Abstract
Background
Several prognostic factors for patients who have undergone esophagectomy owing to esophageal squamous cell carcinoma have been suggested, including intraoperative blood loss. There are few data, however, suggesting such an association with the prognosis following radical esophagectomy.
Methods
Patients with esophageal squamous cell carcinoma who underwent radical esophagectomy were divided into two groups based on the median value of the intraoperative blood loss (510 g). A multivariate Cox proportional-hazard regression analysis was performed to determine if intraoperative blood loss could be an independent prognostic factor for long-term survival following radical esophagectomy. Kaplan–Meier survival analysis with a log-rank test was performed between the groups.
Results
From April 2005 to May 2009, a total of 37 patients underwent radical esophagectomy for the treatment of esophageal squamous cell carcinoma at the Juntendo Shizuoka Hospital and were assigned either to one of two groups: those with ≥510 g blood loss [bleeding group (BG), n = 19] or of those with <510 g blood loss [less bleeding group (LBG), n = 18]. The distribution of the stage of disease, the number of positive lymph nodes, and the presence of lymphatic and vascular invasion was comparable between the groups, but the Kaplan–Meier survival analysis demonstrated that survival was significantly worse in the BG group than in the LBG group (p = 0.00295). This was supported by the multivariate analysis, which indicated that intraoperative blood loss was independently associated with long-term survival after radical esophagectomy.
Conclusions
Intraoperative blood loss could be a useful prognostic factor following radical esophagectomy in patients with esophageal squamous cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, esophageal cancer has been a significantly increasing health problem, being the seventh leading cause of cancer-related death. In 2005, there were approximately 500,000 new cases, and the prevalence is expected to increase by approximately 140% by 2025 [1]. Squamous cell carcinoma (SCC) is responsible for more than 90% of all esophageal cancers worldwide, as it is in Japan [2]. In Western countries, however, adenocarcinoma has exceeded SCC over the past few decades and become the most prevalent form of esophageal cancer [2, 3].
Treatment options for esophageal cancer including the surgical approach, extent of lymphadenectomy, and chemotherapy and radiation therapy have been tailored based on the individual staging, tumor biology and medical co-morbidities. In the West, a transhiatal or transthoracic esophagectomy with limited lymph node dissection has played a major role in the multidisciplinary treatment of resectable esophageal adenocarcinoma [2]. However, in Japan, as a standard of surgical approach, an extended transthoracic esophagectomy with three-field lymphadenectomy has been performed since the 1980s and has been demonstrated to contribute to improved long-term survival of patients with esophageal SCC [4]. Currently, the standard of care for resectable esophageal SCC in Japan is neoadjuvant chemotherapy with 5-fluorouracil/cisplatin followed by an extended intrathoracic esophagectomy with three-field lymphadenectomy [5, 6].
The following factors have been reported to be associated with overall survival in patients with esophageal SCC: age, sex, tumor size, tumor depth, tumor location, tumor stage at the time of presentation, blood vessel invasion, neural invasion, lymph node status, number of positive lymph nodes, resection margins, type of resection, red blood cell transfusion, operating time, and adjuvant chemotherapy [4, 7–10]. Intraoperative blood loss and red blood cell transfusion have been reported as prognostic factors for other types of cancer, such as colorectal cancer, gastric cancer, hepatocellular carcinoma, ductal adenocarcinoma of the pancreas, and prostate cancer [11–15]. Although several studies have examined the impact of blood transfusion on prognosis [16, 17], there are few studies demonstrating the association between the amount of intraoperative blood loss and the long-term outcome of patients with esophageal SCC following radical esophagectomy. The aim of this study was to determine retrospectively the independent prognostic factors, especially intraoperative factors such as blood loss, after curative resection of esophageal SCC.
