Abstract
Background
Allogeneic blood transfusion (ABT) containing packed red blood cells (RBCs) has a known immunosuppressive effect that may affect cancer metastases and recurrence. This study examined whether intraoperative allogeneic RBC transfusion is an independent risk factor of adverse outcome in patients with ampullary carcinoma after curative pancreatoduodenectomy.
Methods
The clinical data of 67 patients with carcinoma of the ampulla of Vatar underwent pancreatoduodenectomy between 1999 and 2004 were analyzed, and long-term follow-up visits were made for all patients. Kaplan–Meier statistics and Cox proportional hazard methodology were used to perform univariate and multivariate analysis to identify independent risk factors for survival. For the meta-analysis, all English-language studies regarding blood transfusion from carcinoma of the ampulla of Vatar or ampullary carcinoma and prognostic factors or factors for survival from 1995 to 2007 were reviewed, and contingency tables were constructed from which a summary relative risk was calculated.
Results
There were 43 patients (64.2%) who received an intraoperative ABT. The amount of intraoperative ABT ranged from 2 to 13 (mean, 4.25) units; there were 18 patients transfused at 2 units, and 25 patients transfused ≥3 units. The follow-up ranged from 2 to 90 (mean, 49) months. Forty-five patients (67.2%) died as a result of tumor progression. For patients transfused ≥3 units, median and cumulative 3-year and 5-year survivals were poorer significantly than that of patients transfused with 2 units and/or nontransfused patients (P < 0.05). After multivariate analysis, except for presence of lymph node metastasis (P = 0.023) and pancreatic invasion (P = 0.024), the intraoperative ABT ≥3 units was found to be an independent poor prognostic factor for those with ampullary cancer after curative pancreatoduodenectomy either (relative risk, 2.082; 95% confidence interval (CI), 1.048–4.135; P = 0.036). Meta-analysis of 346 patients showed the summary relative risk of an adverse outcome after intraoperative ABT in these studies was 2.55 (95% CI, 1.59–4.1).
Conclusions
The amount of intraoperative ABT is one of the important factors that adversely influenced survival in patients with ampullary cancer after curative pancreatoduodenectomy. Healing anemia preoperatively and careful dissection to minimize intraoperative bleeding as much as possible are mandatory for reducing risk of the intraoperative ABT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ampulla of Vater is composed of the intraduodenal portion of bile duct, the pancreatic duct, their common channel, and papilla. Although small, it occupies a strategic part of the body. Ampullary cancer, arising from this small region, is a relatively uncommon tumor with the incidence in men and women approximately 0.038% and 0.027%, respectively, accounting for 0.2% of all tumors in digestive system and 7–19% of periampullary tumors—the second most frequent tumor of this region [1–3]. Pancreatoduodenectomy (PD) with lymphadenectomy remains the only potentially curative treatment for patients with ampullary cancer. Although a role for prognostic factors, such as histology, tumor size, and carcinoembryonic antigen (CEA) level, has been suggested, only nodal involvement and depth of tumor infiltration are commonly accepted and used as prognostic indicators in the daily clinical practice [4].
Emerging evidence during the past decade has suggested that the intraoperative allogeneic blood transfusion (ABT) containing packed red blood cells (RBCs) may be related to poor clinical outcomes, including survival. The importance of the intraoperative ABT as a prognostic and predictive factor for outcome has been examined in a variety of solid tumors, whereas in ampullary carcinoma after pancreatoduodenectomy there are only four published studies to date [5–8]. Talamini et al. [5] first reported in 1997 that the best predictor of prolonged survival for those with ampullary cancer was the absence of intraoperative ABT. A clinical analysis by Park et al. [6] of 130 cases suggested that perioperative ABT, particularly intraoperative ABT, does have an adverse effect on survival of patients with ampullary cancer after pancreatoduodenectomy. As to the amount of transfusion, there was no dose-related risk of transfusion for the survival difference in patients with ampullary cancer in the study by Park et al. [6], but a significant dose-dependent relationship between transfusion and survival has been reported in patients after resection of rectal cancer [9], gastric cancer [10], and patients after hepatic resection for isolated metastatic colorectal cancer [11]. Balachandran et al. [8] demonstrated more recently that the prognosis of patients with intraoperative ABT with RBCs <2 units was better than patients with intraoperative ABT >2 units. However, in the qualitative analysis by Di Giorgio et al. [7], the intraoperative ABT was not found to have any adverse effect on the survival in patients with ampullary cancer. To further explore this effect, we have performed an evaluation to find out the intraoperative allogeneic RBC transfusion on prognosis in patients with carcinoma of the ampulla of Vatar undergoing pancreatoduodenectomy by using univariate, multivariate analysis and meta-analysis.
