreatic drains. Abdominal ultrasound exam was performed as the first level exam in cases of clinical suspicion of intra-abdominal complication and followed by computed tomography if suspicious. Intra-abdominal collections caused by POPF were drained with an interventional ultrasound procedure, usually with placement of a pigtail catheter in the collection in the first instance, with CT-guided pigtail placement reserved for failed ultrasound-guided procedure.
Statistical Analysis
Data were analyzed using SPSS (Statistical Production and Service Solution for Windows, SPSS Inc., Chicago, IL, USA). Continuous variables are given as mean (range). The primary end point was the occurrence of POPF. We analyzed the presence of risk factors associated to the occurrence of POPF by the chi-square test (or Fisher exact test) for categorical measures and by t test for continuous data. Statistical significance was set at 5%.
Results
The cohort consisted of 52 males and 48 females, with a mean age of 68 ± 13 years, range 29 to 90 (Table 2). Clinical manifestations included jaundice in 38 patients (38%), abdominal pain in 20 patients (20%), and digestive symptoms in 9 patients (9%). Twenty-seven patients had undergone previous abdominal surgery. Major comorbidities were cardiopulmonary disease in 30 patients (30%) and diabetes in 16 patients (16%).
In 89 patients, a PJ was performed after a PD without and in 11 patients with pylorus preservation. Concomitant vascular resection was performed in 11 patients (11%). The mean operative time was 431.85 ± 81.53 min (Table 3). Indications for PD were pancreatic adenocarcinoma (n = 44), ampullary adenocarcinoma (n = 7), cholangiocarcinoma (n = 12), neuroendocrine tumor (n = 7), intraductal papillary mucinous neoplasm (n = 4), and other (n = 26) (Table 4). Pancreatic texture was firm in 47 patients (47%) and soft in 53 cases (53%). Wirsung duct size was ≥ 5 mm in 15 patients (15%), 4 mm in 13 patients (13%), 3 mm in 20 patients (20%), 2 mm in 17 patients (17%), and ≤ 1 mm in 35 patients (35%). Mean lymph node harvest was 29 ± 11 (range 11–56), and mean percentage of positive lymph node was 10.0 ± 12.7% (0–52.4%).
Patients were stratified according to the FRS in four groups: negligible risk (4 patients, 0 FRS points), low risk (17 patients, 1–2 FRS points), intermediate risk (48 patients, 3–6 FRS points), and high risk (15 patients, 7–10 FRS points) (Table 5). Mean postoperative length of stay for all patients was 18 ± 9.6 days (range 9 to 56 days). The re-operation rate was 3% (3/100): two of these for bleeding and one for POPF grade C. ISGPFc POPF occurred in 17/100 (17%). Grade A of POPF was found in 10/100 patients (10%) and did not alter clinical management. Grade B of POPF was found in 6/100 patients (6%), and the presence of grade C pancreatic fistulas was documented in 1/100 patients (1%). According to the stratification of patient using the FRS, ISGPFc POPF was documented in 1/19 patient (5.3%) with low FRS, in 13/61 patients (21.3%) with intermediate FRS, and in 3/16 patients (18.8%) with high-risk FRS. All the grade B and grade C pancreatic fistulas occurred in the pancreas with soft texture. According to the revised ISGPSc of POPF, a pancreatic fistula developed in 7/100 patients (7%), of which six cases of POPF grade B (6%) and one case of POPF grade C. The mean age of patients who developed POPF was 71.3 ± 8.0 years (range 48–83) vs. 66.3 ± 14.0 years (range 29–90) in the non-POPF group (p = ns). 3/17 patients (17.6%) in the POPF group were jaundiced compared to 35/83 patients (42.2%) in the non-POPF group (p = ns).
Postoperative complications summarized in Table 6 occurred in 38 patients (38%). The POPF morbidity rate was 17% (17/100); other causes included gastrointestinal hemorrhage (n = 1), intra-abdominal hemorrhage (n = 3), cardiac complications (n = 4), pulmonary complications (n = 6), and other medical complications (n = 20). The overall mortality rate was 2% (2/100) and the specific mortality rate for POPF was 1% (1/100).