Methods
Study design and patient selection
This retrospective review of prospectively collected data was performed with the approval of our institutional review board at the Juntendo Shizuoka Hospital. Eligible subjects included patients with esophageal SCC who underwent radical esophagectomy with three-field lymphadenectomy between April 2005 and May 2009 regardless of neoadjuvant or adjuvant chemotherapy and/or radiation therapy. The patient’s demographic data and procedure data were obtained either from the medical records or a phone interview with patients. Patients who met any of the following criteria were excluded from this study: presence of other coexisting carcinomas, postoperative mortality within 90 days, any pathologic type other than squamous cell carcinoma, presence of distant metastases at the time of the preoperative workup. The patients were divided into two groups based on the median value of the intraoperative blood loss (510 g): bleeding group (BG, ≥510 g) and less-bleeding group (LBG, <510 g).
Clinicopathologic variables
As candidates of prognostic factors for long-term survival after radical esophagectomy, the following clinicopathologic variables were chosen for the univariate and multivariate analyses: intraoperative blood loss, age, sex, tumor location, tumor size, stage of disease, positive lymph nodes, blood transfusion, adjuvant chemotherapy and radiation therapy, lymphatic invasion, vascular invasion. Intraoperative blood loss was calculated at the end of each case by adding the weight of contents in the suction containers to the weight of surgical sponges used during the surgery. Tumor size was defined as the maximum diameter of the tumor, which was obtained from the pathology reports. Lymphovascular invasion was considered present when the presence of tumor cells within lymphatics and vessels was confirmed microscopically. The final pathological staging was determined using the 2002 staging system of the International Union against Cancer (UICC).
Statistical analysis
Data were expressed as a mean ± standard deviation where appropriate. Comparison (univariate analysis) between the groups was performed using the standard Χ2 test and the Mann–Whitney U-test where appropriate. Survival at 3 years was calculated via the Kaplan–Meier method, and a statistical analysis was carried out using the log-rank test for equality of survival curves. To determine a possible independent prognostic factor, a multivariate analysis was performed using a Cox proportional hazards model. A p value <0.05 was considered to indicate a statistically significant difference with a 95% confidence interval (95% CI). Model Χ2 test was used to evaluate the compatibility of this analysis. All statistical analyses were performed using SPSS (version 16.0; SPSS Japan, Tokyo, Japan).
Results
Between April 2005 and May 2009, a total of 43 patients with SCC underwent radical esophagectomy. All patients had complete macroscopic resection of their disease, and SCC was confirmed histopathologically in all patients. There was no obvious evidence of metastatic disease in any of these patients. Most of the patients (n = 41) underwent a right transthoracic subtotal esophagectomy with three-field lymphadenectomy including cervical (bilateral supraclavicular regions), mediastinal (paraesophageal and tracheal regions, including the bilateral recurrent laryngeal nerves), and abdominal (perigastric region and around the celiac axis) lymph nodes followed by reconstruction with either stomach (n = 39) or jejunum (n = 2) by the same surgeon (K.S.). Two patients with SCC of the cervical esophagus underwent esophagectomy in conjunction with laryngectomy followed by reconstruction with stomach; these two patients were included in this study. Four patients who died within 90 days after surgery were excluded to avoid the effects of surgery-related postoperative complications on long-term survival. Two patients with coexisting carcinoma were excluded. Hence, a total of 37 patients were enrolled in the study (Fig. 1). None of 37 patients received neoadjuvant chemotherapy. In all, 4 patients were given adjuvant chemotherapy, 1 patient was given adjuvant radiation therapy, and 20 patients underwent both adjuvant chemotherapy and radiation therapy. Cisplatin (60–80 mg/m2, 1 day/week) and 5-fluorouracil (5-FU) (700–800 mg/m2, 5 days/week) was the most commonly used regimen in these patients. Twelve patients did not receive any adjuvant treatment including four patients with superficial SCC, one patient who was >80 years of age, and seven patients who refused.