Patients and methods
Clinical study
From January 1999 to January 2004, 67 patients underwent curative pancreatoduodenectomy for carcinoma of the ampulla of Vatar at Shanghai Chang Zheng Hospital and Shanghai Chang Hai Hospital, Second Military Medical University, were qualified for voluntary enrollment in the study. Ampullary cancer was defined as carcinoma arising from ampullary region, originating in the intraduodenal portion of the bile duct, the pancreatic duct, their common channel, and papilla, whereas tumors originating in periampullary sites, such as the duodenum, bile duct, or pancreas, were not included in this study. Qualifying patients provided written, informed consent and were scheduled for the clinical and follow-up study.
The medical data of all 67 patients were recorded especially concerning patients’ demographics, clinical manifestations, preoperative hemoglobin level, duration of surgery, operative blood loss, transfusion data, pathologic findings, and adjuvant chemotherapy. Patients with preoperative anemia received the necessary amount of preoperative ABT and underwent operation only if the hemoglobin level ≥10 g/dl and the hematocrit reached ≥30%. Decisions concerning intraoperative ABT were made by surgeon and/or anesthesiologist, depending on the blood loss, hemodynamic status, hemoglobin concentration, age, and comorbidity of the patient. Usually, operative blood loss >500 ml was used as threshold for ABT (i.e., 500–800 ml blood loss transfused with 2 units, 800–1000 ml transfused with 4 units, and >1,000 ml transfused >5 units). All transfusions were performed using units (200 ml/unit) of allogeneic packed RBCs.
Analytic variables for possible prognostic factors were the presence of intraoperative ABT combined with age, sex, smoking and drinking status, preoperative CEA and CA 19–9 level in serum, weight loss, histologic differentiation, tumor size, depth of invasion, lymph node metastasis, and TNM stages defined according to the revised TNM classification issued by the International Union Against Cancer in 2002 [12]. Patients underwent regular follow-up, including sonography or computed tomography/magnetic resonance imaging (CT/MRI) of the abdomen, radiography of the thorax, routine laboratory tests, and tumor markers at regular intervals, that commonly every 6 months for the first 3 years, then annually afterward. Follow-up time was defined as the time from pancreatoduodenectomy to the last clinic visit or the time of patient death.
Statistical analysis
Differences in percentages between groups were evaluated by the χ2 test, and mean values were compared by using Student’s t test. The cumulative survival analyses were calculated by using the Kaplan–Meier method, and the survival difference across the transfused and nontransfuesd groups was ascertained by the log-rank test. A Cox proportional hazards regression model was performed to find variables that had an independent effect on survival. Significance was set at P < 0.05.
Meta-analysis
The PubMed, MEDLINE, Elsevier, and Springer databases were searched for carcinoma of the ampulla of Vatar or ampullary carcinoma from 1995 to 2007 that had an English abstract containing the following key terms: prognostic factors or factors for survival. For each article, the following data were recorded: study design, sample size, the negative outcome used as an end point, number and percentage of patients transfused, number and percentage of patients with positive resected margin, number and percentage of patients with negative outcome, and the type of statistical analysis performed. Studies were included in the meta-analysis only if the four counts (a, b, c, and d) of a standard 2 × 2 contingency table could be extracted or calculated. The four counts were defined as follows: a = the number of transfused patients experiencing the negative outcome; b = the number of nontransfused patients experiencing the negative outcome; c = the number of transfused patients not experiencing the negative outcome; d = the number of nontransfused patients not experiencing the negative outcome.
The data analysis was performed by using the DerSimonian and Laird method (randomized effect model) or the Mantel–Haenszel method (fixed effect model) with the meta-analysis software Review Manager Software (Review Manager 4.2, Cochrane Collaborative, Oxford, England) [13, 14]. The odds ratio (OR) for each clinical event was presented with 95% confidence interval (CI). Heterogeneity between studies was tested with Q test, and the randomized effect model or fixed effect model was selected according to P value of Q test. The summary relative risk (RR) with the 95% confidence interval was calculated with the selected model. A summary relative risk (an estimate of the “average” adverse transfusion effect across all of the studies) was calculated for the studies using the selected model. Significance was set at P < 0.05.
