Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) is categorized as peripheral ICC (PICC) or hilar ICC (HICC). The aims of this study are to clarify clinicopathological differences between PICC and HICC and to determine useful prognostic factors for patients with ICC following aggressive surgical resection.
Methods
Medical records of 44 patients with ICC who underwent surgical resection were retrospectively reviewed. Clinicopathological factors were compared between patients with PICC and HICC. Univariate and multivariate models were used to analyze the effect of clinicopathological factors on disease-specific survival.
Results
Disease-specific survival rates for the 44 patients were 76% at 1 year, 60% at 3 years, and 47% at 5 years. Clinicopathological factors did not differ between patients with PICC and HICC except preoperative jaundice (P < 0.001), preoperative biliary drainage (P = 0.001), postoperative complication (P = 0.046), and macroscopic type (P < 0.001). Multivariate analysis revealed that only lymph node status was an independent prognostic factor of disease-specific survival. The 5-year disease-specific survival rates of patients with or without nodal involvement were 23% and 66%, respectively (P = 0.004).
Conclusions
Clinicopathological characteristics are almost similar between patients with PICC and HICC. Nodal involvement is a potent prognostic factor for patients with ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma is usually divided into three categories based on location: intrahepatic tumors, which originate in the intrahepatic bile duct; perihilar tumors, which originate in the hepatic duct bifurcation; and distal tumors, which originate in the distal extrahepatic or intrapancreatic bile duct.1 Of these three types, intrahepatic cholangiocarcinoma (ICC) represents approximately 10–20% of all cholangiocarcinomas.1,2 The incidence and mortality rates of ICC are gradually increasing in Eastern and Western countries.2–4
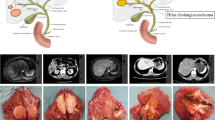
Intrahepatic cholangiocarcinoma often involves the hepatic hilum, and therefore, ICC is generally divided into peripheral ICC (PICC) and hilar ICC (HICC).5 Peripheral ICC requires hepatectomy alone and HICC requires hepatectomy with extrahepatic bile duct resection for surgical management. In previous reports on various aspects of ICC, most analyses included patients with both PICC and HICC,6–29 while some investigators categorized cases of HICC into the perihilar cholangiocarcinoma group because they showed similar clinical features and required similar surgical procedures.30–34 The differences in clinical features, pathological characteristics, and surgical outcome between PICC and HICC have not been fully elucidated.22,27 Identification of the clinicopathological characteristics of patients with PICC and HICC is important to be able to plan a treatment strategy that includes appropriate surgical procedures and adjuvant therapy. The aims of this study are to clarify the clinicopathological differences between patients with PICC and HICC and to determine useful prognostic factors for patients with ICC who have undergone aggressive surgical resection in a single institution.
Patients and Methods
Study Design
Medical records of 44 patients with ICC who were treated at the Department of Surgery, Hiroshima University Hospital between January 1995 and December 2010 were retrospectively reviewed. All patients underwent tumor resection with curative intent and had a confirmed pathological diagnosis.
For this study, ICC was defined as cholangiocarcinoma arising from the intrahepatic bile duct or bile ductules and located in the hepatic parenchyma. The tumors were classified as either PICC or HICC. Tumors classified as PICC were confined to the liver without involvement of the hepatic hilum and required hepatectomy alone, whereas tumors classified as HICC involved the hepatic duct confluence and required hepatectomy with extrahepatic bile duct resection (Fig. 1). All patients with HICC developed invasion of the bile duct in the hepatic hilum pathologically while all patients with PICC did not. Cases of cholangiocarcinoma arising from a bile duct confluence or from the first bifurcation were excluded, as were cases of intraductal ICC, or cases with combined hepatocellular and cholangiocarcinoma.
Eighteen clinicopathological factors, including patient demographics, perioperative factors, tumor characteristics, and patient survival, were compared between the two types of ICC. In addition, univariate and multivariate survival analyses were performed to determine useful prognostic factors of patients with ICC. Written informed consent was obtained from all patients for surgical treatment and pathological examinations according to the institutional guidelines.