A total of 43 patients underwent radical esophagectomy without neoadjuvant therapy. Four patients who died within 90 days and two patients with coexisting carcinoma were excluded. The remaining 37 patients were enrolled in this study and divided into two groups: less-bleeding group (n = 18) and bleeding group (n = 19). In all, 4 patients underwent chemotherapy, 1 patient underwent radiation therapy, and 20 patients underwent both chemotherapy and radiation therapy
These 37 patients were then assigned to the BG group (n = 19) or the LBG group (n = 18) based on the criteria. Patient demographics and the clinicopathologic variables of each group are summarized in Table 1. The distribution of sex and age was comparable between the groups. The mean amount of blood loss in each group was 317 g (range 160–500 g) in the LBG group and 1017 g (range 510–2780 g) in the BG group. In 29% (11/37) of patients, blood transfusion with a median of 500 g (range 280–1650 g) was required. Only one patient in the LBG group, whose intraoperative blood loss was 500 g, required blood transfusion. The mean tumor size of all patients was 5 cm (range 1.5–9.0 cm). However there was a trend that the tumor size in the LBG group was larger than that in the BG group (5.3 ± 1.5 vs. 4.7 ± 2.3 cm, respectively). In 32 of 37 (86%) patients, the primary tumor was located in the thoracic esophagus. The amount of blood transfusion required was significantly higher in the BG group (p = 0.009), whereas depth of tumor, the number of positive nodes, tumor size, TNM staging, and the presence of lymphovascular invasion were comparable between the groups.
Most of the patients had a history of smoking and alcohol consumption. Postoperatively, pulmonary complications such as atelectasis, pleural effusion, and pneumonia were the most commonly observed (BG group, n = 16; LGB group, n = 8). Worsening pulmonary function was seen in three patients after recovery from the surgery (BG group, n = 3; LBG group, n = 0). These three patients had recovered well from surgery for a while, but their pulmonary function got worse several months after surgery because they developed interstitial pneumonia. Two of them required oxygen therapy. Stricture of an anastomosis was found in three patients (BG group, n = 2; LBG group, n = 1). No anastomotic leakage was reported.
During a mean follow-up of 48 months (range 4–60 months), lymph node recurrence and/or remote organ metastasis occurred in eight patients in the BG group [cervical lymph node (n = 2), bronchial lymph node (n = 2), liver metastasis (n = 2), and both bronchial lymph node and lung metastasis (n = 2)] and in eight patients in the LBG group [bronchial lymph node (n = 6), cervical lymph node (n = 1), and both bronchial lymph node and lung metastasis (n = 1)] (Table 2). There was a significant difference in the incidence of liver metastasis between the BG and LBG groups (2 vs. 0, p = 0.011), whereas there was no difference in the combined data of remote organ metastasis (liver and lung) between the groups (4 vs. 1, p = 0.147). There was no difference in the mode of lymph node recurrence between the groups. Among them, all eight patients in the BG group and three patients in the LBG group died of recurrent and/or metastatic disease. On the other hand, two patients in the LBG group died of other underlying diseases such as liver cirrhosis and chronic obstructive lung disease. Eventually, a total of 13 patients died during the follow-up period for this study.
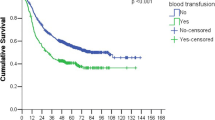
The median survival of patients in the BG group was significantly shorter than that of patients in the LBG group (10.4 vs. 26.4 months, respectively; p = 0.00295). The Kaplan–Meier analysis demonstrated that the survival was significantly worse in the BG group than in the LBG group. The 3-year survival rates for the BG and LBG groups were 37 and 77%, respectively (Fig. 2). This suggests that the amount of intraoperative blood loss is highly associated with survival after radical esophagectomy even though the distribution of the stage of disease, the number of positive lymph nodes, and presence of lymphovascular invasion are comparable between the groups.
To determine if the amount of intraoperative blood loss and/or other variables could be an independent prognostic factor, a multivariate analysis was performed (Table 3). Multivariate analysis demonstrated that the stage of the disease, positive lymph nodes, and blood transfusion in addition to intraoperative blood loss were independent prognostic factors (p < 0.05). The hazard ratio was the highest in positive nodes, followed by the stage of disease, intraoperative blood loss, and blood transfusion.