Results
Clinical study
The mean age of the 67 patients at the time of surgery was 59.2 (range, 35–77) years. Twenty-seven patients (40.3%) were men, and 40 (59.7%) were women. The most common initial symptoms were the presence of jaundice (50/67, 75.2%), followed by weight loss (28/67, 41.8%), abdominal pain (26/67, 38.1%), and fever (20/67, 30.2%). Five patients had other malignant tumor previously. Thirty patients (44.8%) had associated with other diseases at presentation, in which primary hypertension (15/67, 22.4%) was the most common, followed by cardiac disease (6/67, 9%), diabetes mellitus (12/67, 17.9%), hepatocirrhosis (3/67, 4.5%), and chronic obstructive pulmonary disease (2/67, 3%). All patients underwent ultrasonography (US), 45 patients had CT, and 40 patients had MRI and/or MRCP. Biliary dilation was found in 89.5% of the patients (60/67) on US. Periampullary mass was detected in 5 patients (7.5%) by US, 8 patients (17.8%) by CT, and 15 patients (37.5%) by MRI, respectively. Endoscopic retrograde cholangiopancreatography (ERCP) was performed in 30 patients, and the multiple biopsies with positive rate in these patients was 46.7% (14/30). Endoscopic ultrasonography was performed in 15 patients, and 9 patients showed malignant tumor in the multiple biopsies.
All patients underwent curative pancreatoduodenectomy, and the mean surgical time was 6.2 (range, 4–12.2) hours. Forty-three patients (64.2%) received an intraoperative ABT. The amount of operative blood loss ranged from 200 to 3,200 (mean, 712) ml, and the amount of ABT ranged from 2 to 13 (mean, 4.25) units. Eighteen patients with 500–800 ml of operative blood loss received ABT at 2 units, and 25 patients with ≥800 ml operative blood loss received ABT ≥3 units. None of patients in the group donated autologous blood preoperatively and received this blood intraoperatively or postoperatively. One or more surgical complications developed in 14 patients who underwent pancreaticoduodenectomy, for an overall postoperative morbidity rate of 20.9%, including pancreatic fistula (4/67, 6%), intra-abdominal bleeding (3/67, 4.5%), intra-abdominal abscess (6/67, 9%), and delayed gastric emptying (8/67, 12%). Three patients (3/67, 4.5%) received postoperative RBC transfusion due to early postoperative intra-abdominal bleeding, all three had ≥3 units ABT intraoperatively. No postoperative deaths occurred. Postoperative adjuvant chemotherapy was performed in 57 patients (85.1%), and none of patients received postoperative radiotherapy.
Histologic examinations of the resected specimens confirmed that adenocarcinoma was found in 62 patients (92.5%), papillary carcinoma in 4 patients, and adenosquamous carcinoma in 1 patient. The mean tumor size was 2.4 (range, 0.3–4.5) cm. Altogether, 33 patients (49.3%) showed lymph node metastasis with a mean of three positive lymph nodes (range 1–9) for each patient, and 34 patients (50.7%) showed no lymph node metastasis. Tumor staging according to the 6th edition of the American Joint Committee on Cancer (AJCC) staging manual was as follows [12]: IA: 14 patients (T1N0M0, T1: limited to the ampulla of Vater or sphincter of Oddi); IB: 8 patients (T2N0M0, T2: invading the duodenal wall); IIA: 12 patients (T3N0M0, T3: invading the pancreas); IIB: 33 patients (any TN1M0). A total of 53 tumors (79.1%) were well or moderately differentiated (G1, G2), and 14 (20.9%) were poorly differentiated (G3). Margins were negative for all pancreatic resections.
The follow-up ranged from 2 to 90 (mean, 49) months, and none of patients were lost during the follow-up. The mean survival time after curative resection of the 67 patients was 49 (95% CI, 35–77) months. The overall 3-year survival rate was 54.8% and the overall 5-year survival rate was 50.8%. There are 19 patients still alive to date, and 3 of them suspected recurrence. Forty-eight patients died during follow-up: 45 patients (67.2%) due to tumor progression, and 3 patients died of uncertain causes.