Discussion
Pancreatic reconstruction after PD is a crucial task because POPF, which is the most frequent complication of this operation,20 remains the primary cause of morbidity, contributing to prolonged hospital stay, increased costs, and mortality.21 Despite multiple randomized studies and meta-analyses, there is no level I evidence or universally accepted optimal pancreatic anastomosis after PD, and the end-to-side PJ remains the most common type of reconstruction.12,18 The two widely used methods to accomplish an end-to-side PJ are duct-to-mucosa PJ or invagination PJ.9 In duct-to-mucosa PJ, mucosa-to-mucosa sutures through optimal approximation facilitate anastomotic healing, in addition to providing support to the pancreatic remnant by the jejunal serosa. Moreover, another advantage of this technique is the small incision of the jejunum. However, this technique may leave a dead space between the pancreatic stump and jejunal wall, with accumulation of pancreatic juice from the accessory or tiny pancreatic ducts. In addition, duct-to-mucosa PJ is difficult if the duct of Wirsung is of small diameter, and is prone to obstruction. In contrast, end-to-side PJ invagination is easier to perform, and theoretically, all the pancreatic juice drains into the jejunum.9 However, the disadvantage of the classic invagination technique is related to the wide enterotomy of the jejunum anastomosed because, in the event of pancreatic fistula, the wide communication is likely to result in a high-output grade C fistula. Moreover, with this technique, knots are usually tied across the jejunum and the pancreas, and this can cause laceration of pancreatic tissue during knot tying, which may lead to a secondary leak of pancreatic juice from the capsule.
The end-to-end PJ invagination is another technique proposed to reduce POPFs incidence,22 its advantage being that it enables intussusception of the jejunum over the pancreas to create an invaginated anastomosis. However, possible issues include risk of devascularization of the pancreatic stump during the intussusception by the jejunum with the risk of ischemic pancreatitis and necrosis. Furthermore, the different caliber between the diameter of the pancreatic stump and the lumen of jejunum may be an issue in the creation of a leak-proof closure.18 The other issue with this technique concerns the wide dehiscence from the jejunum in the event of a pancreatic fistula, as this is likely to be a high-output grade C.
A variant of end-to-side PJ was proposed by Blumgart,19 and subsequently modified by German14 and Japanese groups.13 Specifically, the technique is a duct-to-mucosa anastomosis with invagination of the pancreas by the jejunum. Its primary advantages include the use of interrupted full pancreatic thickness mattress sutures, which firmly anchor the gland to the jejunum. In addition, the placement of the duct-to-mucosa stitches before securing the posterior seromuscular jejunum permits a tension-free approximation, with excellent visualization of the pancreatic duct during the duct-to-mucosa anastomosis. Possible weaknesses of the Blumgart technique and its variants are those of the classic duct-to-mucosa PJ, as well as the full thickness sutures and the positioning of the final knots on the pancreas surface. For this reason, Fujii et al. in their modified Blumgart’s anastomosis do not tie the two penetrating sutures on the pancreas but continue them through the seromuscular layer of the jejunum before tying them to approximate the pancreas and the jejunum.13
The mPJ technique described in this communication combines the advantages of the previous techniques and could mitigate their weaknesses. Specifically, the modifications of the described mPJ include small incision of the jejunal wall, similar to the duct-to-mucosa PJ, while like the two classic invagination techniques, the pancreatic juice from the secondary duct can drain into the jejunum, as the Wirsung duct is not sutured to the jejunum but just stented. Despite the disputed benefit of pancreatic duct stenting in reducing pancreatic fistula after PD, we think that in our technique, it is useful in keeping the alignment of the Wirsung duct with the small incision in the jejunal wall. Two other technical details of the proposed technique are the type of the sutures and the careful choice of the widest segment of the jejunum, which ensure easier invagination of the pancreatic stump. We think that the choice of the 5/0 Prolene, which is sliding, thin, and durable at the same time, as well as less prone to infection than a braided suture, could contribute to the successful healing of the anastomosis, by reducing pancreatic trauma and securing lasting seal.23,24 The running suture for the inner layer creates a waterproof suture line, and in the outer layer, the knots tied on the jejunum protect the first layer and minimize the risk of pancreatic capsule laceration. In fact, regardless of the type of pancreatic anastomosis performed, a certain degree of laceration and/or damage to the pancreatic parenchyma is unavoidable. This is especially true for patients with soft and friable pancreatic tissue.6
Some retrospective studies showed that the duct-to-mucosa PJ was associated with a lower rate of POPF in the low-risk patients with dilated pancreatic duct or hard pancreas, whereas the invagination PJ technique was safer in the high-risk patients with small pancreatic duct or soft pancreas.18 With the new ISGPS 2016 classification,5 used because it is the most current and it will be one that will replace the ISGPFc from now on, we registered the very low POPF rate of 7%. However, because this is one of the first published study using this new classification, we decided to used previous versions of the classification, to make our results more comparable with the previously reported data.