Discussion
In Japan, a subtotal esophagectomy with three-field lymph node dissection has been performed routinely as a curative procedure in patients with esophageal SCC and reasonable functional status because the prognosis is highly associated with the presence of positive lymph nodes [7]. In contrast, true benefits of the extended lymph node dissection for patients with esophageal cancer remain controversial, especially in Western countries [2]. The concept of three-field lymphadenectomy has been established for the treatment of esophageal SCC in which lymph node metastases are found to locate almost equally at the abdomen through the neck. On the other hand, two-field lymphadenectomy is commonly performed in Western countries because of the high incidence of esophageal adenocarcinoma, which usually arises from the distal esophagus due to underlying gastroesophageal reflux disease followed by Barrett’s esophagus; and the extension of lymph node involvement is often limited to the intrathoracic area. However, it is clear that the greater the number of lymph nodes obtained, the more accurately is the staging, and the prognosis can be determined [18]. In addition, it has been demonstrated that the number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors [19].
Although proponents of a three-field lymphadenectomy believe it leads to better staging, decreased risk of local recurrence, and improvement of overall survival, most of previous reports regarding the outcomes of three-field lymphadenectomy were written based on single-institution experiences in a retrospective fashion, which was certainly subject to bias and obvious limitations [20, 21]. There is only one randomized controlled trial to compare two-field versus three-field lymphadenectomy in patients with esophageal SCC. It was conducted by Nishihira et al. [22], in which 62 patients were randomly assigned by a double-blind method to either the two-field group (n = 30) or the three-field group (n = 32). The authors concluded that three-field lymphadenectomy may reduce local recurrence and prolong survival after resection of thoracic esophageal carcinoma, although it was not shown statistically. However, it is expected that the extension of lymph node dissection would be highly associated with the amount of intraoperative blood loss and the extended surgery is more likely to require blood transfusion.
Postoperative pulmonary complications are extremely common after esophagectomy, as in this study; and they potentially affect patients’ quality of life and the prognosis. In this present study, most of the patients had a history of smoking and were expected to have poor pulmonary function preoperatively. To prevent postoperative pulmonary complications and improve the functional status, preoperative assessment of pulmonary functions is critical, and preoperative smoking cessation and aggressive pulmonary rehabilitation should be implemented. In addition, it is important to control the fluid balance between the intravascular and third space, avoiding failure of many organs such as lung, heart, and kidney due to volume overload. The perioperative management of fluid balance would be more difficult in patients with excessive intraoperative blood loss and/or blood transfusion. Two patients developed late pulmonary dysfunction due to interstitial pneumonia even at 3 months after surgery, suggesting that a prolonged time to recover from surgery is required, especially for patients with little pulmonary reserve.
There are several explanations for the negative effects of excessive blood loss on oncologic outcomes after surgery. Excessive intraoperative blood loss may cause tumor dissemination and potentially lead to local or systemic recurrence [23]. Some reports have suggested that intraoperative blood loss during curative gastrectomy for gastric cancer is a critical risk factor of recurrence [24]. Kamei et al. [25] reported that intraoperative blood loss, not blood transfusion, may have a specific association with peritoneal recurrence of gastric cancer—but not with other types of recurrence. In addition, Bruns et al. [26] demonstrated that >700 ml of blood loss significantly reduced natural killer cell function in patients who underwent gastrointestinal surgery. These studies suggested that intraoperative blood loss may impair the immune system locally and systemically, potentially leading to peritoneal recurrence of gastric cancer. In the present study, intrathoracic lymph node (bronchial lymph node) recurrence and lung metastasis were most commonly observed. Although peritoneal and/or pleural metastasis of esophageal cancer is not common, locally and/or systemically impaired immune system due to blood loss may be associated with lymph node recurrence and organ metastasis in the thoracic cavity.
