Abstract
Introduction
In this multi-institutional study of patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma, we sought to identify factors associated with perioperative transfusion requirement as well as the association between blood transfusion and perioperative and oncologic outcomes.
Methods
The surgical databases across six high-volume institutions were analyzed to identify patients who underwent pancreaticoduodenectomy for pancreatic adenocarcinoma from 2005 to 2010. For statistical analyses, patients were then stratified by transfusion volume according to whether they received 0, 1–2, or >2 units of packed red blood cells.
Results
Among 697 patients identified, 42 % required blood transfusion. Twenty-three percent received 1–2 units, and 19 % received >2 units. Factors associated with an increased transfusion requirement included older age, heart disease, diabetes, longer operative time, higher blood loss, tumor size, and non-R0 margin status (all p < 0.05). The median disease-free survival (13.8 vs. 18.3 months, p = 0.02) and overall survival (14.0 vs. 21.0 months, p < 0.0001) durations of transfused patients were shorter than those of transfusion-free patients. Multivariate modeling identified intraoperative transfusion of >2 units (hazard ratio, 1.92, p = 0.009) and postoperative transfusions as independent factors associated with decreased disease-free survival.
Conclusions
This multi-institutional study represents the largest series to date analyzing the effects of perioperative blood transfusion on patient outcomes following pancreaticoduodenectomy for pancreatic adenocarcinoma. While blood transfusion was not associated with increased rate of infectious complications, allogeneic blood transfusion did confer a negative impact on disease-free and overall survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Awareness of the immunomodulatory effect of allogeneic blood transfusions is certainly not novel. Initial publications from the early 1970s demonstrated a protective benefit of blood transfusions on transplanted renal allograft survival.1 Red blood cell transfusions have also been shown to decrease the frequency of autoimmune-driven inflammatory bowel disease exacerbations.2
Despite these initial reports of the beneficial effects of immunological inhibition following transfusion, most recent reports have focused on the deleterious effects of transfusion-related immunosuppression (TRIM). The first report of TRIM-related poor outcomes in oncologic patients was reported by Burrows and Tartter,3 demonstrating a decrease in overall survival in patients receiving perioperative blood transfusions around the time of surgical resection of colon cancer. Since then, similar findings have been reported for patients being treated for primary malignancies of the esophagus,4 stomach,5 lung,6 prostate,7 breast,8 and bone,9 as well as colorectal cancer metastases to the liver.10 Accumulation of lipid mediators,11 pro-inflammatory cytokines,12 and immunosuppressive proteins13 in the fractions of stored blood have been implicated as potential mechanisms for this TRIM-induced negative effect on survival. Following transfusion, these components of stored blood may promote suppression of natural killer cell activity and decreased IL-2 production, thus inhibiting the body's innate immunosurveillance system typically responsible for cancer cell detection and elimination.14
Patients undergoing pancreaticoduodenectomy (PD) for treatment of pancreatic adenocarcinoma often require perioperative blood transfusion. Factors including extensive dissection within a highly vascular operative field as well as potential chronic anemia within patients suffering an underlying malignancy result in recently published perioperative transfusion rates of 40–60 %.15 , 16 Despite reports that TRIM is associated with worse outcomes in this patient population, controversy still remains.17 Moreover, even the largest published reports investigating this association in patients with pancreatic adenocarcinoma have utilized patient data limited to surgeons operating at single institutions.18 , 19 We therefore sought to investigate long term survival outcomes in patients who received perioperative blood transfusions on a multi-institutional basis. We hypothesized that perioperative blood transfusion in patients undergoing PD for surgical treatment of adenocarcinoma of the head of the pancreas was associated with decreased disease-free and overall survival.