Preoperative Workup and Surgical Procedures
Preoperative workup included ultrasonography, computed tomography, endoscopic ultrasonography, endoscopic retrograde cholangiography, and percutaneous transhepatic cholangiography to evaluate the local or distant tumor extensions. If jaundice was identified preoperatively, percutaneous transhepatic biliary drainage or endoscopic retrograde biliary drainage was performed to reduce the cholestatic liver damage. If the estimated liver resection volume exceeded 60% of the whole liver as calculated by computed tomography, then preoperative percutaneous transhepatic portal embolization was performed on the liver segment to be resected, in order to induce compensatory hypertrophy of the future remnant liver.
All surgical resections included hepatectomy with or without resection of the extrahepatic bile duct. Patients with PICC underwent hepatectomy alone while patients with HICC underwent hemihepatectomy or trisectionectomy with resection of the caudate lobe and extrahepatic bile duct. All patients underwent dissection of the regional lymph nodes, which included the nodes along the common hepatic artery, nodes in the hepatoduodenal ligament, and posterior pancreaticoduodenal nodes. Intraoperative pathological assessment of the distal bile duct transection lines was performed with frozen tissue sections. If the bile duct transection line was positive for malignant cells, further resection of the bile duct was performed to the maximum extent possible. When the extrahepatic bile duct was resected, biliary continuity was restored by a Roux-en-Y hepaticojejunal anastomosis.
Pathological Investigations
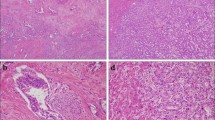
Following tumor resection, tumor size and macroscopic type were evaluated. The macroscopic type of ICC was determined based on the following three categories defined by the Liver Cancer Study Group of Japan:36 mass-forming (MF) type, periductal infiltrating (PI) type, and MF + PI type. Permanent sections with hematoxylin and eosin staining were prepared. All specimens were examined pathologically, and each tumor was classified as well-differentiated, moderately differentiated, or poorly differentiated adenocarcinoma according to the predominant pathological grading of differentiation. Presence of intrahepatic metastasis, serosal invasion, portal vein invasion, and lymph node metastasis were all pathologically examined. Intrahepatic metastasis, serosal invasion, portal vein invasion, and lymph node metastasis were defined pathologically in this study. Surgical margins were considered positive if infiltrating adenocarcinoma was present at the hepatic transection line, the distal bile duct transection line, or the dissected periductal soft tissue margins. The final stage of ICC was determined according to the TNM classification system of malignant tumors published by the International Union Against Cancer (UICC), 7th edition.37
Postoperative Adjuvant Chemotherapy
Beginning in 2002, postoperative adjuvant chemotherapy was routinely administered to patients with ICC, using a regimen that has been described previously.6,11,38 In summary, patients were treated with 10 cycles of gemcitabine plus S-1 every 2 weeks. Each chemotherapy cycle consisted of intravenous gemcitabine at a dose of 700 mg/m2 on day 1 and orally administered S-1 at a dose of 50 mg/m2 for seven consecutive days, followed by a 1-week break from chemotherapy. Neither external beam radiation nor intraoperative irradiation was administered to any patient during the study period.
Survival
Patients were followed regularly in outpatient clinics at 3-month intervals by undergoing a blood test, ultrasonography, and computed tomography for up to 5 years after surgery. Information after 5 years was collected by telephone or personal interview. For patients who died, survival time after surgery and the cause of death were recorded. For surviving patients, postoperative survival time and status of recurrence were recorded. Only cancer-related deaths were considered as events and disease-specific survival was calculated. The median follow-up time after operation was 43 months (range, 1–196 months).