Other studies have suggested that a large amount of blood loss may be associated with a longer period of systemic and local hypoperfusion, potentially leading to impaired oxygen delivery to vital organs such as lung, liver, and kidney. This may promote systemic inflammation, which may prevent antitumor immunity [11]. Specifically, hemorrhagic shock has been reported to be associated with the elevated levels of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α, all of which could increase the risk for early postoperative mortality [11, 27, 28]. Younes et al. [29] reported that the number of intraoperative hypotensive episodes may negatively impact the long-term outcome after resection of colorectal cancer with liver metastasis. In the present study, no patients had a prolonged period of hypotension or hypovolemic shock during the surgery, and the intraoperative blood pressure was properly maintained with fluid resuscitation. However, patients who required blood transfusion may have a short period of local hypoperfusion in some organs such as liver and lung, potentially enhancing the recurrence or metastatic process via depression of the immune system.
Several reports have suggested that blood transfusion is a significant prognostic factor of outcomes in patients who underwent surgical resection of cancer [15, 16, 30, 31]. The mechanisms involved in the adverse effects of red blood cell transfusion may be related to impaired immunity or enhanced inflammation, which may lead to tumor growth or recurrence. The immunosuppressive effects with blood transfusion have been reported in patients with transplantation [32]. Kaplan et al. [33] demonstrated the increased activity and number of T-suppressor cells as well as the diminished natural killer cell activity in recipients of blood transfusions. The exact mechanisms of effects on the prognosis by both intraoperative blood loss and blood transfusion remain unknown. There may be unknown effects of intraoperative blood loss on the association between blood transfusion and decreased survival. Because patients with excessive intraoperative blood loss often require blood transfusion, intraoperative blood loss may be a more sensitive factor than blood transfusion. However, there is a possibility of measurement and selection bias regarding these two variables. Further studies with a larger sample size are required to determine the true impact of intraoperative blood loss and transfusion on the long-term outcome after radical esophagectomy in patients with SCC
Finally, although the incidence of lymph node recurrence and/or remote organ metastasis was comparable between the BG (8/19) and LBG (8/18) groups, the prognosis for the BG group was significantly worse than that for the LBG group. This may be explained by the fact that more patients in the BG group had remote metastasis compared to those in the LBG group. Osugi et al. [34] reported that patients with local lymphatic recurrence survived longer than patients with remote metastasis, suggesting that remote metastasis contributes more to survival compared to local lymph node recurrence. This further supports our results.
Conclusions
The present study demonstrates that the survival rate is significantly worse among patients with a large intraoperative blood loss (≥510 g) requiring blood transfusion compared to that in patients with less blood loss (<510 g) even though the distribution of the stage of disease, the number of positive lymph nodes and presence of lymphovascular invasions is comparable between the groups. Systemic inflammation and impaired immunity induced by local and/or systemic hypoperfusion due to blood loss and the following blood transfusion may promote growth of any residual tumor after surgery. Although surgical resection should be the primary choice for patients with a resectable esophageal SCC and reasonable functional status, every effort should be made to reduce intraoperative blood loss requiring blood transfusion as it may be associated with decreased long-term survival.
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Cohen DJ, Ajani J (2011) An expert opinion on esophageal cancer therapy. Expert Opin Pharmacother 12:225–239
Demeester SR (2009) Epidemiology and biology of esophageal cancer. Gastrointest Cancer Res 3(Suppl):S2–S5
Osugi H, Takemura M, Takada N et al (2002) Prognostic factors after oesophagectomy and extended lymphadenectomy for squamous oesophageal cancer. Br J Surg 89:909–913
Kitagawa Y (2008) Therapeutic strategies for advanced respectable esophageal cancer. Nippon Geka Gakkai Zasshi 109:333–337
Higuchi K, Koizumi W, Tanabe S et al (2009) Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res 3:153–161