Methods
Data Collection
A retrospective review of prospectively collected institutional databases was initially performed across six high-volume academic surgical institutions within the Central Pancreas Consortium to identify patients who underwent PD for all diagnoses between 1st January 2005 and 31st December 2010. Approval was granted by each respective institutional review board prior to data collection. Each institution provided data on patient demographics and medical history, operative and pathological statistics, perioperative blood transfusion requirements, and postoperative course, including various surgical complications. Blood transfusions were counted if they were administered from the onset of surgery until discharge from index admission. Of note, for the current study, only units of packed red blood cells (pRBCs) were included in the analysis. Additional sources of products, including fresh frozen plasma, platelets, albumin, and coagulation factors were not investigated. Hospital length of stay, time to most recent follow-up, and time to disease recurrence and/or death (where applicable) were noted. These data were then collated into a common multi-institutional database. An analysis of this entire cohort has previously been published.20 From this entire cohort, a subset of patients undergoing PD for treatment of pancreatic adenocarcinoma was identified, whereas the remaining patients who underwent PD for other diagnoses were excluded from further analysis. For statistical analyses, patients were then stratified according to transfusion quantity (0, 1–2, or greater than 2 units of pRBCs), as well as transfusion timing (intraoperative vs. postoperative).
Operative Technique, Postoperative Complications, and Long-Term Follow-up
All operative cases were performed by fellowship-trained pancreatic surgeons with assistance from senior surgical residents or surgical oncology fellows as appropriate. Patient selection, operative conduct, transfusion requirement, and postoperative course were determined at the discretion of the attending surgeon and his or her surgical team. Given the multi-institutional nature of this study, specific transfusion triggers varied across institutions. However, common triggers included ongoing intraoperative blood loss, need for additional intraoperative vascular volume with or without additional expected blood loss, hemoglobin levels <7.0 g/dL (or higher in the setting of prior heart disease), or symptomatic anemia. Specific postoperative complications including pancreatic fistula and delayed gastric emptying were identified by the definitions provided by the International Study Group of Pancreatic Surgery (ISGPF).21 , 22 Postoperatively, patients received follow-up under protocols specific to each institution. In general, after several immediate postoperative office visits, patients traditionally underwent surveillance with serum tumor markers (e.g., CA 19-9) and CT scan imaging every 3–6 months for approximately 3 years at the discretion of the attending surgeon. Tumor recurrence was defined as a significant elevation in baseline tumor markers or evidence of tumor on radiologic imaging, whichever occurred first.
Statistical Analyses
SAS software (SAS version 9.3; SAS Institute Inc., Cary, NC) was used to perform all statistical analyses. Analysis of variance (ANOVA) was performed to compare all continuous variables (e.g., age, body mass index (BMI), procedure length, and hospital length of stay) between transfusion groups. Chi-square analyses were performed on all remaining nonparametric variables. To identify independent factors associated with decreased disease-free and overall survival, univariate analyses were first performed with disease-free survival and overall survival as dependent endpoints. Patient demographic variables included in the regression analyses included patient age, gender, race, and comorbidities (e.g., BMI, smoking status, and presence or absence of diabetes or heart disease). Clinicopathologic variables included receipt of neoadjuvant therapy, estimated blood loss, operative time, type of Whipple performed (pylorus-preserving PD vs. standard PD), tumor size, lymph node involvement, margin status, postoperative complications, length of hospital stay, and transfusion requirements (timing and volume). Data on receipt of adjuvant therapy were not available for these analyses. All variables with p values <0.15 on univariate analysis were then included in the respective stepwise multivariate logistic regression model.
For survival analyses, Kaplan-Meier survival curves were generated with death/most recent follow-up and cancer recurrence as endpoints and statistically analyzed via the log-rank test. All deaths were included in the analysis, regardless of whether they occurred perioperative (i.e., within 30 days of index surgery) or longer term. To account for differences in patient demographics and perioperative variables, an additional survival analysis was performed following a propensity-matched analysis utilizing a “greedy-matching” algorithm in which patients undergo computerized matching of cases to controls using a number of patient variables. In the current analysis, the variables within the logistic regression used to generate propensity scores included age (continuous), gender (male/female), race (White/Black/Other), Whipple type (standard PD vs. pylorus-preserving PD, BMI (continuous), smoking status (yes/no), diabetes (yes/no), and heart disease (yes/no). With the exception of the initial univariate analyses to determine statistically and/or clinically significant variables to include in the multivariate model, all p values <0.05 were considered statistically significant.
