Abstract
Background and aims
Significant progress in surgical technique and perioperative management has substantially reduced the mortality rate of pancreatic surgery. However, morbidity remains considerably high, even in expert hands and leakage from the pancreatic stump still accounts for the majority of surgical complications after pancreatic head resection. For that reason, management of the pancreatic remnant after partial pancreatoduodenectomy remains a challenge. This review will focus on technique, pitfalls, and complication management of pancreaticoenteric anastomoses.
Materials and methods
A medline search for surgical guidelines, prospective randomized controlled trials, systematic metaanalysis, and clinical reports was performed with regard to surgical technique and complication management of pancreatic anastomoses.
Results
Pancreaticojejunostomy appears to be most widely performed, but pancreaticogastrostomy is a reasonable alternative. Postoperative treatment with octreotide can be recommended only for patients with soft pancreatic tissue, and neither stents of the pancreatic duct nor drainages have proven to effectively reduce anastomotic complications. Gastroparesis remains the most common complication after pancreatic surgery and should be treated conservatively. However, it may be a symptom of other local complications, such as anastomotic leakage, pancreatic fistula or abscess. All septic complications may finally result in late postoperative hemorrhage, which requires immediate diagnostic workup and therapy. Today, interventional radiology has emerged as a standard tool in the management of local septic complications and bleeding. Therefore, relaparotomy has become less frequent and salvage pancreatectomy is now a rare procedure in case of local complications.
Conclusion
The surgeon’s experience with one or the other technique of pancreatic anastomosis appears to be more important than the technique itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic surgery has improved dramatically within the past 15 years. While in the 1980s mortality rates after Whipple’s procedure still exceeded 20%, today, mortality has been reduced to less than 5% in high volume centers. Some authors even report mortality rates as low as 0 to 3% (own experience 2001–2006: 2.3%) [1–12]. Due to improved security of the procedure, pancreatic head resection is also applied for the treatment of benign conditions like chronic pancreatitis, large adenomas, diverticula and benign periampullary tumors. Partial pancreatoduodenectomy has become a standard procedure, and the critical step in pancreatic surgery is no longer the resection itself but the reconstruction of the pancreaticoenteric anastomosis. This is highlighted by the fact that in spite of significantly reduced mortality, morbidity remains as high as 30 to 50%, even in large series (own experience: 30.4%) [1, 3, 4, 6, 8–10, 12–18]. Complications related to the pancreatic remnant, such as pancreatic fistula, anastomotic dehiscence, abscess formation, and septic hemorrhage are the main causes of morbidity and mortality following pancreatic head resection [1–4, 12, 15, 17, 19, 20]. Some authors have named the pancreatic anastomosis the “Achilles heel” of pancreatic surgery because it has the highest rate of surgical complications among all abdominal anastomoses [21]. The following article will focus on different reconstructive techniques after pancreatic resection as well as on management approaches for anastomotic complications.
The pancreatic remnant
Resection of the pancreatic head using the scalpel might be superior to diathermia, ultrasound dissection or stapler pancreatectomy [22]. However, management of the pancreatic remnant after partial pancreaticoduodenectomy is still controversially discussed. More than 80 different methods of pancreaticoenteric reconstruction have been proposed, illustrating the complexity of surgical technique as well as the absence of a gold standard for all patients [20]. Simple closure of the pancreatic duct by ligation, fibrin, or tissue glue without performing a pancreatic anastomosis resulted in high rates of fistulas, pancreatitis, and postoperative insulin-dependent diabetes, and therefore has been widely abandoned [23, 24]. Anastomoses of the pancreatic remnant with the jejunum or the stomach are common practice [11, 20, 22, 25]. Numerous methods for pancreatojejunostomy and—to a lesser extent—for pancreatogastrostomy have been described, and the most important principles will be discussed here.
Pancreatojejunostomy vs. pancreaticojejunostomy
Pancreatic anastomosis using a jejunal loop is probably the most commonly used method of surgical reconstruction after pancreatic head resection [20, 22]. Apart from different positions of the jejunal loop (antecolic, retrocolic, retromesenteric) and other variants like the isolated loop technique (separate Roux-Y loops for biliary and pancreatic anastomosis) [26, 27], mainly two types of anastomosis can be performed. First, in so-called invagination anastomoses, the cross section of the pancreatic remnant is completely immersed into the jejunal loop. Second, in “duct-to-mucosa” anastomoses, the pancreatic duct is primarily sutured to the mucosa of the jejunal loop [21, 28–30]. Here, pancreatic secretion is drained only via the pancreatic duct [31–35] and the parenchymatous cross-section of the pancreatic stump is covered by the jejunal wall, which has to be fixed with a second suture line to the pancreatic capsula [36]. The invagination technique is also called pancreatojejunostomy, whereas pancreaticojejunostomy is an alternative name for “duct-to-mucosa” techniques. However, prospective randomized trials have found no differences between both methods regarding fistula rates, morbidity, or mortality [28, 33].
Dunking vs. telescopic anastomosis
Since most complications after pancreatic head resection originate from the pancreatic cross-section (e.g., pancreatic fistula), several variants of invagination or “dunking” techniques have been developed. The advantage of covering all possible leaks and injuries of the pancreatic capsula by deep insertion of the stump into the loop is limited by the risk of ischemic pancreatitis and necrosis following extensive devascularization of the stump. Most authors deem mobilization by 1.5–2 cm to be sufficient [20, 35]. Out of concerns that overlapping mucosa might lead to fistula formation and that wound healing between the jejunal mucosa and the pancreatic capsule is impaired, various methods such as mucosectomy, cauterization of the mucosa [37], and so called “telescopic” anastomoses have been developed. In “telescopic” anastomoses, the jejunal loop is inverted so that the surface of the pancreatic remnant is now covered by serosa instead of mucosa. Jejunal inversion can be prepared in advance or it can be achieved simultaneously with suturing the anastomosis using multilayer stitches [38]. The first method bears the advantage that traction and shear forces at the pancreatic remnant can be avoided during invagination. The methods of “dunking” and “telescopic” anastomosis vary from simple “parachute” techniques to sophisticated three- and four-layer techniques [20, 29, 39]. The so called “binding anastomosis” in which a circular ligature is looped around the entire circumference of the anastomosis in order to balance differences in diameter between jejunal loop and pancreatic remnant is another option [37].