The results of the present study compare favorably with those from other recent series reporting on pancreatic anastomotic failure after PD using the FRS. In our series, the fistula rate in patients with high risk (FRS 7–10) was lower than that of the expected from the literature (ISGPFc 18.8 vs 50.0% as reported by Miller in a multi-institutional external validation of the FRS comprising 594 cases of PDs), while with low and intermediate risks, FRS was quite lower or comparable to that expected (ISGPFc 5.3 vs 11.4% and ISGPFc 21.3 vs 29.9%, respectively).15,16 Also, the comparison of the only clinically relevant POPFs between our results and those recently published by Wang with the modified Blumgart PJ is encouraging, as with ISGPSc, we reported 6.3 vs 14.3% of POPFs in high-risk FRS, 9.8 vs 6.0% in intermediate-risk FRS, 0 vs 4.8% in low-risk FRS, and 7 vs 7% of overall POPFs.10
The lower than expected incidence of POPF encountered in the present study has some limitations including the consistent use of a single technique for the pancreatic anastomosis, which by increasing proficiency in execution accounts for the benefit observed rather than a direct benefit of the technique. Indeed, there is indirect evidences for this “beneficial effect” from consistent use in the literature.12 Secondly, the retrospective nature of the study and the single institution experience may lead to some bias. Despite these limitations, the reported mPJ technique appears to be a simple, safe technique for PJ and reproducible in all pancreatic types including cases with small Wirsung duct and soft gland. When compared with conventional procedures, this technique resulted in low leakage rates especially in patients with high risk of POPF.
Conclusion
This communication describes our mPJ technique which is safe and resulted in a lower post PD pancreatic fistula rate than expected especially for the “difficult” pancreas with high FRS, including soft gland texture and small pancreatic duct diameter. Comparative prospective studies with other techniques are necessary to draw definitive conclusions.
References
Yoshioka R, Yasunaga H, Hasegawa K, Horiguchi H, Fushimi K, Aoki T, Sakamoto Y, Sugawara Y, Kokudo N. Impact of hospital volume on hospital mortality, length of stay and total costs after pancreaticoduodenectomy. Br J Surg 2014; 101:523–529.
Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014; 259(4):773–780.
Zhang X, Dong X, Liu P, Yan Y, Wei Y, Zechner D, Gong P, Vollmar B. Binding versus Conventional Pancreaticojejunostomy in Preventing Postoperative Pancreatic Fistula: A Systematic Review and Meta-Analysis. Dig Surg 2017; 34(4):265–280.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery 2005; 138(1):8–13.
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fisula: 11 Years After. Surgery 2017; 161(3):584–591.
Chen Y, Ke N, Tan C, Zhang H, Wang X, Mai G, Liu X. Continuous versus interrupted suture techniques of pancreaticojejunostomy after pancreaticoduodenectomy. J Surg Res 2005; 93(2):590–7.
El Nakeeb A, El Hemaly M, Askr W, Abd Ellatif M, Hamed H, Elghawalby A, Attia M, Abdallah T, Abd ElWahab M. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: A prospective randomized study. Int J Surg 2015; 16:1–6.
Schoellhammer HF, Fong Y, Gagandeep S. Techniques for prevention of pancreatic leak after pancreatectomy. Hepatobiliary Surg Nutr 2014; 3(5):276–87.
Hua J, He Z, Qian D, Meng H, Zhou B, Song Z. Duct-to-Mucosa Versus Invagination Pancreaticojejunostomy Following Pancreaticoduodenectomy: a Systematic Review and Meta-Analysis. J Gastrointest Surg 2015; 19(10):1900–9.
Wang SE, Chen SC, Shyr BU, Shyr YM. Comparison of Modified Blumgart pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. HPB (Oxford) 2016; 18(3):229–35.
Ji W, Shao Z, Zheng K, Wang J, Song B, Ma H, Tang L, Shi L, Wang Y, Li X, Song B, Zhang Y, Jin G. Pancreaticojejunostomy with double-layer continuous suturing is associated with a lower risk of pancreatic fistula after pancreaticoduodenectomy: a comparative study. Int J Surg 2015; 13:84–9.