A comparison was made between patients with ampullary cancer undergoing pancreatoduodenectomy who received transfusions and those who did not (Table 1). There was no significant difference in age, sex, preoperative total bilirubin, associated illnesses, tumor histology, tumor size, TNM stage, pancreas invasion, and lymph node metastasis. It seemed that there was a trend toward higher percentage of patients with preoperative anemia in the transfused patients or transfused ≥3 units patients, but this did not reach statistical difference compared with nontransfused patients (P = 0.082, 0.062).
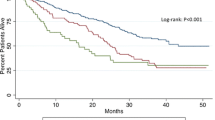
Survivals for all patients are presented in Table 2 and Figs. 1 and 2. For patients transfused ≥3 units, median and cumulative 3-year and 5-year survivals were only 25 months and 36.7% and 30.2%, respectively, which were poorer significantly than that of patients transfused with 2 units and/or nontransfused patients (P < 0.05). However, there was no significant difference of the survivals between the patients transfused with 2 units and nontransfused. When the univariate analysis was performed to assess the impact of various clinical parameters, operative data, transfusion, histology, and TNM stage on survivals after pancreatoduodenectomy, it revealed that presence of lymph node metastasis (P < 0.001), larger tumor size (P < 0.001), presence of pancreatic invasion (P = 0.003), later TNM stage (P = 0.004), poorer histologic differentiation (P = 0.028), as well as intraoperative ABT ≥3 units (P = 0.001) were the significant factors that adversely influenced the 3-year and 5-year survivals of patients with log-rank test (Table 2). On multivariate analysis by the Cox regression hazard model, presence of lymph node metastasis (P = 0.023), presence of pancreatic invasion (P = 0.024), and intraoperative ABT ≥3 units (P = 0.036) were the significant independent predictors of survival. The relative risk of intraoperative ABT ≥3 units was 2.082 (i.e., 108.2% increase in the risk of negative outcome for patients transfused ≥3 units), with a 95% confidence interval of 1.048 to 4.135 (Table 3).
Meta-analysis
Sixty-four earlier articles identified from 1995 to 2007 dealt with the prognostic factors or factors for survival of ampullary carcinoma or carcinoma of the ampulla of Vatar, but only four of them primarily considering blood transfusion [5–8]. Meanwhile, all references of the four articles were searched and none of the references related to the blood transfusion and prognosis of ampullary carcinoma. On the other hand, although Balachandran’s study dealt with intraoperative ABT and progress of patients with ampullary cancer after pancreatoduodenectomy [8], it could not extract four counts of a standard 2 × 2 contingency table and had to exclude from meta-analysis. Meta-analysis on 346 patients from the other three earlier studies [5–7] and our present study was further performed with the Review Manager 4.2 program to identify the influence for 5-year survival made by the intraoperative ABT (Table 4). Death was used as the end point in all of the above-mentioned studies. The randomized effect model was finally selected for the analysis, as P = 0.97, >0.05 with the Q test. The summary relative risk of an adverse outcome after intraoperative ABT was 2.55 (i.e., a 155 percent increase in the risk of negative outcome in the transfused patients), with a 95% confidence interval of 1.59 to 4.1.
Discussion
Since the first suggestion that ABT has a negative effect on survival after surgical resection of colorectal cancer in 1982 [15], several other cancers, including prostate cancer [16], lung cancer [17], cervical cancer [18], osteosarcoma [19], breast cancer [20, 21], renal cancer [22], and pancreatic cancer [23], have been reported with similar findings. However, a number of studies do not support this causative relationship, such as in patients with breast cancer who underwent immediate breast reconstruction with a transverse rectus abdominis musculocutaneous flap [24].
The mechanism of the adverse effect of ABT on the survival in patients with cancer is still incompletely known, and the immunosuppressive effect of transfusion has been proposed as a main possible. The immune system is important for preventing the occurrence, metastases, or recurrence of solid tumors, and any manipulation that impairs the immune response has the potential to affect cancer metastases and recurrence. Animal studies have linked transfusion to both tumor regression and tumor growth, depending on the model used [25–27]. In Lieberman’s animal studies, A/J mice were randomized to receive two weekly transfusions of washed whole blood cells from C57 Bl/6 or A/J donors or saline. Compared with syngeneic transfusions and saline infusion, the effect of multiple allogeneic blood transfusions on cellular immunity, tumor growth, and host survival in a murine C1300 neuroblastoma model was observed. Animals transfused with allogeneic blood had a significantly diminished lymphocyte response to mitogen, reduced donor-specific and third party alloantigen, and reduced cytotoxicity against a natural killer (NK) cell-sensitive target. These in vitro deficits in cellular immunity correlated with a significantly greater tumor weight to total body weight ratio on day 21, and reduced median host survival in the allogeneic group. ABT had an adverse affect on NK and T-lymphocyte function, which was associated with enhanced tumor growth and reduced survival in tumor-bearing mice [25]. In the clinical setting, packed allogeneic RBC transfusion has been shown to enhance both inflammatory and immunosuppressive systemic responses in patients who underwent colorectal cancer resection. The impairment of postoperative immunity due to ABT seems to be more marked than the enhanced inflammatory response [28].