End-to-end versus end-to-side anastomosis
The problem of differing diameters between the jejunal loop and the pancreatic remnant in end-to-end anastomosis can only partially be resolved by mechanical distension or inversion of the loop and different suture or biding techniques. Therefore, end-to-side anastomoses are more and more favored over end-to-end anastomoses [20, 21]. Size and shape of the antimesenteriale opening of the jejunal wall can be adjusted to the cross-section of the pancreatic remnant. Although numerous technical variants have been published, prospective randomized studies are rare [28, 33].
Pancreaticogastrostomy
Some authors have proposed pancreaticogastrostomy as an alternative to pancreaticojejunostomy, assuming that postoperative complications are less frequent [40–42]. Excellent blood supply to the stomach, easy surgical technique, accessibility for postoperative endoscopy, and separate drainage of biliary and pancreatic juice were considered advantageous. It appears, however, that bleeding from the anastomosis is a typical complication of this technique. Most comparative studies are retrospective analyses of different cohorts and therefore of limited value (Table 1) [11, 25, 42–52]. Furthermore, excellent results of pancreaticogastrostomy were often compared to unsatisfactory results after pancreaticojejunostomy with uncommonly high rates of fistula and mortality. These authors probably had more experience with gastric anastomoses. A recently published survey in Japanese hospitals found no difference between pancreaticojejunostomy and pancreaticogastrostomy for the rates of fistula, bleeding, abscess, and mortality in 3,109 patients after pancreatic head resection [22]. Three prospective randomized trials comparing the two methods have been published (Table 1) [11, 53, 54], and none of them found significant differences. Consequently, the surgeon’s experience with one or the other technique appears to be more important than the technique itself.
Own technique
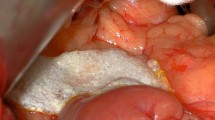
In our department, we use a modified end-to-side duct-to-mucosa technique, which was originally devised by Leslie H. Blumgart, New York (Fig. 1). Three to four transpancreatic U-sutures are placed in addition to the “duct-to-mucosa” sutures to fix the jejunal wall on the pancreatic remnant. Herewith, the pancreatic cross-section is completely covered by jejunal serosa, and tangential shear forces at the pancreatic stump seem to be widely eliminated. Since the introduction of this technique in our department, postoperative rates of pancreatic fistulas and overall morbidity have been reduced significantly (unpublished data).
Pancreaticojejunostomy as performed at the Surgical Department of Munich University, Klinikum-Grosshadern: an end-to-side “duct to mucosa” technique according to L.H. Blumgart, New York. Following 3–4 transpancreatic U-sutures, which approximate the posterior jejunal wall to the pancreatic remnant (a, b), the “duct-to-mucosa” anastomosis is performed by 4–8 single stitches (c). Finally, U-sutures are completed on the anterior wall of the loop (d). This technique avoids tangential shear forces on the pancreatic capsule and may reduce the occurrence of fistulas
Drainage and stenting
All of the aforementioned anastomotic techniques can be combined with stenting or drainage of the pancreatic duct [31, 32, 34, 35]. Three different types of drainages need to be discriminated. The external jejunal loop drainage is an endoluminal transcutaneous drainage that derives biliary and pancreatic juice from the anastomosis in order to prevent postoperative accumulation of aggressive fluids in the afferent loop. “Lost drainages” or pancreatic stents are short tubes which are inserted or sutured into the pancreatic duct and typically surpass the anastomosis by a few centimeters [33]. They are intended to splint the duct-to-mucosa anastomosis, to prevent accumulation of pancreatic juice in the pancreatic stump and to avoid direct contact of the juice with the anastomosis. The third option is an external drainage of the pancreatic duct, using a long but thin silicon tube, which directly inserts the pancreatic duct via the jejunal loop [32, 34]. In contrast to the “lost drainage,” this tube has to be removed after a while. Since none of the drainages and stents has proven to be of significant benefit, but pancreatic duct drainages may cause acute postoperative pancreatitis of the remnant, their use has been concluded in our department.
Octreotide
Prophylactic use of somatostatin and synthetic somatostatin analogues (octreotide, vapreotide) during and after pancreatic surgery remains controversial [55]. The matter of interest is a suppression of exocrine pancreatic secretion to prevent postoperative complications [56, 57].Until recently, however, it was not clear whether the suppression of exocrine pancreatic secretion significantly reduces the rate of pancreatic fistula. Several randomized, placebo-controlled multicentre trials came to contradictory results (Table 2) [58–62]. Only recently, Connor et al. published a meta-analysis of prospective randomized controlled trials with at least 50 included patients per trial (1,918 patients, 10 studies). The authors report that somatostatin analogues were able to reduce the rates of biochemical fistula, pancreas-specific complications, and overall morbidity but not the incidence of clinically relevant anastomotic disruption or mortality after pancreatic surgery [55]. Regarding pancreas-specific complications, one in nine patients (11%) did profit from the treatment with octreotide. Today, prophylactic treatment with octreotide is common practice in patients with soft pancreatic tissue, with a filiform pancreatic duct and without fibrosis, particularly in oncological surgery [own prophylactic regimen: 100 μg subcutaneously every 8 h for 5 days]. However, there are no clear data for this subgroup, and the decision should be left to the individual surgeon.
Delayed gastric emptying
Delayed gastric emptying (DGE) occurs in approximately 30% (10–70%) of patients after partial pancreaticoduodenectomy, and therefore is the most common complication after pancreatic head resection [1, 4, 5, 11, 12, 16, 63–67]. Defining DGE by the need of a nasogastric tube for more than 3 days following surgery or reinsertion of the nasogastric tube after day 3 appears reasonable because this significantly impairs postoperative oral intake [67]. A relationship to the preservation of the pylorus has not been established [63–67]. Likewise, problems with the gastrojejunostomy or pylorojejunostomy appear to be only rarely the cause of DGE. However, two recently published trials could demonstrate an association of DGE and retrocolic reconstruction of the pylorojejunostmy in patients undergoing pylorus preserving pancreatoduodenectomy [68, 69]. Therefore, the antecolic route for reconstruction should be recommended. If DGE occurs, the patency of the gastrojejunostomy or pylorojejunostomy should be confirmed and small bowel obstruction excluded, as mechanical complications need to be distinguished from gastroparesis [67]. The main causes of postoperative gastroparesis are extended retroperitoneal lymphadenectomy and abdominal septic complications, which need to be treated first [6, 65, 70, 71]. Gastro-duodenal denervation and decreased serum levels of motilin after duodenopancreatectomy may also play a role in postoperative gastroparesis, since motilin secretion is primarily located in the duodenum. DGE significantly prolongs hospitalization [11, 63, 67] but is not life-threatening and can most often be managed conservatively using erythromycin (an agonist on the motilin receptor), prokinetic drugs (e.g., metoclopramide, neostigmine), a nasogastric tube, and total parenteral nutrition [72].