Shrikhande SV, Sivasanker M, Vollmer CM, Friess H, Besselink MG, Fingerhut A, Yeo CJ, Fernandez-del Castillo C, Dervenis C, Halloran C, Gouma DJ, Radenkovic D, Asbun HJ, Neoptolemos JP, Izbicki JR, Lillemoe KD, Conlon KC, Fernandez-Cruz L, Montorsi M, Bockhorn M, Adham M, Charnley R, Carter R, Hackert T, Hartwig W, Miao Y, Sarr M, Bassi C, Büchler MW; International Study Group of Pancreatic Surgery (ISGPS). Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2016; 161(5):1221–1234.
Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 2014; 18(6):1108–15.
Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg 2009; 96(7):741–50.
Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, Vollmer CM Jr. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg 2014; 18(1):172–79.
Mark P Callery, Wande B Pratt, Tara S Kent, Elliot L Chaikof, Charles M Vollmer Jr. A Prospectively Validated Clinical Risk Score Accurately Predicts Pancreatic Fistula after Pancreatoduodenectomy. J Am Coll Surg 2013; 216(1):1–14.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213.
Kleespies A, Albertsmeier M, Obeidat F, Seeliger H, Jauch K-W, Bruns CJ. The challenge of pancreatic anastomosis. Langenbeck's Archives of Surgery 2008; 393(4):459–471.
Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 2010; 210(1):54–9.
McMillan MT, Soi S, Asbun HJ, Ball CG, Bassi C, Beane JD, Behrman SW, Berger AC, Bloomston M, Callery MP, Christein JD, Dixon E, Drebin JA, Castillo CF, Fisher WE, Fong ZV, House, MG, Hughes SJ, Kent TS, Kunstman JW, Malleo G, Miller BC, Salem RR, Soares K, Valero V, Wolfgang CL, Vollmer CM Jr. Risk-adjusted out- comes of clinically relevant pancreatic fistula following pancreatoduodenectomy: a model for performance evaluation. Ann Surg 2016; 264:344–52.
Xiong JJ, Altaf K, Mukherjee R, Huang W, Hu WM, Li A, Ke NW, Liu XB. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during Pancreaticoduodenectomy. Br J Surg 2012; 99(8):1050–61.
Peng S, Mou Y, Cai X, Peng C. Binding Pancreaticojejunostomy is a new technique to minimize leakage. Am J Surg 2002; 183(3):283–5.
Chen Y, Tan C, Zhang H, Mai G, Ke N Liu X. Novel entirely continuous running suture of two-layer pancreaticojejunostomy using only one polypropylene monofilament suture. J Am Coll Surg. 2013; 216(2):e17–21.
Dhom J, Bloes DA, Peschel A, Hofmann UK. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J Orthop Res 2017 35(4):925–933.
Funding
The study was supported by the ARPA Foundation (www.fondazionearpa.it).
Author information
Authors and Affiliations
Contributions
Study conception and design: Prof. Morelli Luca, Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, Dott. Gianardi Desirée, Prof. Del Chiaro Marco, Prof. Di Candio Giulio, and Prof. Mosca Franco; acquisition of data: Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, and Dott. Gianardi Desirée; analysis and interpretation of data: Prof. Morelli Luca, Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, and Dott. Gianardi Desirée; drafting of manuscript: Prof. Morelli Luca, Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, and Dott. Gianardi Desirée; critical revision of manuscript: Prof. Morelli Luca, Prof. Del Chiaro Marco, Prof. Giulio Di Candio, and Prof. Mosca Franco; final approval of the version to be published: Prof. Morelli Luca, Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, Dott. Gianardi Desirée, Prof. Del Chiaro Marco, Prof. Di Candio Giulio, and Prof. Mosca Franco; agreement for all aspects of the work: Prof. Morelli Luca, Dott. Di Franco Gregorio, Dott. Guadagni Simone, Dott. Palmeri Matteo, Dott. Furbetta Niccolò, Dott. Gianardi Desirée, Prof. Del Chiaro Marco, Prof. Di Candio Giulio, and Prof. Mosca Franco.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morelli, L., Di Franco, G., Guadagni, S. et al. Technical Details and Results of a Modified End-to-Side Technique of Pancreatojejunostomy: a Personal Series of 100 Patients. J Gastrointest Surg 21, 2090–2099 (2017). https://doi.org/10.1007/s11605-017-3587-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3587-7