On the other hand, the deleterious effects of transfusion on tumor recurrence also may be mediated by the increased mitogenic activity observed in stored blood. Patients with colorectal cancer with stored blood transfused exhibited a 100 percent increase in mitogenic activity over preoperative values compared with no significant change in nontransfused patients, and the mitogenic activity increased with storage time [29, 30]. In addition, a beneficial effect of pretransplant ABTs on graft outcome in renal transplantation was repeatedly demonstrated in both human and animal models [31–35]. In a large, prospective, multicenter study, a significantly improved graft survival rate was reported in recipients transfused pretransplant compared with those not transfused [32]. Immunosuppressive effect is most likely responsible for the well-established beneficial relationship between ABT and improved renal transplant survival. These have led to speculate that ABT may have a deleterious effect on the immune surveillance for micrometastases and result in an increased risk of metastatic disease or local recurrence after surgical resection of cancer.
The ABT had been linked to cancer recurrence or cancer-related death in 28 retrospective studies on colorectal cancer. Unlike colorectal cancer, the published data for carcinoma of the ampulla of Vatar are even less to date. In our study, the intraoperative ABT was decided by surgeon and/or anesthesiologist, depending on the blood loss, hemodynamic status, and hemoglobin concentration. The mean operative blood loss of the 67 patients was 712 (range, 200–3,200) ml, and the intraoperative ABT rate was 64.2%, which was basically in accordance with the range of curative pancreatoduodenectomy reported previously [6–8, 36, 37]. Similar to the previous report in 64 patients with carcinoma of the ampulla of Vatar by Di Giorgio et al. [7], our study on the 67 patients has not shown significant different survival rates between transfused and nontransfused groups. However, the meta-analysis of 346 patients from three previous studies and our present data demonstrated a clear link between intraoperative ABT and poor survival of ampullary cancer patients. There was a 155 percent increase in risk of an adverse outcome for patients who received intraoperative ABT compared with patients who did not, and the difference was statistically significant. The most possible reason for above difference might be due to the low sample size studied as well as the omission of the necessary dose-related subgroup analysis, which results in this underestimation and discrepancy of the intraoperative ABT.
Results arising from our further subgroup analysis suggest that ≥3 units intraoperative ABT does significantly influences the prognosis of patient with ampullary cancer after pancreatoduodenectomy, which is demonstrated as an independent significant predictor when evaluated by the Cox proportional hazard analysis. Similar results were found previously in patients undergoing surgery for rectal cancer that type (ABT) and number (>3 units) of blood units associated with reduced overall survival [9], and in patients undergoing curative gastrectomy for gastric cancer that a significant difference in the survival rates according to the amount of ABT, in which the 5-year survival rates were 74.1% for nontransfused group, 60.7% for transfused with 1–2 units, 55.4% for transfused with 3–4 units, and 38.2% with more than 4 units [10]. In addition, in the prospective study from Toronto General Hospital for evaluation the independent risk factors associated with postoperative mortality, which consisted of 2,996 patients having noncardiac surgery, the perioperative RBC transfusion had been shown to be independently associated with death, with the strongest overall predictor of death being transfusion of ≥3 units RBC [38]. The mechanism of these aforementioned phenomena is not entirely clear to date, but it seems possible involved in a cumulative dose-response relationship between the ABT and impairment of postoperative immunity, because the volume of ABT has been related to the magnitude of the immunosuppressant effect in a number of studies [28, 39–41]. Moreover, the dose-related risk of ABT also had been demonstrated previously in patients who had hepatic resection for colorectal liver metastasis [11] and patients with renal carcinoma after surgery [22].