Results
Patient and Perioperative Demographics
A total of 697 patients were identified from the original database for further analysis. Of these, 293 patients (42 %) required blood transfusion throughout the course of their index admission while 404 patients (58 %) remained transfusion free. One hundred sixty-one patients (23 %) received a total of 1–2 units of pRBCs and 132 patients (19 %) received a total of greater than 2 units (range, 0–25 units). Eighteen percent of patients required transfusions intraoperatively only, 14 % required their transfusions postoperatively only, and 10 % received both intra- and postoperative transfusions. Of those patients receiving transfusions, the average transfusion volume of those receiving intraoperative blood was 2.69 ± 0.18 units of pRBCs, whereas the average volume for those receiving postoperative units was 3.02 + 0.25 units of pRBCs. Table 1 lists the patient demographics and perioperative variables included within the analysis. The average age of all patients was 65.8 years with an equal male/female ratio. Roughly half of all patients (48 %, n = 377) endorsed a significant active or past smoking history, 30 % (n = 212) were diabetic, and 16 % (n = 113) carried a diagnosis of heart disease. The overall estimated blood loss was 672 ± 29 mL across all patients.
The most common postoperative complications across all patients were wound infection (12 %, n = 83), pancreatic fistula or intra-abdominal abscess (11 %, n = 74), delayed gastric emptying (8 %, n = 54), and urinary tract infection (4 %, n = 29). Overall length of stay averaged 10 days; a longer length of stay was associated with receipt of blood transfusion (p < 0.001). Those patients who received blood also demonstrated higher 30-day reoperation (p = 0.02) and 90-day readmission (p = 0.001) rates as compared with those patients who remained transfusion free. A total of ten patients (1.4 %) died within 30 days of their initial operation. The median follow-up duration for all patients was 1.5 years, and there was no difference in follow-up time between transfused and nontransfused groups.
Factors Associated with Blood Transfusion
Patient demographics associated with higher transfusion requirement included increasing age, heart disease, and diabetes (all p < 0.05). Not surprisingly, higher estimated blood loss (EBL) was associated with an increased transfusion requirement (p < 0.001), with an EBL of patients receiving 0, 1–2, or >2 units averaging 468 ± 17, 679 ± 43, and 1,291 ± 121 mL of blood loss, respectively. Sex (p = 0.03) and race (p = 0.02) were also associated with differences in transfusion requirements, but these findings are likely clinically insignificant. Drain placement, longer operative time, larger tumor size, and non-R0 margin status were also associated with a higher transfusion requirement (all p < 0.03). Of all complications identified, only delayed gastric emptying (p = 0.02) and myocardial infarction (p = 0.006) were associated with a higher transfusion requirement. Receipt of blood transfusion was also associated with a longer length of stay (p < 0.001) as well as a higher 30-day mortality rate (p = 0.039).
BMI and smoking status were not associated with blood transfusion. Additionally, receipt of neoadjuvant therapy, type of Whipple performed (standard vs. pylorus preserving), and nodal metastases were not associated with an increased transfusion requirement. The overall intraoperative vein resection rate was 13.6 % (n = 95). Not surprisingly, there was an increased transfusion rate in those patients who underwent vein resection compared with those who did not (51 vs. 41 %), but this difference did not reach statistical significance (p = 0.09).
Survival Analyses
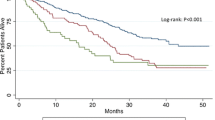
Figure 1a, b depicts the Kaplan-Meier analyses of disease-free survival and overall survival, respectively, stratified by transfusion status (i.e., whether the patient received a transfusion or not). The median disease-free survival durations for transfusion-free patients and for those who received blood were 18.3 and 13.8 months, respectively. The survival curve of patients who received 0 units differed from that of patients receiving any transfusion (log-rank p = 0.02). Similarly, the median overall survival for patients receiving 0 units of pRBCs vs. those patients who received blood was 21.0 and 14.0 months, respectively. A decrease in overall survival was noted between these two groups (log-rank p < 0.0001).