Clinical management of anastomotic complications
Although gastroparesis is the most common complication after duodenopancreatectomy, leakage from the pancreatic anastomosis with subsequent local sepsis is the major cause for postoperative morbidity and mortality [1, 3–5, 8, 12, 14, 19, 45]. In the following, the diagnostic and therapeutic approach to pancreatic fistula, anastomotic breakdown, abscess formation, and septic hemorrhage will be discussed. During the last decade, further development of interventional radiology has lead to a more conservative management of septic complications. Most high volume centers report only slightly decreasing rates of postoperative complications, but a significant reduction in relaparotomies [7, 10, 12, 14, 16].
Pancreatic fistula and anastomotic dehiscence
Definition
The terms pancreatic fistula, pancreatic leakage, and anastomotic dehiscence are not unequivocally defined in the literature. All terms signify a leakage in the area of the pancreatic anastomosis and could, therefore, be used synonymously. Contrary definitions and usage of these terms, however, have led to confusion. Hence, diverging rates of postoperative fistula (1–24%) were reported by different teams that use the same surgical technique [1, 4, 10, 12, 15, 19, 29, 73]. A uniform definition is therefore urgently needed to make results comparable. It appears reasonable to discriminate a biochemical fistula from anastomotic breakdown. We define pancreatic fistula as a persisting amylase-rich effluent via upper abdominal drainages for more than 3 days after surgery (> 10 ml/day containing threefold levels of serum-amylase) [74]. Anastomotic breakdown, though, should only be diagnosed when clinical signs of abdominal sepsis are apparent (increasing pain, peritonism, fever >38.5°C, leukocytosis >15 × 109/l and evacuation of infected fluid or intestinal content via local drains) or leakage is radiologically or operatively verified. In general, pancreatic fistula is not dangerous and occurs in up to 20% after pancreaticojejunostomy [45, 48, 49, 75]. Clinical relevant anastomotic disruption is less common but critical since it constitutes the primary cause of postoperative mortality [2, 7]. Large trials have shown mortality to be as high as 40% when anastomotic dehiscence is present [1, 3, 9, 12]. In case of survival, therapy is complicated, and hospital stay is prolonged.
Etiology
Various risk factors for anastomotic dehiscence and pancreatic fistula have been described. It is generally accepted that tissue consistency, exocrine function of the pancreatic remnant, and the diameter of the pancreatic duct are important determinants of pancreatic fistula [76–78]. Bartoli et al. reviewed 2,664 patients with postoperative pancreatic fistula with respect to the primary diagnosis [76]. Only 5% of patients with chronic pancreatitis, concomitant pancreatic fibrosis and exocrine insufficiency developed postoperative pancreatic fistula, while this rate was 12% for patients with pancreatic carcinoma, 15% for ampullary carcinoma and 33% for distal bile duct cancer. In the latter group, the pancreatic duct should not be distended at all. Therefore, the rate of pancreatic fistula was higher in patients with small duct and minimal fibrosis. Technical differences in anastomosis and drainage are thought to influence the rate of pancreatic fistula and have been discussed above. Results in the literature, however, are not consistent, and the choice of surgical method should be based on individual experience. Nonetheless, general surgical skill does matter in the prevention of pancreas-specific complications. Preparation and resection of the pancreas should been done with extreme care and any unnecessary manipulation and mobilization should be avoided. Furthermore, we believe that shear forces on the anastomosis through tangentially placed sutures may promote pancreatic leakage, and that transpancreatic U-sutures as applied for the Blumgart anastomosis (Fig. 1) and other mattress techniques [33] might be a reasonable strategy to prevent pancreatic fistula.
Diagnosis
Most pancreatic fistula and anastomotic disruptions occur 3 to 7 days postoperatively, and early diagnosis is crucial. Patients sometimes complain about dyspnea, tachycardia, and progressive abdominal pain (in case of anastomotic dehiscence) but may show just as well only weak symptoms like gastroparesis, bowel atony, or be completely asymptomatic (in case of pancreatic fistula). Proper physical examination is fundamental to the detection of anastomotic dehiscence. An increase in pancreatic secretion that becomes milky before turning turbid brownish is a characteristic finding. Plain abdomen and chest radiographs may hint at abdominal sepsis by showing ileus or pleural effusion. The latter occurs in 75% of patients with anastomotic dehiscence following pancreatic surgery [79, 80]. Free abdominal air, however, is a rare finding and indicates a serious anastomotic breakdown. In the early phase of anastomotic failure, CT scan often shows unspecific changes like pancreatic edema and peripancreatic fluid collections. However, late complications such as abscess formation or septic pseudoaneurysm of visceral arteries can easily be diagnosed with CT scan. In some cases, even the application of a contrast agent via the peritoneal drain may reveal a necrotic cavity or even the anastomotic dehiscence itself (Fig. 2).