The life-saving effects of ABT are well established, as well as the ability to recover more quickly from a major operation when low blood volumes are corrected. The safety of donated blood has improved through newer viral screening techniques; however, the transfusion of blood products is not without risk. The surgeon should counsel patients about all potential risks of ABT, but concern over the risk of metastatic disease or recurrence should probably not dissuade him or her from using allogeneic blood when it is clinically indicated. When possible, ABT should be avoided through careful surgical hemostasis and the use of autogenous blood. The surgical techniques should be improved to avoid over bleeding and intraoperative ABT. It also is very important to stick to the clinical indications for ABT strictly and to avoid unnecessary or over ABT. If patient does have anemia preoperatively, it is strongly recommended to correct anemia with ABT preoperatively, because the preoperative correction with ABT does not worsen the prognosis in ampullary cancer [6], esophageal cancer [42], and colorectal cancer [43, 44].
Conclusions
Our study confirms that the amount of intraoperative ABT is one of the important factors that adversely influenced survival in patients with ampullary cancer after curative pancreatoduodenectomy. On the basis of present works, ≥3 units of ABT in pancreatoduodenectmy seems to have more oncological risk in general. Healing anemia preoperatively and careful dissection to minimize intraoperative bleeding as much as possible are mandatory for reducing risk of the intraoperative ABT.
References
Yamaguchi K, Enjoji M (1987) Carcinoma of the ampulla of Vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer 59:506–515
Nakase A, Matsumoto Y, Uchida K, Honjo I (1977) Surgical treatment of cancer of the pancreas and the periampullary region: cumulative results in 57 institutions in Japan Ann Surg 185:52–57
Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF (1998) Factors predictive of survival in ampullary carcinoma. Ann Surg 228:87–94
Qiao QL, Zhao YG, Ye ML et al (2007) Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg 31:137–143
Talamini MA, Moesinger RC, Pitt HA et al (1997) Adenocarcinoma of the ampulla of Vatar: a 28-year experience. Ann Surg 225:590–600
Park SJ, Kim SW, Jang JY, Lee KU, Park YH (2002) Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg 26:487–492
Di Giorgio A, Alfieri S, Rotondi F et al (2005) Pancreatoduodenectomy for tumors of Vater’s ampulla: report on 94 consecutive patients. World J Surg 29:513–518
Balachandran P, Sikora SS, Kapoor S et al (2006) Long-term survival and recurrence patterns in ampullary cancer. Pancreas 32:390–395
Jagoditsch M, Pozgainer P, Klingler A et al (2006) Impact of blood transfusions on recurrence and survival after rectal cancer surgery. Dis Colon Rectum 49:1116–1130
Hyung WJ, Noh SH, Shin DW et al (2002) Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol 9:5–12
Stephenson KR, Steinberg SM, Hughes KS, Vetto JT, Sugarbaker PH, Chang AE (1988) Perioperative blood transfusions are associated with decreased time to recurrence and decreased survival after resection of colorectal liver metastases. Ann Surg 208:679–687
Greene FL, Page DL, Fleming ID (2002) American Joint Committee on cancer staging manual, 6th edn. Springer-Verlag, New York, pp 151–156
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trial 7:177–188
Fleiss JL, Gross AJ (1991) Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol 44:127–139
Burrows L, Tartter PI (1982) Effect of blood transfusions on colonic malignancy recurrent rate. Lancet 2(8299):662
Heal JM, Chuang C, Blumberg N (1988) Perioperative blood transfusions and prostate cancer recurrence and survival. Am J Surg 15:374–380
Moores DW, Piantalosi S, MeKneally MF (1989) Effect of perioperative blood transfusion on outcome in patients with surgically resected lung cancer. Ann Thorac Surg 48:746–747
Blumberg N, Agarwal MM, Chuang C (1988) A possible association between survival time and transfusion in cervical cancer. Yale J Biol Med 61:493–500
Chesi R, Cazzola A, Bacci G, Borqhi B, Balladelli A, Urso G (1989) Effect of perioperative blood transfusion on survival in osteosarcoma treated by multimodal therapy. Cancer 64:1727–1737