Statistical Analysis
The χ 2 test or Fisher exact test was used for comparison between the two groups. Survival curves were constructed using the Kaplan–Meier method, and differences in survival curves were compared by univariate log-rank (Mantel–Cox) test. Factors found to be P < 0.05 on univariate analysis were subjected to multivariate analysis using a Cox proportional hazards model. P < 0.05 was considered statistically significant. Statistical analysis was performed with the Macintosh version of StatView version 5.0 (SAS Institute, Cary, NC, USA).
Results
Patient Demographics and Operative Procedures Performed
Of the 44 eligible patients, 25 had PICC and 19 had HICC. The patient group included 29 men (66%) and 15 women (34%) whose median age was 68 years (range, 37–81 years): 19 patients (43%) were more than 70 years old. Six patients (14%) were positive for hepatitis B virus (HBV) surface antigen or hepatitis C virus (HCV) antibody and eight patients (18%) had preoperative jaundice. For reduction of serum bilirubin levels or preoperative workup, percutaneous transhepatic biliary drainage was performed in seven (16%) patients and endoscopic retrograde biliary drainage in three (7%) patients. Percutaneous transhepatic portal embolization was performed in six patients (14%). Depending on the local extension of the tumor, a wide variety of hepatic resection procedures was performed. Major hepatectomy, including hemihepatectomy and trisectionectomy, was performed for 41 (93%) patients. All patients with HICC underwent caudate lobectomy and extrahepatic bile duct resection (Table 1). There was one perioperative death (2%) within 30 days of surgery. Postoperative complication occurred in 18 patients (41%). Biliary fistula was the most common complication (n = 9), followed by intraabdominal abscess (n = 3), chylous ascites (n = 2), and miscellaneous complications (n = 4). Postoperative adjuvant chemotherapy was performed for 23 patients (52%).
Tumor Characteristics
Mean tumor size was 45.8 mm (range, 15–140 mm). The macroscopic categories of the tumors were 17 (39%) of MF type, 9 (20%) of PI type, and 18 (41%) of MF + PI type. Tumors were pathologically identified as well-differentiated adenocarcinoma in 15 patients (34%), moderately differentiated adenocarcinoma in 18 patients (41%), and poorly differentiated adenocarcinoma in 11 patients (25%). Intrahepatic metastasis, serosal invasion, and portal vein invasion were identified in 10 (23%), 10 (23%), and 20 patients (45%), respectively. There were 20 tumors (45%) with lymph node metastasis and 24 (55%) without lymph node metastasis. Twenty-seven patients (61%) had negative surgical margins. According to the UICC staging system, 10 (23%), 10 (23%), 5 (11%), and 19 patients (43%) had pT1, pT2a, pT2b, and pT3 tumors, respectively, and 8 (18%), 9 (20%), 7 (16%), 17 (39%), and 3 patients (7%) were diagnosed with stage I, II, III, IVA, and IVB disease, respectively.
Comparison of Clinicopathological Factors Between PICC and HICC
Table 2 compares several clinicopathological factors between patients with PICC and HICC. No significant difference was found between the two groups in terms of gender, age, HBV or HCV infection, use of percutaneous transhepatic portal embolization, type of hepatic resection, use of adjuvant chemotherapy, tumor size, tumor differentiation, intrahepatic metastasis, serosal invasion, portal vein invasion, lymph node status, surgical margin status, UICC pT factor, or UICC stage. However, the rates of preoperative jaundice (P < 0.001), preoperative biliary drainage (P = 0.001), postoperative complication (P = 0.046), and PI or PI + MF type (P < 0.001) were significantly lower in patients with PICC than in patients with HICC (Table 2). Disease-specific survival was not different between patients with PICC and HICC (Fig. 2): the 5-year disease-specific survival rates of patients with PICC and HICC were 59% and 35%, respectively (P = 0.560, Fig. 2).
Prognostic Factors of Patients with ICC
Overall survival rates for the 44 patients were 78% at 1 year, 52% at 3 years, and 31% at 5 years, and disease-specific survival rates were 76% at 1 year, 60% at 3 years, and 47% at 5 years. The median survival time was 27.8 months (range, 1–196 months). Tumor recurrence occurred in 22 patients. The sites and nature of recurrence in these patients included peritoneal dissemination (n = 14), liver metastases (n = 4), local disease (n = 3), and lung metastasis (n = 1). Nineteen patients died of recurrent disease, and five patients died of other diseases. Two patients with liver metastasis and one patient with local recurrence were still alive at the time of this writing.