Contrast medium given through a peritoneal drainage displays the anastomotic dehiscence after limited pancreatic head enucleation and longitudinal pancreaticojejunostomy according to Frey. The anastomotic leakage was well drained and could therefore be treated conservatively. Jejunal loop, drainage and distended pancreatic duct can be identified
Therapy
Therapeutic strategies for pancreatic fistula and anastomotic dehiscence have changed in recent years. Today, treatment is individualized, depending on the clinical state of the patient [7]. In 80% of cases so-called “low output” fistula are present, which are mostly asymptomatic and should be treated conservatively with drainage, irrigation, and temporary TPN or nutrition through a feeding jejunostomy for 2–3 weeks [81]. Proper fixation of peritoneal drainages is crucial to prevent their accidental loss. A follow-up CT scan might be useful to exclude late abscess formation. Experienced groups report fistula rates of 9–15%, but surgical intervention is required in only 4% of patients [1, 7]. Percutaneous CT-guided drainage has significantly reduced the rate of relaparotomies [82–84]. Only a small group of patients develops abdominal sepsis and needs another surgical procedure. Intraoperative management depends on the clinical state of the patient, the vitality of the pancreatic remnant and the degree of anastomotic dehiscence. Nowadays, completion pancreatectomy is only rarely performed [1]. In case of a sufficient anastomosis but a small fistula of the pancreatic tail or a small leakage of the anastomosis, irrigation and insertion of additional drains into the lesser sac is the proper clinical approach (similar to acute pancreatitis). In the case of anastomotic disruption or subtotal dehiscence, surgical repair of the anastomosis should usually not be attempted because blood supply is often not adequate and the inflammatory tissue cannot be securely sutured. In these cases, the anastomosis should be resolved and the jejunal loop shortened and closed [1]. In some cases, when extensive necrosis is present, salvage pancreatectomy including splenectomy is the only life-saving option, and it should be done before septic shock develops [71, 79, 85, 86]. However, the hospital mortality for those patients still remains high [1]. If the pancreatic tail shows no signs of major pancreatitis or necrosis, shortening and closure of the pancreatic stump and extensive surgical drainage can be performed [87]. This approach may lead not only to a reduction in postoperative diabetes but also to a higher incidence of pseudocysts. Only in selected cases of early technical failure, suturing a new anastomosis is feasible. For this procedure, the absence of local sepsis and a sufficient blood supply are obligatory [88].
Biliary fistula
Dehiscence of the bilioenteric anastomosis and consequent biliary fistula are rare (1–9%) and can usually be managed conservatively by adequate drainage. Only in some patients percutaneous transhepatic drainage of the biliary system is necessary in addition to maintenance and irrigation of intraoperatively placed peritoneal tubes. Using combined intra- and extraluminal biliary drainage, the fistula generally closes spontaneously. Surgical intervention is usually not necessary and limited to major leakage during the first postoperative days caused by technical problems [6, 7, 14, 81, 85, 89]. Late onset of septic bilioma should be treated by CT-guided drainage and irrigation.
Abscess
Intra-abdominal abscess formation occurs in approximately 10% of patients following duodenopancreatectomy, mainly as a consequence of pancreatic fistula or dehiscence of the pancreatic anastomosis [6, 7, 14, 81, 85, 89]. A real abscess should not be confounded with early postoperative fluid collections diagnosed on CT scan. Fluid collections occur frequently and regress spontaneously after a few weeks without any specific therapy. Biliary fistula and dehiscence of the gastrointestinal or entero-enteric anastomosis are rarely the cause for postoperative abscesses. Principally, CT or ultrasound-guided drainage and i.v. antibiotics are the therapy of choice. With respect to potential secondary complications, any abscess diagnosed must be drained. Only if an abscess cannot be drained sufficiently by interventional radiology or if the patient does not improve in spite of sufficient drainage, surgical intervention may be necessary to prevent further complications.
Postoperative bleeding
Bleeding occurs in around 2–18% of patients after pancreatic surgery [1, 12, 14, 64, 66, 90–95] and is associated with a mortality rate of 15–60% [81, 85, 90, 92, 94–96]. Different causes may be responsible. We should discriminate early from late postoperative bleeding and abdominal from gastrointestinal (intraluminal) bleeding [95]. Based on the onset, localization and severity of bleeding the International Study Group of Pancreatic Surgery (ISGPS) has recently proposed a classification of postpancreatectomy hemorrhage (PPH) into three grades. PPH grade A is defined as a mild early postoperative bleeding without major clinical impact, PPH grade B as a severe early postoperative bleeding with a consecutive change in a given clinical pathway, and PPH grade C as a severe late postoperative hemorrhage with immediate diagnostic and therapeutic consequences [95].
Early abdominal bleeding
Early abdominal bleeding is the most frequent type of postoperative hemorrhage (PPH grade A/B). It occurs within 24–48 h after surgery and is usually caused by either inadequate hemostasis or a slipped ligature. Technical causes are responsible for 30–60% of cases [90, 92]. Nonetheless, coagulopathies, e.g., due to major intraoperative blood loss or preoperative hyperbilirubinaemia, might also be responsible for diffuse postoperative hemorrhage. In large series, however, no significant difference in bleeding rates between patients with or without jaundice was found. For that reason, the advantage of preoperative biliary decompression is questionable [92]. Early abdominal bleeding can be easily detected through early blood loss via the peritoneal drainages, a drop in hemoglobin levels and alteration of vital signs. Depending on the intensity of bleeding and the patient’s cardio-circulatory function (PPH grade A/B), blood transfusions and pro-coagulatory drugs are combined with timely surgery for hemostasis and removal of hematoma [7, 90, 92].
Early gastrointestinal bleeding
Early gastrointestinal bleeding may appear as coffee ground vomitus, change of color in the naso-gastric tube or melena. Depending on severity, the bleeding can be associated with a drop in hemoglobin levels or with hemorrhagic shock. Postoperative GI-bleeding is usually caused by mucosal bleeding or bleeding from the suture-line of the gastrointestinal or pylorointestinal anastomosis, and can be controlled endoscopically in most cases [90]. Early bleeding from the pancreaticojejunostomy or the bilioenteric anastomosis is rare [97], and postoperative stress ulcer bleeding is—contrary to the common belief—extremely rare after duodenopancreatectomy [92]. Immediate but cautious endoscopy is the diagnostic and therapeutic approach of choice (Fig. 4a). In most cases, anastomotic bleeding can be controlled endoscopically by injection of fibrin glue, sclerosants or application of clips [90]. Relaparotomy only has to be performed in cases of insufficient hemostasis despite endoscopical treatment, limited cardio-circulatory function, unknown origin of GI-bleeding due to large amounts of endoluminal blood or suggested bleeding from the afferent loop (Fig. 4a) [97]. Intraoperatively, most endoluminal bleedings can be controlled directly via gastrotomy proximal to the gastro-enterostomy. Some authors also report good results for enterotomy of the jejunal loop in case of bleeding from the pancreatic or biliary anastomosis [92, 97].