Tartter PI, Burrows L, Papatestas AE, Lesnick G, Aufses AH (1985) Perioperative blood transfusion has prognostic significance for breast cancer. Surgery 97:225–230
Pysz M (2000) Blood transfusions in breast cancer patients undergoing mastectomy: possible importance of timing. J Surg Oncol 75:258–263
Edna TH, Vada K, Hessesberg F, Mjolnerod OK (1992) Blood transfusion and survival following surgery for renal carcinoma. Br J Urol 70:135–138
Cameron JL, Crist DW, Sitzmann JV et al (1991) Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 161:120–125
Rinker BD, Bowling JT, Vasconez HC (2007) Blood transfusion and risk of metastatic disease or recurrence in patients undergoing immediate TRAM flap breast reconstruction: a clinical study and meta-analysis. Plast Reconstr Surg 119:2001–2007
Liberman MD, Shou J, Sigal RK, Yu J, Goldfine J, Daly J (1990) Transfusion-induced immunosuppression results in diminished host survival in a murine neuroblastoma model. J Surg Res 48:498–503
Blajchman MA, Bordin JO (1995) The tumor growth-promoting effect of allogeneic blood transfusions. Immunol Invest 24:311–317
Lin HS, Samy RN, Lum J, Dorie MJ, Terris DJ (2002) Effect of blood transfusion in an experimental sarcoma model. Arch Otolaryngol Head Neck Surg 128:308–312
Ydy LR, Slhessarenko N, de Aguilar-Nascimento JE (2007) Effect of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response after colorectal cancer resection. World J Surg 31:2044–2051
Hoh H, Umpleby H, Cooper A, Taylor I (1990) Recurrence of colorectal cancer and perioperative blood transfusion: is blood storage time important? Dis Colon Rectum 33:127–130
Mynster T, Nielsen HJ, Danish RANX05 Colorectal Cancer Study Group (2001) Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum 44:955–964
Ames SA, Shelby J, Roberts LK, Nelson EW (1988) Factors in transfusion-related enhanced tumor growth. Transplant Proc 20:1121–1124
Opelz G, Varenterghem Y, Kirste G et al (1997) Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation 63:964–967
Obertop H, Bijnen AB, Vriesendorp HM, Westbroek DL (1978) Prolongation of renal allograft survival in DLA tissue typed beagles after third-party blood transfusions and immunosuppressive treatment. Transplantation 26:255–259
van Es AA, Marquet RL, van Rood JJ, Kalff MW, Balner H (1977) Blood transfusions induce prolonged kidney allograft survival in rhesus monkeys. Lancet 1(8010):506–509
van Es AA, Balner H (1979) Effect of pretransplant transfusions on kidney allograft survival. Transplant Proc 11:127–137
Diener MK, Knaebel HP, Heukaufer C et al (2007) A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg 245:187–200
Chan C, Franssen B, Uscanga L et al (2006) Pancreatoduodenectomy: results in a large volume center. Rev Gastroenterol Mex 71:252–256
O’Farrell R, Wijeysundera D, Karkouti K et al (2006) Perioperative transfusion increases mortality. Can J Anesth 53(Suppl 1):26456
Ikuta S, Miki C, Hatada T et al (2003) Allogenic blood transfusion is an independent risk factor for infective complications after less invasive gastrointestinal surgery. Am J Surg 185:188–193
Bordin JO, Chiba AK, Carvalho KI et al (1999) The effect of unmodified or prestorage white cell-reduced allogeneic red cell transfusions on the immune responsiveness in orthopedic surgery patients. Transfusion 39:718–723
Kaplan J, Sarnaik S, Gitlin J et al (1984) Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood 64:308–310
Hirai T, Yamashita Y, Kuwahara M, Inoue H, Toge T (1998) Poor prognosis in esophageal cancer patients with postoperative complications. Surg Today 230:576–579
Laurent C, Sa Cunha A, Coudrec P, Rullier E, Saric J (2003) Influence of postoperative morbidity on long-term survival following liver reaction for colorectal metastasis. Br J Surg 90:1131–1136
Fujita S, Teramoto T, Watanabe M, Kodaira S, Kitajima M (1993) Anastomotic leakage after colorectal surgery: a risk factor for recurrence and poor prognosis. Jpn J Clin Oncol 23:299–302
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yao, H.S., Wang, Q., Wang, W.J. et al. Intraoperative Allogeneic Red Blood Cell Transfusion in Ampullary Cancer Outcome after Curative Pancreatoduodenectomy: A Clinical Study and Meta-Analysis. World J Surg 32, 2038–2046 (2008). https://doi.org/10.1007/s00268-008-9675-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-008-9675-9