Kaplan-Meier analyses of disease-free and overall survival by transfusion status. a The median disease-free survival durations for transfusion-free patients (solid line) and for those who received blood (dotted line) were 18.3 and 13.8 months, respectively. Transfused patients demonstrated significantly shorter disease-free survival compared with transfusion-free patients. b The median overall survival durations for transfusion-free patients (solid line) and for those who received blood (dotted line) were 21.0 and 14.0 months, respectively. Transfused patients demonstrated significantly shorter overall survival compared with transfusion-free patients
Figure 2a, b depicts the Kaplan-Meier analyses of disease-free survival and overall survival, respectively, stratified by transfusion volume. The median disease-free survival durations for patients having received 0, 1–2, or >2 units were 18.3, 17.8, and 10.2 months, respectively. The survival curves of patients who received 0 units or 1–2 units differed from those of patients receiving a large transfusion volume of >2 units (log-rank p < 0.001 and log-rank p = 0.014, respectively). The survival curves of patients receiving 0 units and 1–2 units were not statistically different. A similar volume-dependent effect was also observed for overall survival. The median overall survival for patients receiving 0, 1–2, or >2 units of pRBCs was 21.0, 16.0, and 11.1 months, respectively. A dose-dependent decrease in overall survival across all groups was demonstrated (log-rank p = 0.003 for 0 vs. 1–2 units, log-rank p < 0.0001 for 0 vs. >2 units, and log-rank p = 0.003 for 1–2 vs. >2 units).
Kaplan-Meier analyses of disease-free and overall survival stratified by transfusion volume. a The median disease-free survival durations for patients having received 0 (solid line), 1–2 (dashed line), or >2 units (dotted line) were 18.3, 17.8, and 10.2 months, respectively. Those patients receiving a large volume transfusion of >2 units of blood demonstrated a significantly decreased survival vs. those receiving 0 or 1–2 units. b The median overall survival durations for patients receiving 0 (solid line), 1–2 (dashed line), or >2 units (dotted line) of pRBCs were 21.0, 16.0, and 11.1 months, respectively. A significant dose-dependent decrease in overall survival across all groups was observed
Survival analyses were repeated following a propensity matching across transfusion volume cohorts. A total of 136 matched pairs were identified between the 0- and 1- to 2-unit cohorts, 104 pairs between the 1- to 2- and >2-unit cohorts, and 111 pairs between the 0- and >2-unit cohorts. After propensity matching, those patients who received >2 units of pRBCs within the perioperative period demonstrated a worsened disease-free survival compared with those patients who remained transfusion free (log-rank p = 0.028; Fig. 3).
Factors Associated with Decreased Disease-Free Survival
To identify independent variables associated with decreased disease-free survival, a univariate analysis was first performed on all clinically relevant patient, operative, and postoperative variables. The initial univariate analysis (Table 2) identified sex, diabetes, pylorus-preserving PD, estimated blood loss, nodal metastases, margin status, deep-vein thrombosis and/or pulmonary embolism (DVT/PE), and length of stay as potential independent predictors of decreased disease-free survival (all p < 0.15). Additionally, transfusion volume and transfusion timing again demonstrated significance and were included in further analysis. A stepwise multivariate model was then run with the abovementioned variables included within the analysis. Under this model, intraoperative transfusion of >2 units (hazard ratio (HR), 1.92 (95 % confidence interval (CI), 1.18–3.13), p = 0.009) as well as postoperative transfusion of 1–2 units (HR, 1.55 (95 % CI, 1.05–2.28), p = 0.026) or >2 units (HR, 2.06 (95 % CI, 1.31–3.26), p = 0.002) were independently associated with worsened disease-free survival (Table 3). Additional independent factors associated with disease-free survival included nodal metastases (HR, 1.38 (95 % CI, 1.00–1.92), p = 0.051), pylorus-preserving PD (HR, 1.51 (95 % CI, 1.05–2.18), p = 0.028), and DVT/PE (HR, 3.54 (95 % CI, 1.21–10.33), p = 0.021).