Late postoperative bleeding
Late postoperative bleeding is a rare (1.5–5%) but serious complication after pancreatic surgery (PPH grade C) [7, 98–100]. It is defined as postoperative hemorrhage more than 48 h after surgery and is associated with a mortality rate higher than 60% [90, 92–94, 99–102]. If the bleeding occurs intraluminally, the pancreatic anastomosis or marginal ulcers should be considered as the source of bleeding [90, 93, 94, 97, 101]. Most often, however, late postoperative bleeding occurs as abdominal hemorrhage due to local septic complications with consecutive erosion of large retroperitoneal vessels [92, 98, 100–103]. The hepatic artery or the stump of the gastroduodenal artery are regularly involved [99, 100]. Dissection of major vessels during lymphadenectomy in tumor patients [90, 99, 100] as well as neoadjuvant radiochemotherapy [104] are suggested risk factors. The dissected vessels are prone to erosion in a septic environment, especially when pancreatic fistula or anastomotic leakage is present. Septic pseudoaneurysm (e.g., of the gastroduodenal artery) or rupture (e.g., of the hepatic artery) can be the consequence. Pancreatic fistulas are found in up to 90% of patients with late postoperative hemorrhage [92, 99, 101, 103]. Biliary fistula or dehiscence of the bilioenteric anastomosis is a less common cause of septic hemorrhage. Pre-existing chronic inflammatory pseudoaneurysms might be involved in late postoperative bleeding after surgery for chronic pancreatitis, where no lymphadenectomy was performed. No association could be established between the occurrence of bleeding and the placement of drainages [105]. However, prophylaxis seems to be the best therapy. Anastomotic leakage needs to be diagnosed early and drained sufficiently, late abscesses have to be treated by CT-guided drainages and irrigation, and all patients with diffuse peritonitis need manual irrigation in the operating theatre. Some authors have suggested a subtotal pancreatic resection shifted to the left with removal of the head and corpus instead of classical Whipple’s procedure in order to avoid contact between major blood vessels and the pancreatic anastomosis [104]. Others have proposed covering of the vessels with the falciform ligament [90] or omentum [101, 106]. Suturing of the gastroduodenal artery instead of ligation and preserving a vessel stump of no less than 1 cm are recommended to avoid slipping of the knot in the presence of local sepsis and to allow catheter-embolization of the gastroduodenal artery in case of bleeding later on [100, 104].
Diagnosis
Bloody secretion via maintained peritoneal drainages days to weeks after surgery, suddenly deteriorated vital signs of the patient, and spontaneous drop in hemoglobin levels are highly suspicious of a late septic hemorrhage. Early diagnosis of a so-called “sentinel bleed” is very important [101] because in 30 to 80% of patients, this little bleeding episode will precede a massive erosive hemorrhage by several hours or days [90, 99, 101, 103–105]. Particularly due to undetected septic complications like silent anastomotic breakdown or an undiscovered abscess, late anastomotic bleeding or preliminary septic hemorrhage may occur as a “sentinel bleed” and find its way either via the maintained drainages or via the dehiscent anastomosis into the intestine. This way, it may mimic upper GI-bleeding. Consequently, late gastrointestinal bleeding is always suspect of an anastomotic failure and a local septic complication, which needs immediate diagnostic workup [92]. If the source of bleeding cannot be identified endoscopically, a “sentinel bleed” must be suspected and its origin needs to be urgently sought after by contrast-enhanced high-resolution CT scan (Fig. 4b) [99–101]. Interventional angiography may follow for precise localization of the bleeding and catheter-embolization (coiling/stenting; Fig. 3) [99, 100, 102, 105].
Erosive bleeding from the right hepatic artery due to a sub-hepatic abscess 4 weeks after Whipple’s procedure (PPH grade C) (a, b). Drop in hemoglobin levels and cardio-circulatory instability of the patient led to the diagnostic work-up with primary CT-scan (a, b) and immediate angiography of the celiac trunk for precise localization of the bleeding (c). Selective catheter-embolization distal (d) and proximal to the site of erosion was achieved, with final occlusion of the right hepatic artery (e). CT-guided drainage of the sub-hepatic abscess could initially avoid surgery, but as a consequence of coiling a second intrahepatic abscess developed due to ischemia (f)
Therapy
Angiography and interventional hemostasis (coiling, stenting) are preferable to open surgery in patients with stable cardio-circulatory conditions (Fig. 4b) [99, 100]. Surgery, in the scenario of late septic hemorrhage, is associated with high mortality and should be limited to patients with hemorrhagic shock or patients where embolization was not sufficient [7, 90, 92, 98, 105]. Several studies have demonstrated good results for catheter-embolization of septic bleeding in acute pancreatitis as well as in patients after partial pancreatoduodenectomy [99, 100, 103, 107, 108]. Success of this method, however, largely depends on local availability and expertise. Often, complete coiling of the eroded vessel (e.g. the hepatic artery) is necessary. This in turn may lead to secondary complications such as liver necrosis, hepatic abscess and biliary complications [99], particularly when accessory vessels are absent (Fig. 3f). In near future, stenting of the celiac arteries may possibly prevent this [109]. However, interventional hemostasis has to be followed necessarily by causative treatment of the septic focus. For that reason, some authors still prefer open surgery as the first-line therapy to interventional “bridging” [92, 98]. On the other hand, mortality of surgical revision in case of late septic hemorrhage remains high and the intraoperative management is still a matter of debate [98]. In most patients, hemostasis may only be achieved through ligation of the whole blood vessel concerned, which may lead to severe ischemic consequences, as described above. Some authors perform salvage pancreatectomy in the same session, for definite control of the septic focus [2, 79, 90, 110]. This benefit might be counterbalanced by the disadvantage of a large surgical trauma and postoperative insulin-dependent diabetes [92]. Figure 4 shows our department’s diagnostic and therapeutic algorithm for postoperative bleeding following pancreatic surgery.