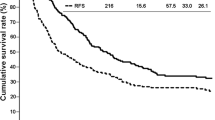
Eighteen clinicopathological factors were investigated using the log-rank test to determine their prognostic significance. Gender, age, type of ICC, HBV or HCV infection, preoperative jaundice, preoperative biliary drainage, use of percutaneous transhepatic portal embolization, type of hepatic resection, use of adjuvant chemotherapy, macroscopic type, tumor size, tumor differentiation, intrahepatic metastasis, serosal invasion, surgical margin status, and UICC pT factor did not influence postoperative disease-specific survival by univariate survival analysis. In contrast, univariate analysis revealed that portal vein invasion (P = 0.022), lymph node status (P = 0.004), and UICC stage (P = 0.006) were significantly associated with disease-specific survival. These factors were entered into multivariate analysis with a Cox proportional hazards model, and only lymph node metastasis (P = 0.032) remained independently associated with disease-specific survival. UICC stage was not used as a dependent variable in multivariate survival analysis to avoid confounding with nodal status (Table 3). The 5-year disease-specific survival rates of patients with or without lymph node metastasis were 23% and 66%, respectively (P = 0.004, Fig. 3a). The 5-year disease-specific survival rates of patients with or without portal vein invasion were 0% and 60%, respectively (P = 0.022, Fig. 3b).
Discussion
In the current study, we focused on identifying clinicopathological and outcome differences between patients with PICC and those with HICC. We found that the rates of preoperative jaundice, preoperative biliary drainage, PI or PI + MF type, and postoperative complication in patients with PICC were lower than those in patients with HICC. These results are reasonable because HICC involves the hepatic hilum and requires resection of the extrahepatic bile duct and hepaticoenteric anastomosis for surgical treatment. However, other clinicopathological factors, including lymph node metastasis, vascular invasion, and UICC stage, did not differ between the two groups.
A few other comparative studies on clinicopathological differences between patients with PICC and HICC have been published. Nakagohri et al.27 reported that in contrast with our findings, lymph node status and margin status were different between PICC and HICC, and that HICC was the more aggressive disease in a retrospective review of 40 patients with ICC. In addition, Aishima et al.22 reported that in an analysis of 53 patients with ICC, lower frequency of lymph node metastasis was found in patients with PICC compared with those with HICC. One potential reason for the difference in results between this study and previous reports might be the disparity of HBV or HCV infection in the patient populations. Patients with HBV or HCV infection reportedly have more indolent tumors than do patients without HBV or HCV infection.9,39 Zhou et al.39 reported that the rate of lymph node metastasis in ICC patients with HBV infection was significantly lower than that in ICC patients without HBV infection. Hepatitis B virus or HCV infection in this series and in Aishima’s series was found in 20% and 47% of patients with PICC, respectively, which might explain the more aggressive features of PICC in the current study.
Several investigators have compared the 5-year survival rate of patients with PICC with that of patients with HICC. According to these reports, some investigators reported that the 5-year survival rate of patients with PICC was significantly higher than that of patients with HICC,11,14,20–22,25 although others reported that the 5-year survival rates between the two groups did not differ.7,12,15,18,27,35 Aishima et al.20 reported that the disease-specific 5-year survival rate of patients with PICC was significantly better than that of patients with HICC (60% vs. 36%, P = 0.012). Guglielmi et al.11 reported that among 52 patients with ICC, patients with PICC had a better overall 5-year survival rate than those with HICC (26% vs. 0%, P = 0.02). In contrast, Lang et al.14 mentioned that there was no significant difference in recurrence-free survival between patients with PICC and HICC in an analysis of 53 patients with ICC. However, these reports were based on a small number of patients. Uchiyama et al.35 analyzed 341 patients with ICC who underwent surgical resection at nine Japanese high-volume centers and found no significant difference in overall 5-year survival rate between patients with PICC and HICC (overall 5-year survival rate, 26% vs. 33%, P = 0.090). In the current study, no significant difference was seen in disease-specific survival between patients with PICC and HICC. Further studies on a larger number of patients are needed to clarify a survival difference between patients with PICC and HICC.