Factors Associated with Decreased Overall Survival
A similar analysis was performed to identify variables associated with decreased overall survival. Significant patient variables in this model on univariate analysis included age, race, BMI, heart disease, diabetes, and smoking status (Table 4). Operative statistics included receipt of neoadjuvant therapy, Whipple type, EBL, tumor size, nodal metastases, and margin status. Significant postoperative factors included DVT/PE, delayed gastric emptying, uncontrolled hyperglycemia, and length of stay. Transfusion volume (0, 1–2, or >2 units) and transfusion timing (intra- vs. postoperative) both reached statistical significance on univariate analysis and were therefore also included in the multivariate model. Next, the stepwise multivariate analysis model was performed. Under this model, receipt of transfusions postoperatively was independently associated with decreased overall survival (1–2 units: HR, 1.35 (95 % CI, 0.99–1.85), p = 0.056; >2 units: HR, 2.14 (95 % CI, 1.41–3.23), p < 0.001), while receipt of blood transfusions intraoperatively was not (Table 5). Additional independent predictors of decreased survival within this model included an R1 (HR, 1.36 (95 % CI, 1.01–1.85), p = 0.04) or R2 (HR, 8.2 (95 % CI, 2.40–27.98), p < 0.001) margin status, smoking status (HR, 1.54 (95 % CI, 1.18–2.00), p = 0.002), tumor size (HR, 1.04 (95 % CI, 1.00–1.08), p = 0.04), nodal metastases (HR, 1.4 (95 % CI, 1.06–1.85), p = 0.02), DVT/PE (HR, 2.71 (95 % CI, 1.29–5.69), p = 0.009), and delayed gastric emptying (HR, 2.13 (95 % CI, 1.35–3.36), p = 0.001). Higher BMI was found to be slightly protective (HR, 0.98 (95 % CI, 0.94–0.99), p = 0.047).
Discussion
This multi-institutional report represents the largest study to date investigating the deleterious association between perioperative blood transfusion and survival on patients with adenocarcinoma of the head of the pancreas undergoing curative PD. Within this heterogeneous oncologic patient population, we report an overall transfusion rate of 42 %—well within recent historical norms for patients undergoing this procedure. By limiting our analysis to patients undergoing PD for a diagnosis of adenocarcinoma, we likely selected for a group of patients which ultimately required more blood product as compared with those undergoing PD for nonmalignant diagnoses. A study by Chu et al.23 confirms this, demonstrating that undergoing PD for cancer was associated with higher estimated blood loss and increased transfusion rate compared with patients undergoing the same procedure for surgical management of chronic pancreatitis. Additionally, a single institution audit of the transfusion requirements of all oncology patients24 identified pancreatic cancer as the malignancy with the highest increase in transfusion rate over their 3-year study period, a factor the authors attributed to the difficulty of the surgery as well as the increasing rate of patients receiving neoadjuvant and adjuvant chemotherapy.
In addition to the known immunosuppressive effects of chemotherapy and radiotherapy, allogeneic blood transfusion has previously been shown to be immunosuppressive. While initial reports suggested a benefit of immunosuppression in transplanted graft survival,1 current reports discuss the adverse effects of undergoing allogeneic blood transfusion. Despite the precipitous decline in the transmission of blood-borne viruses such as human immunodeficiency virus (HIV) and hepatitis C that once plagued allogeneic blood transfusion, blood transfusion still remains a significant source of infection-related morbidity.25 Early reports implicated the leukocytes within transfused blood in the suppression of T lymphocyte and natural killer-driven host cellular responses.14 Interestingly, despite the association between blood transfusion and increased rate of recipient infection, our transfused patient cohort did not demonstrate an increased infection rate as compared with the transfusion-free cohort.