References
Beger HG, Gansauge F, Schwab M, Poch B (2007) Pancreatic head resection: the risk for local and systemic complications in 1315 patients - a monoinstitutional experience. Am J Surg 194:S16–S19
Berberat PO, Friess H, Kleeff J, Uhl W, Buchler MW (1999) Prevention and treatment of complications in pancreatic cancer surgery. Dig Surg 16:327–336
Bottger TC, Junginger T (1999) Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg 23:164–171 discussion 171–2
Büchler MW, Friess H, Wagner M, Kulli C, Wagener V, Z’Graggen K (2000) Pancreatic fistula after pancreatic head resection. Br J Surg 87:883–889
Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’Graggen K (2003) Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg 138:1310–1314 discussion 1315
Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J (1993) One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 217:430–435 discussion 435–8
de Castro SM, Busch OR, Gouma DJ (2004) Management of bleeding and leakage after pancreatic surgery. Best Pract Res Clin Gastroenterol 18:847–864
Neoptolemos JP, Russell RC, Bramhall S, Theis B (1997) Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg 84:1370–1376
Trede M, Saeger HD, Schwall G, Rumstadt B (1998) Resection of pancreatic cancer–surgical achievements. Langenbecks Arch Surg 383:121–128
Trede M, Schwall G, Saeger HD (1990) Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 211:447–458
Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA (1995) A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 222:580–588 discussion 588–92
Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J, Zahurak ML, Grochow LB, Abrams RA (1997) Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 226:248–257 discussion 257–60
Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL (1997) Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg 225:590–599 discussion 599–600
Miedema BW, Sarr MG, van Heerden JA, Nagorney DM, McIlrath DC, Ilstrup D (1992) Complications following pancreaticoduodenectomy. Current management. Arch Surg 127:945–949 discussion 949–50
Geer RJ, Brennan MF (1993) Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 165:68–72 discussion 72–3
Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232:786–795
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD (2000) Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567–579
Bruns CJ, Jauch KW (2007) Surgical treatment of pancreatic cancer. Dtsch Med Wochenschr 132:798–802
Bakkevold KE, Kambestad B (1993) Morbidity and mortality after radical and palliative pancreatic cancer surgery. Risk factors influencing the short-term results. Ann Surg 217:356–368
Shrikhande SV, Qureshi SS, Rajneesh N, Shukla PJ (2005) Pancreatic anastomoses after pancreaticoduodenectomy: do we need further studies? World J Surg 29:1642–1649
Batignani G, Fratini G, Zuckermann M, Bianchini E, Tonelli F (2005) Comparison of Wirsung-jejunal duct-to-mucosa and dunking technique for pancreatojejunostomy after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int 4:450–455
Watanabe M, Usui S, Kajiwara H, Nakamura M, Sumiyama Y, Takada T, Nagakawa T (2004) Current pancreatogastrointestinal anastomotic methods: results of a Japanese survey of 3109 patients. J Hepatobiliary Pancreat Surg 11:25–33
Goldsmith HS, Gosh BC, Hivos AG (1971) Ligation versus implantation of the pancreatic duct after pancreaticoduodenectomy. Surg Gynecol Obstet 132:87–92
Tran K, Van Eijck C, Di Carlo V, Hop WC, Zerbi A, Balzano G, Jeekel H (2002) Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg 236:422–428 discussion 428
McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, Vollmer CM Jr, Dixon E (2006) Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg 93:929–936
Kingsnorth AN (1989) Duct to mucosa isolated Roux loop pancreaticojejunostomy as an improved anastomosis after resection of the pancreas. Surg Gynecol Obstet 169:451–453
Sutton CD, Garcea G, White SA, O’Leary E, Marshall LJ, Berry DP, Dennison AR (2004) Isolated Roux-loop pancreaticojejunostomy: a series of 61 patients with zero postoperative pancreaticoenteric leaks. J Gastrointest Surg 8:701–705
Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, Salvia R, Pederzoli P (2003) Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery 134:766–771
Marcus SG, Cohen H, Ranson JH (1995) Optimal management of the pancreatic remnant after pancreaticoduodenectomy. Ann Surg 221:635–645 discussion 645–8
Tani M, Onishi H, Kinoshita H, Kawai M, Ueno M, Hama T, Uchiyama K, Yamaue H (2005) The evaluation of duct-to-mucosal pancreaticojejunostomy in pancreaticoduodenectomy. World J Surg 29:76–79
Biehl T, Traverso LW (1992) Is stenting necessary for a successful pancreatic anastomosis? Am J Surg 163:530–532
Dai XW, Ma K, Wang FX, Yang FQ, Wang BS, Zhao HY, Sun W, Liu BL, Qiu F, Pu XM, Wang L, Dai Y (2003) Prevention of pancreaticojejunal anastomotic leakage after pancreaticoduodenectomy with separate internal drainage of bile and pancreatic fluid. Hepatobiliary Pancreat Dis Int 2:131–134
Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P (2005) Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg 29:1111–1119 discussion 1120–1
Roder JD, Stein HJ, Bottcher KA, Busch R, Heidecke CD, Siewert JR (1999) Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy: a prospective study. Ann Surg 229:41–48
Z’Graggen K, Uhl W, Friess H, Buchler MW (2002) How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg 9:733–737
Warren KW, Cattell RB (1956) Basic techniques in pancreatic surgery. Surg Clin North Am 36:707–724
Peng SY, Mou YP, Liu YB, Su Y, Peng CH, Cai XJ, Wu YL, Zhou LH (2003) Binding pancreaticojejunostomy: 150 consecutive cases without leakage. J Gastrointest Surg 7:898–900
Rao AC, Gabriel G, Serrano J, Benedicto R (2004) Inkwell pancreaticojejunal anastomosis after pancreaticoduodenectomy. Am J Surg 187:410–412
Ohwada S, Iwazaki S, Nakamura S, Ogawa T, Tanahashi Y, Ikeya T, Iino Y Morishita Y (1997) Pancreaticojejunostomy-securing technique: duct-to-mucosa anastomosis by continuous running suture and parachuting using monofilament absorbable thread. J Am Coll Surg 185:190–194
Delcore R, Thomas JH, Pierce GE, Hermreck AS (1990) Pancreatogastrostomy: a safe drainage procedure after pancreatoduodenectomy. Surgery 108:641–645 discussion 645–7
Icard P, Dubois F (1988) Pancreaticogastrostomy following pancreatoduodenectomy. Ann Surg 207:253–256
Mason GR, Freeark RJ (1995) Current experience with pancreatogastrostomy. Am J Surg 169:217–219
Adloff M, Schloegel M, Ollier JC, Cuvelier G (1992) Pancreatojejunostomy or pancreatogastrostomy after cephalic pancreatoduodenectomy. Chirurgie 118:63–70
Andivot T, Cardoso J, Dousset B, Soubrane O, Bonnichon P, Chapuis Y (1996) Complications of two types of pancreatic anastomosis after pancreaticoduodenectomy. Ann Chir 50:431–437
Aranha GV, Hodul P, Golts E, Oh D, Pickleman J, Creech S (2003) A comparison of pancreaticogastrostomy and pancreaticojejunostomy following pancreaticoduodenectomy. J Gastrointest Surg 7:672–682