Many investigators have used multivariate analysis to determine useful prognostic factors for ICC after surgical resection (Table 4).7–10,12–20,23–26,28–30,34,35 According to these reports, potentially significant factors include intrahepatic metastasis,12,16,17,19,20,24,28,35 serum carbohydrate antigen 19-9 level,8,35 pathological grading of differentiation,8,17,30 vascular invasion,13,29 tumor size,9,18,20 and pathologically curative resection.10,14,15,19,24–26,35 In addition, lymph node metastasis was reported to be one of the most significant independent prognostic factors for patients with ICC by multivariate survival analysis.8,9,13,15,17,19,20,26,35 In the current study, the disease-specific survival of patients with nodal involvement was significantly worse than that of patients without nodal involvement, and multivariate analysis revealed that only lymph node status was an independent prognostic factor for patients with ICC.
The reported frequency of nodal involvement in patients with ICC who underwent surgical resection ranges from 6% to 65% (Table 4).7–10,12–20,23–26,28–30,34,35 Frequency of nodal involvement in the current study was comparable to that of previous reports, at 45% of patients. However, previously published 5-year survival rates for patients with lymph node metastasis differ substantially from those in the current study. Several investigators reported that no patients with nodal involvement had survived for more than 5 years,7,9,15,16,23,30 and others reported a 5-year survival rate that ranged from 6% to 17%.13,14,17,19,22,25,26,29 A multicenter analysis conducted by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery35 showed that of 139 patients with ICC who had lymph node involvement, only seven patients survived for more than 4 years, and three patients survived for more than 5 years: the 5-year survival rate was 7%. Based on these results, some investigators reported that radical resection should be contraindicated for patients with nodal involvement,40 or that lymph node dissection might not improve survival of patients with ICC.41 However, in the current study, two patients with nodal involvement have survived for more than 4 years without recurrence42 and the overall 5-year survival rate of patients with nodal involvement was 21%, which was an excellent result compared with that of previous reports. In our institution, dissection of the regional lymph nodes is routinely performed for all patients with ICC. We believe that routine lymph node dissection may contribute to a relatively favorable prognosis for patients with nodal involvement.7
We have already reported the prognostic impact of adjuvant chemotherapy for patients with biliary carcinoma in the recent reports.6,11,38,43 However, we could not demonstrate a beneficial effect of adjuvant chemotherapy for patients with ICC in this study. The reasons for this result are probably that this study was based on a small number of patients and follow-up time of patients was short. However, survival of patients who received adjuvant chemotherapy tended to be better than that of patients who did not (3-year disease-specific survival rate, 63% vs. 33%). Further studies on a larger number of patients with long-term follow-up are needed to confirm the prognostic impact of adjuvant chemotherapy for patients with ICC.
In conclusion, the clinicopathological characteristics of patients with PICC were almost similar to those of patients with HICC, and long-term outcome was similar between the two groups. Lymph node status was a potent prognostic factor for patients with ICC. However, the limitations of this study are its retrospective design and the small number of patients studied. Further studies on larger numbers of patients are required to confirm the results of this study.
References
Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-473.
Nathan H, Pawlik TM, Wolfgang CL, et al. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 2007;11:1488-1496.
Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepato. 2004;40:472-477.
Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77-82.
Okuda K, Kubo Y, Okazaki N, et al. Clinical aspects of intrahepatic bile duct carcinoma including hilar carcinoma: a study of 57 autopsy-proven cases. Cancer 1977;39:232-246.
Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651-658.