Tsurumaru M, Kajiyama Y, Udagawa H et al (2001) Outcomes of extended lymph node dissection for squamous cell carcinoma of the thoracic esophagus. Ann Thorac Cardiovasc Surg 7:325–329
Tachibana M, Kinugasa S, Dhar DK et al (1999) Prognostic factors after extended esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Surg Oncol 72:88–93
Tachibana M, Kinugasa S, Dhar DK et al (1999) Prognostic factors in T1 and T2 squamous cell carcinoma of the thoracic esophagus. Arch Surg 134:50–54
Hu Y, Zheng B, Rong TH et al (2010) Prognostic analysis of the patients with stage-III esophageal squamous cell carcinoma after radical esophagectomy. Chin J Cancer 29:178–183
Katz SC, Shia J, Liau KH et al (2009) Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 249:617–623
Nagai S, Fujii T, Kodera Y et al (2011) Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas 40:3–9
Oefelein MG, Colangelo LA, Rademaker AW et al (1995) Intraoperative blood loss and prognosis in prostate cancer patients undergoing radical retropubic prostatectomy. J Urol 154(Pt 1):442–447
Tartter PI (1992) The association of perioperative blood transfusion with colorectal cancer recurrence. Ann Surg 216:633–638
Ojima T, Iwahashi M, Nakamori M et al (2009) Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg 13:1821–1830
Takemura M, Osugi H, Higashino M et al (2005) Effect of substituting allogenic blood transfusion with autologous blood transfusion on outcomes after radical oesophagectomy for cancer. Ann Thorac Cardiovasc Surg 11:293–300
Tachibana M, Tabara H, Kotoh T et al (1999) Prognostic significance of perioperative blood transfusions in resectable thoracic esophageal cancer. Am J Gastroenterol 94:757–765
Peyre CG, Hagen JA, DeMeester SR et al (2008) Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 248:979–985
Mariette C, Piessen G, Briez N et al (2008) The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 247:365–371
Altorki N, Kent M, Ferrara C et al (2002) Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 236:177–183
Lerut T, Nafteux P, Moons J et al (2004) Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome—a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg 240:962–972; discussion 972–964
Nishihira T, Hirayama K, Mori S (1998) A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 175:47–51
Kobayashi S, Asano T, Ochiai T (2001) A proposal of no-touch isolation technique in pancreatoduodenectomy for periampullary carcinomas. Hepatogastroenterology 48:372–374
Dhar DK, Kubota H, Tachibana M et al (2000) Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg 24:588–593; discussion 593–584. doi:10.1007/s002689910099
Kamei T, Kitayama J, Yamashita H et al (2009) Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg 33:1240–1246. doi:10.1007/s00268-009-9979-4
Bruns CJ, Schafer H, Wolfgarten B et al (1996) Einfluss des intraoperativen Blutverlustes auf die Funktion der naturlichen Killerzellen bei Tumoren des oberen Gastrointestinaltraktes [Effect of intraoperative blood loss on the function of natural killer cells in tumors of the upper gastrointestinal tract]. Langenbecks Arch Chir Suppl Kongressbd 113:146–149
Roumen RM, Hendriks T, van der Ven-Jongekrijg J et al (1993) Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma: relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg 218:769–776
Cue JI, Peyton JC, Malangoni MA (1992) Does blood-transfusion or hemorrhagic-shock induce immunosuppression. J Trauma 32:613–617
Younes RN, Rogatko A, Brennan MF (1991) The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg 214:107–113
Ydy LR, Slhessarenko N, de Aguilar-Nascimento JE (2007) Effect of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response after colorectal cancer resection. World J Surg 31:2044–2051. doi:10.1007/s00268-007-9159-3
Dresner SM, Lamb PJ, Shenfine J et al (2000) Prognostic significance of peri-operative blood transfusion following radical resection for oesophageal carcinoma. Eur J Surg Oncol 26:492–497
Lieberman MD, Shou J, Sigal RK et al (1990) Transfusion-induced immunosuppression results in diminished host survival in a murine neuroblastoma model. J Surg Res 48:498–503
Kaplan J, Sarnaik S, Gitlin J et al (1984) Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood 64:308–310
Osugi H, Takemura M, Higashino M et al (2003) Causes of death and pattern of recurrence after esophagectomy and extended lymphadenectomy for squamous cell carcinoma of the thoracic esophagus. Oncol Rep 10:81–87
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Komatsu, Y., Orita, H., Sakurada, M. et al. Intraoperative Blood Transfusion Contributes to Decreased Long-Term Survival of Patients With Esophageal Cancer. World J Surg 36, 844–850 (2012). https://doi.org/10.1007/s00268-012-1433-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1433-3