This same suppression of the innate immune response is thought to underplay the association of blood transfusion and cancer recurrence. Barnett and colleagues have published several studies investigating the accumulation of pro-cancer cytokines within the plasma fraction of stored pRBCs.12 In a similar study, they discovered that delivery of the acellular plasma fraction of stored pRBCs to mice previously infected with pancreatic cancer demonstrated increased tumor growth and lymph node metastases compared with transfusion-free mice previously inoculated with the same pancreatic cell line. Though the presence of leukocytes and their associated cytokines certainly play a role in TRIM, sufficient evidence exists for non-leukocyte products within the stored units such as ubiquitin13 to play an immunosuppressive role. It is hypothesized that this perioperative state of immunosuppression could temporarily handicap the innate immune system and allow for renegade micrometastatic tumor cells to go undetected at the time of surgical resection, allowing for local and distant metastatic spread. This is a factor of great significance in pancreas cancer, in which a significant number of patients may have systemic disease at the time of surgery. Despite these proposed mechanisms, no clear and indisputable source has yet to be identified within units of pRBCs which confer such an immunosuppressive effect. The specific source underlying this association remains elusive, and we continue to challenge our basic science and translational research colleagues to identify the mechanism(s) involved.
In our study, we have demonstrated that patients who received a perioperative transfusion volume of >2 units of pRBCs experienced significantly earlier disease recurrence as well as significantly decreased overall survival on Kaplan-Meier survival analyses. Additionally, receiving any amount of transfusion postoperatively was found to be independent predictor of earlier disease recurrence on multivariate analysis. Only receipt of a high volume postoperative transfusion was associated with a negative prognosis for overall survival on multivariate analysis, while receipt of intraoperative transfusion was not found to be an independent predictor of decreased overall survival. Two of the largest single-center studies published to date investigating transfusion-related outcomes following PD, one from Emory18 and the other from Memorial Sloan Kettering,19 both also identified postoperative transfusion as an independent predictor of decreased survival. While the Memorial Sloan Kettering study only looked at the primary endpoint of overall survival, the Emory study also identified postoperative transfusion as an independent predictor of disease-free survival within their cohort. The mechanism underlying the discrepant outcomes dependent upon timing of transfusion (i.e. intra- vs. postoperatively) is currently unclear. One may hypothesize that postoperative transfusion provides the patient an additional immunosuppressive episode following the initial immunosuppressive surgery itself, thus providing a “two-hit” model and resulting in synergistic suppression of endogenous immunosurveillance. This phenomenon of the impact of transfusion warrants future study to truly elucidate the underlying mechanism.
Unique to this current study compared with the abovementioned studies was the negative prognostic association of receipt of a high-volume (>2 units) intraoperative transfusion on disease-free survival (HR, 1.92 (95 % CI, 1.18–3.13), p = 0.009). This effect was absent in the patient population that received a smaller intraoperative transfusion of only 1–2 units and also had no effect on overall survival. Neither the Memorial Sloan Kettering study nor the Emory study demonstrated such an effect. The data from Emory demonstrated a slight trend towards decreased overall survival in patients who received any intraoperative transfusion; however, due to their relatively smaller sample size as well as shorter survival/follow-up duration, this effect was not significant. Additionally, unlike the current study, those patients were analyzed in an all-or-none fashion, whereas the current study was stratified by transfusion volume. Only the receipt of a larger transfusion volume (i.e., >2 units of pRBCs) was identified as a significant predictor of decreased overall survival; a transfusion of 1–2 units was not significant on multivariate analysis.
The negative prognostic effect of intraoperative transfusions has been studied previously. A study from Cameron et al. from The Johns Hopkins University26 initially reported the finding that intraoperative blood transfusion was a significant prognostic factor among 81 patients undergoing PD for pancreatic cancer. However, in their follow-up report17 released several years later, this initial finding was no longer significant with the addition of more patients and a longer follow-up period. Alternatively, one study found that an intraoperative transfusion of >3 units pRBCs was an independent poor prognostic factor for patients with ampullary cancer undergoing curative PD.16 Similarly, another study from Korea27 identified intraoperative transfusion as an independent poor prognostic factor for patients with ampullary cancer but not adenocarcinoma of the head of the pancreas. Despite the current controversy between studies, it is clear that significant intraoperative blood transfusion volume is associated with long-term morbidity and mortality. An appropriately designed randomized trial may provide a more definitive answer of a causal relationship, but its design would be challenged by the logistical and ethical limitations of withholding blood transfusions from severely anemic patients.