Arnaud JP, Tuech JJ, Cervi C, Bergamaschi R (1999) Pancreaticogastrostomy compared with pancreaticojejunostomy after pancreaticoduodenectomy. Eur J Surg 165:357–362
Kim SW, Youk EG, Park YH (1997) Comparison of pancreatogastrostomy and pancreatojejunostomy after pancreatoduodenectomy performed by one surgeon. World J Surg 21:640–643
Miyagawa S, Makuuchi M, Lygidakis NJ, Noguchi T, Nishimaki K, Hashikura Y, Harada H, Hayashi K, Kakazu T (1992) A retrospective comparative study of reconstructive methods following pancreaticoduodenectomy–pancreaticojejunostomy vs. pancreaticogastrostomy. Hepatogastroenterology 39:381–384
Oussoultzoglou E, Bachellier P, Bigourdan JM, Weber JC, Nakano H, Jaeck D (2004) Pancreaticogastrostomy decreased relaparotomy caused by pancreatic fistula after pancreaticoduodenectomy compared with pancreaticojejunostomy. Arch Surg 139:327–335
Schlitt HJ, Schmidt U, Simunec D, Jager M, Aselmann H, Neipp M, Piso P (2002) Morbidity and mortality associated with pancreatogastrostomy and pancreatojejunostomy following partial pancreatoduodenectomy. Br J Surg 89:1245–1251
Takano S, Ito Y, Watanabe Y, Yokoyama T, Kubota N, Iwai S (2000) Pancreaticojejunostomy versus pancreaticogastrostomy in reconstruction following pancreaticoduodenectomy. Br J Surg 87:423–427
Wente MN, Shrikhande SV, Muller MW, Diener MK, Seiler CM, Friess H, Buchler MW (2007) Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta-analysis. Am J Surg 193:171–183
Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P (2005) Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg 242:767–771 discussion 771–3
Duffas JP, Suc B, Msika S, Fourtanier G, Muscari F, Hay JM, Fingerhut A, Millat B, Radovanowic A, Fagniez PL (2005) A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg 189:720–729
Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ (2005) Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg 92:1059–1067
Gyr KE, Meier R (1993) Pharmacodynamic effects of Sandostatin in the gastrointestinal tract. Digestion 54(Suppl 1):14–19
Raptis S, Schlegel W, Lehmann E, Dollinger HC, Zoupas C (1978) Effects of somatostatin on the exocrine pancreas and the release of duodenal hormones. Metabolism 27:1321–1328
Büchler M, Friess H, Klempa I, Hermanek P, Sulkowski U, Becker H, Schafmayer A, Baca I, Lorenz D, Meister R et al (1992) Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 163:125–130
Montorsi M, Zago M, Mosca F, Capussotti L, Zotti E, Ribotta G, Fegiz G, Fissi S, Roviaro G, Peracchia A et al (1995) Efficacy of octreotide in the prevention of pancreatic fistula after elective pancreatic resections: a prospective, controlled, randomized clinical trial. Surgery 117:26–31
Pederzoli P, Bassi C, Falconi M, Camboni MG (1994) Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Italian Study Group. Br J Surg 81:265–269
Sarr MG (2003) The potent somatostatin analogue vapreotide does not decrease pancreas-specific complications after elective pancreatectomy: a prospective, multicenter, double-blinded, randomized, placebo-controlled trial. J Am Coll Surg 196:556–564 discussion 564–5; author reply 565
Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, Campbell KA, Choti MA (2000) Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 232:419–429
Braasch JW, Deziel DJ, Rossi RL, Watkins E Jr, Winter PF (1986) Pyloric and gastric preserving pancreatic resection. Experience with 87 patients. Ann Surg 204:411–418
Patel AG, Toyama MT, Kusske AM, Alexander P, Ashley SW, Reber HA (1995) Pylorus-preserving Whipple resection for pancreatic cancer. Is it any better? Arch Surg 130:838–842 discussion 842–3
Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, Pitt HA, Lillemoe KD (1999) Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 229:613–622 discussion 622–4
Zerbi A, Balzano G, Patuzzo R, Calori G, Braga M, Di Carlo V (1995) Comparison between pylorus-preserving and Whipple pancreatoduodenectomy. Br J Surg 82:975–979
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Buchler MW (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142:761–768
Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Muller MW, Friess H, Buchler MW (2005) Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg 140:1094–1099
Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, Yamaue H (2006) Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg 243:316–320
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F (1998) Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 228:508–517
van Berge Henegouwen MI, van Gulik TM, DeWit LT, Allema JH, Rauws EA, Obertop H, Gouma DJ (1997) Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg 185:373–379
Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, Cameron JL (1993) Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg 218:229–237 discussion 237–8
Tsao JI, Rossi RL, Lowell JA (1994) Pylorus-preserving pancreatoduodenectomy. Is it an adequate cancer operation. Arch Surg 129:405–412
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13
Connor S, Alexakis N, Garden OJ, Leandros E, Bramis J, Wigmore SJ (2005) Meta-analysis of the value of somatostatin and its analogues in reducing complications associated with pancreatic surgery. Br J Surg 92:1059–1067
Bartoli FG, Arnone GB, Ravera G, Bachi V (1991) Pancreatic fistula and relative mortality in malignant disease after pancreaticoduodenectomy. Review and statistical meta-analysis regarding 15 years of literature. Anticancer Res 11:1831–1848
Friess H, Malfertheiner P, Isenmann R, Kuhne H, Beger HG, Buchler MW (1996) The risk of pancreaticointestinal anastomosis can be predicted preoperatively. Pancreas 13:202–208
Hamanaka Y, Nishihara K, Hamasaki T, Kawabata A, Yamamoto S, Tsurumi M, Ueno T, Suzuki T (1996) Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery 119:281–287
Farley DR, Schwall G, Trede M (1996) Completion pancreatectomy for surgical complications after pancreaticoduodenectomy. Br J Surg 83:176–179
van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ (1997) Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 185:18–24
Yeo CJ (1995) Management of complications following pancreaticoduodenectomy. Surg Clin North Am 75:913–924
Gervais DA, Fernandez-del Castillo C, O’Neill MJ, Hahn PF, Mueller PR (2001) Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics 21:673–690
vanSonnenberg E, Wittich GR, Chon KS, D’Agostino HB, Casola G, Easter D, Morgan RG, Walser EM, Nealon WH, Goodacre B, Stabile BE (1997) Percutaneous radiologic drainage of pancreatic abscesses. AJR Am J Roentgenol 168:979–984
vanSonnenberg E, Wittich GR, Goodacre BW, Casola G, D’Agostino HB (2001) Percutaneous abscess drainage: update. World J Surg 25:362–369 discussion 370–2
Cullen JJ, Sarr MG, Ilstrup DM (1994) Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg 168:295–298
Smith CD, Sarr MG, vanHeerden JA (1992) Completion pancreatectomy following pancreaticoduodenectomy: clinical experience. World J Surg 16:521–524
Beger HG, Rau B, Gansauge F, Poch B. The risk of pancreatic head resection - a monoinstitutional experience. in American Pancreatic Club Meeting. 2006. Los Angeles, CA.