Ercolani G, Vetrone G, Grazi GL, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg 2010;252:107-114.
Saxena A, Chua TC, Sarkar A, et al. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg 2010;14:1128-1138.
Zhang L, Cai JQ, Zhao JJ, et al. Impact of hepatitis B virus infection on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2010;101:233-238.
Cho SY, Park SJ, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol 2010;17:1823-1830.
Murakami Y, Uemura K, Sudo T, et al. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. J Gastrointest Surg 2009;13:1470-1479.
Shimada K, Sano T, Sakamoto Y, et al. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol 2007;96:160-165.
Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-1254.
Lang H, Sotiropoulos GC, Sgourakis G, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg 2009;208:218-228.
Yedibela S, Demir R, Zhang W, et al. Surgical treatment of mass-forming intrahepatic cholangiocarcinoma: an 11-year Western single-center experience in 107 patients. Ann Surg Oncol 2009;16:404-412.
Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg 2008;32:2675-2680.
Shirai K, Ebata T, Oda K, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2008;32:2395-2402.
Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-2794.
Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg 2008;15:417-422.
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96.
Sano T, Shimada K, Sakamoto Y, et al. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol 2008;15:590-599.
Aishima S, Kuroda Y, Nishihara Y, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol 2007;31:1059-1067.
Miwa S, Miyagawa S, Kobayashi A, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol 2006;41:893-900.
Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-733.
Tajima Y, Kuroki T, Fukuda K, et al. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg 2004;91:99-104.
Morimoto Y, Tanaka Y, Ito T, et al. Long-term survival and prognostic factors in the surgical treatment for intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg 2003;10:432-440.
Nakagohri T, Asano T, Kinoshita H, et al. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg 2003;27:289-293.
Ohtsuka M, Ito H, Kimura F, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 2002;89:1525-1531.
Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-391.
Shirabe K, Mano Y, Taketomi A, et al. Clinicopathological prognostic factors after hepatectomy for patients with mass-forming type intrahepatic cholangiocarcinoma: relevance of the lymphatic invasion index. Ann Surg Oncol 2010;17:1816-822.
Guglielmi A, Ruzzenente A, Campagnaro T, et al. Does intrahepatic cholangiocarcinoma have better prognosis compared to perihilar cholangiocarcinoma? J Surg Oncol 2010;101:111-115.
Tamandl D, Kaczirek K, Gruenberger B, et al. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg 2009;96:919-925.
Ebata T, Kamiya J, Nishio H, et al. The concept of perihilar cholangiocarcinoma is valid. Br J Surg 2009;96:926-934.
Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-3056.
Uchiyama K, Yamamoto M, Yamaue H, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2011;18:443–52.
The Japan Liver Cancer Study Group. Classification of primary liver cancer. 1st ed. Tokyo: Kanehara; 1997.
Sobin LH, Gospodarowicz MK, Wittekind C (eds). International Union Against Cancer (UICC): TNM Classification of Malignant Tumors. 7th ed. New York: Wiley-Blackwell; 2010.
Murakami Y, Uemura K, Sudo T, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 2009;250:950-956.
Zhou H, Wang H, Zhou D, et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer 2010;46:1056-1061.
Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery 2000;127:498-505.
Shimada M, Yamashita Y, Aishima S, et al. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg 2001;88:1463-1466.
Murakami Y, Yokoyama T, Takesue Y, et al. Long-term survival of peripheral intrahepatic cholangiocarcinoma with metastasis to the para-aortic lymph nodes. Surgery 2000;127:105-106.
Murakami Y, Uemura K, Sudo T, et al. Prognostic factors of patients with advanced gallbladder carcinoma following aggressive surgical resection. J Gastrointest Surg 2011;15:1007–1016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murakami, Y., Uemura, K., Sudo, T. et al. Intrahepatic Cholangiocarcinoma: Clinicopathological Differences Between Peripheral Type and Hilar Type. J Gastrointest Surg 16, 540–548 (2012). https://doi.org/10.1007/s11605-011-1730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1730-4