Some researchers propose the negative prognostic survival effects associated with perioperative blood transfusions are associated with patient and operative factors (e.g., more aggressive tumor biology associated with local invasion and potential for vascular resection) rather than the immunosuppressive effect of the transfusion itself. For example, in a study published in the Annals of Surgery in 1994, Busch and colleagues investigated the patterns of tumor recurrence in 420 patients undergoing curative-intent operations for colorectal cancer.28 These authors concluded that the association between blood transfusion and prognosis in colorectal cancer resulted from the perioperative circumstances that necessitate transfusions, leading to the development of local recurrences but not of distant metastases. It should be noted, however, that the tumor biology and disease processes of colorectal cancers differ greatly from those of pancreatic cancer. Specifically, pancreatic cancer is often systemic at the time of initial diagnosis, and the vast majority of pancreatic cancer patients who recur harbor evidence of distant metastases. Additionally, although patient demographics did vary between transfusion cohorts, the propensity-matched analysis still identified a disease-free survival advantage for those patients who remained transfusion-free in the perioperative period.
Like most retrospective studies, ours is not without limitations. Despite the multi-institutional nature of this study and subsequently large sample size, data from individual institutions are subject to individual practice biases. For example, surgeons from different institutions may have varying triggers for transfusion; as such, specific transfusion triggers and preoperative hemoglobin levels were not investigated. We feel, however, that the potential for various transfusion thresholds creates a more heterogeneous patient population, much more representative of pancreatic cancer patients at-large. This patient heterogeneity may therefore strengthen the correlation between transfusion and decreased survival. Within our study, transfusion rates across institutions varied from 15 to 67 % with an average transfusion volume of 0.4–2.6 units per patient across institutions. While data regarding receipt of neoadjuvant therapy were included in this study, data regarding receipt of adjuvant therapy were not available for all institutions and therefore were not included in the analyses. Given that these patients were all treated at institutions which perform high volumes of pancreaticoduodenectomies, the authors presume an equal number from each transfusion study cohort did and did not receive adjuvant therapy postoperatively. Additionally, given the retrospective nature of this study, it is possible that infectious complications were under-reported and therefore underestimated the true infection rates. Several studies have identified significant intraoperative blood loss (cutoffs of 400 mL29 or 2,000 mL30) as independent predictors of decreased survival, attributing the anemia and associated local trauma and inflammation to the physiologic insult predisposing to poorer early (i.e., infectious) and late (i.e., survival) outcomes. While higher EBL was significant in our univariate models, it did not reach significance on our final multivariate models for either disease-free or overall survival. Despite this, it is clear that higher EBL is associated with higher intraoperative (and likely postoperative) transfusion requirements, and that the poor prognosis of transfused patients is multifactorial and likely related to blood loss, local and systemic tissue inflammation, and the immunosuppressive effects of allogeneic blood transfusion.
Conclusions
Our current study represents the first multi-institutional report analyzing the effects of perioperative blood transfusion on outcomes of patients undergoing PD. Significant perioperative anemia within oncologic patients is multifactorial. Preoperative factors including anemia of chronic disease, malnutrition, and anemia secondary to neoadjuvant chemoradiotherapy; intraoperative blood loss secondary to operating within a highly vascularized surgical field; and postoperative chemoprophylaxis and chemotherapy, all contribute to the anemia experienced by oncologic patients. Despite the controversy within the literature regarding blood transfusion and patients with pancreatic adenocarcinoma undergoing PD, our multi-institutional study confirms the association between perioperative blood transfusion and deleterious long-term survival effects on this patient population. Across all institutions, 42 % of patients underwent perioperative blood transfusion. This was not associated with increased infection rates, yet was associated with significantly decreased disease-free and overall survival. We certainly would not advocate withholding transfusions in severely anemic patients in need of blood to mitigate this risk; however, a significant effort should be made to address and optimize all modifiable risk factors. Nutritional optimization, growth factors, meticulous surgical technique, and novel blood conservation techniques can all be utilized to minimize anemia and reduce the risks associated with blood transfusion in this already vulnerable patient population.