Wu CC, Hwang CR, Yeh DC, Hwang YC, Liu TJ, P’Eng FK (1996) Treatment for dehiscence of pancreaticojejunostomy after pancreaticoduodenectomy: is resection of the residual pancreas necessary? Hepatogastroenterology 43:271–274
Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T (1999) Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg 6:303–311
Koukoutsis I, Bellagamba R, Morris-Stiff G, Wickremesekera S, Coldham C, Wigmore SJ, Mayer AD, Mirza DF, Buckels JA, Bramhall SR (2006) Haemorrhage following pancreaticoduodenectomy: risk factors and the importance of sentinel bleed. Dig Surg 23:224–228
Meinke WB, Twomey PL, Guernsey JM, Frey CF, Farias LR, Higgins G, Keehn R (1983) Gastrointestinal bleeding after operation for pancreatic cancer. Am J Surg 146:57–60
Rumstadt B, Schwab M, Korth P, Samman M, Trede M (1998) Hemorrhage after pancreatoduodenectomy. Ann Surg 227:236–241
Shankar S, Russell RC (1989) Haemorrhage in pancreatic disease. Br J Surg 76:863–866
van Berge Henegouwen MI, Allema JH, van Gulik TM, Verbeek PC, Obertop H, Gouma DJ (1995) Delayed massive haemorrhage after pancreatic and biliary surgery. Br J Surg 82:1527–1531
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW (2007) Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 142:20–25
Balladur P, Christophe M, Tiret E, Parc R (1996) Bleeding of the pancreatic stump following pancreatoduodenectomy for cancer. Hepatogastroenterology 43:268–270
Wente MN, Shrikhande SV, Kleeff J, Muller MW, Gutt CN, Buchler MW, Friess H (2006) Management of early hemorrhage from pancreatic anastomoses after pancreaticoduodenectomy. Dig Surg 23:203–208
de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Lameris JS, van Gulik TM, Obertop H, Gouma DJ (2005) Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 241:85–91
Makowiec F, Riediger H, Euringer W, Uhl M, Hopt UT, Adam U (2005) Management of delayed visceral arterial bleeding after pancreatic head resection. J Gastrointest Surg 9:1293–1299
Yamashita Y, Taketomi A, Fukuzawa K, Tsujita E, Harimoto N, Kitagawa D, Kuroda Y, Kayashima H, Wakasugi K, Maehara Y (2007) Risk factors for and management of delayed intraperitoneal hemorrhage after pancreatic and biliary surgery. Am J Surg 193:454–459
Brodsky JT, Turnbull AD (1991) Arterial hemorrhage after pancreatoduodenectomy. The ‘sentinel bleed’. Arch Surg 126:1037–1040
Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI (2004) Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 199:186–191
Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka M (1998) Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg 133:1099–1102
Turrini O, Moutardier V, Guiramand J, Lelong B, Bories E, Sannini A, Magnin V, Viret F, Blache JL, Giovannini M, Delpero JR (2005) Hemorrhage after duodenopancreatectomy: impact of neoadjuvant radiochemotherapy and experience with sentinel bleeding. World J Surg 29:212–216
Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM (2002) Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg 137:55–59
Kurosaki I, Hatakeyama K (2004) Omental wrapping of skeletonized major vessels after pancreaticoduodenectomy. Int Surg 89:90–94
de Perrot M, Berney T, Buhler L, Delgadillo X, Mentha G, Morel P (1999) Management of bleeding pseudoaneurysms in patients with pancreatitis. Br J Surg 86:29–32
Stosslein F, Zimmermann L, Bulang T (1998) Embolization treatment of bleeding complications in pancreatitis. J Hepatobiliary Pancreat Surg 5:344–347
Gebauer T, Schulz HU, Tautenhahn J, Halloul Z, Effenberger O, Lippert H, Burger T (2004) [Interventional and vascular surgical management for inflammatory arrosion hemorrhage from visceral arteries following pancreatic surgery]. Chirurg 75:1021–1028
Tien YW, Lee PH, Yang CY, Ho MC, Chiu YF (2005) Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg 201:554–559
Acknowledgements
We would like to thank Markus Rentsch, M.D., for preparing the illustrations (Fig. 1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kleespies, A., Albertsmeier, M., Obeidat, F. et al. The challenge of pancreatic anastomosis. Langenbecks Arch Surg 393, 459–471 (2008). https://doi.org/10.1007/s00423-008-0324-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-008-0324-4