References
Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusion on subsequent kidney transplants. Transfusion Proc 1973;5:253
Peters WR, Fry RD, Fleshman JW, Kodner IJ. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum 1989;32:749–753
Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet 1982;2:662
Komatsu Y, Orita H, Sakurada M, Maekawa H, Hoppo T, Sato K. Intraoperative blood transfusion contributes to decreased long-term survival of patients with esophageal cancer. World J Surg. 2012;36(4):844–850
Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, Shimoyama H, Chohno S, Okabayashi T, Kagawa S, Orita K. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. 1987;27(5):375–357
Moores DW, Piantadosi S, McKneally MF. Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg. 1989;47(3):346–351
Heal JM, Chuang C, Blumberg N. Perioperative blood transfusions and prostate cancer recurrence and survival. Am J Surg 1998;156:374–380
Tartter PI, Burrows L, Papatestas AE, Lesnick G, Aufses AH Jr. Perioperative blood transfusion has prognostic significance for breast cancer. Surgery. 1985;97(2):225–230.
Chesi R, Cazzola A, Bacci G, Borghi B, Balladelli A, Urso G. Effect of perioperative transfusions on survival in osteosarcoma treated by multimodal therapy. Cancer. 1989;64(8):1727–1737
Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237(6):860–869; discussion 869–870
Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Investig 1998;101:1458–1467
Benson DD, Beck AW, Burdine MS, Brekken R, Silliman CC, Barnett CC Jr. Accumulation of pro-cancer cytokines in the plasma fraction of stored packed red cells. J Gastrointest Surg 2012;16:460–468
Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res. 2006;135:226–232.
Jensen LS, Andersen AJ, Christiansen PM, Hokland P, Juhl CO, Madsen G, Mortensen J, Møller-Nielsen C, Hanberg-Sørensen F, Hokland M. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79(6):513–516
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH, Lillemoe KD. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579
Yao HS, Wang Q, Wang WJ, Hu ZQ. Intraoperative allogeneic red blood cell transfusion in ampullary cancer outcome after curative pancreatoduodenectomy: a clinical study and meta-analysis. World J Surg. 2008;32(9):2038–2046
Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221(6):721–731; discussion 731–733
Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, Staley CA 3rd, Kooby DA. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18(5):1327–1334
Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg. 2007;94(4):466–472
Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, Patel SH, Bentram DJ, Weber SM, Cho CS, Winslow ER, Scoggins CR, Martin RC, Kim HJ, Baker JJ, Merchant NB, Parikh AA, Kooby DA. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256(3):529–537
Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142(5):761–768
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8–13
Chu CK, Sarmiento JM, Park J, Staley CA, Galloway JR, Adsay NV, Kooby DA. Differences in presentation and perioperative outcome after pancreaticoduodenectomy for cancer and benign pancreatitis. Am Surg. 2010;76(6):606–613
Dernedde U, Dernedde R, Shepstone L, Barrett A. Three-year single institution audit on transfusion requirements in oncology patients. Clin Oncol (R Coll Radiol). 2007;19(4):223–237
Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137(6):711–716; discussion 716–717
Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161(1):120–124; discussion 124–125
Park SJ, Kim SW, Jang JY, Lee JY, Park YH. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002;26(4):487–492
Busch OR, Hop WC, Marquet RL, Jeekel J. Blood transfusions and local tumor recurrence in colorectal cancer. Evidence of a noncausal relationship. Ann Surg. 1994;220(6):791–797
Kazanjian KK, Hines OJ, Duffy JP, Yoon DY, Cortina G, Reber HA. Improved survival following pancreaticoduodenectomy to treat adenocarcinoma of the pancreas: the influence of operative blood loss. Arch Surg. 2008;143(12):1166–1171
Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, Yamada S, Sugimoto H, Nomoto S, Takeda S, Morita S, Nakao A. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas. 2011;40(1):3–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutton, J.M., Kooby, D.A., Wilson, G.C. et al. Perioperative Blood Transfusion Is Associated with Decreased Survival in Patients Undergoing Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: a Multi-institutional Study. J Gastrointest Surg 18, 1575–1587 (2014). https://doi.org/10.1007/s11605-014-2567-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2567-4