Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) constitutes a group of heterogeneous malignancies within the liver. We sought to subtype ICC based on anatomical origin of tumors, as well as propose modifications of the current classification system.
Methods
Patients undergoing curative-intent resection for ICC, hilar cholangiocarcinoma (CCA), or hepatocellular carcinoma (HCC) were identified from three international multi-institutional consortia of databases. Clinicopathological characteristics and survival outcomes were assessed.
Results
Among 1264 patients with ICC, 1066 (84.3%) were classified as ICC-peripheral subtype, whereas 198 (15.7%) were categorized as ICC-perihilar subtype. Compared with ICC-peripheral subtype, ICC-perihilar subtype was more often associated with aggressive tumor characteristics, including a higher incidence of nodal metastasis, macro- and microvascular invasion, perineural invasion, as well as worse overall survival (OS) (median: ICC-perihilar 19.8 vs. ICC-peripheral 37.1 months; p < 0.001) and disease-free survival (DFS) (median: ICC-perihilar 12.8 vs. ICC-peripheral 15.2 months; p = 0.019). ICC-perihilar subtype and hilar CCA had comparable OS (19.8 vs. 21.4 months; p = 0.581) and DFS (12.8 vs. 16.8 months; p = 0.140). ICC-peripheral subtype tumors were associated with more advanced tumor features, as well as worse survival outcomes versus HCC (OS, median: ICC-peripheral 37.1 vs. HCC 74.3 months; p < 0.001; DFS, median: ICC-peripheral 15.2 vs. HCC 45.5 months; p < 0.001).
Conclusions
ICC should be classified as ICC-perihilar and ICC-peripheral subtype based on distinct clinicopathological features and survival outcomes. ICC-perihilar subtype behaved more like carcinoma of the bile duct (i.e., hilar CCA), whereas ICC-peripheral subtype had features and a prognosis more akin to a primary liver malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cholangiocarcinoma (CCA) constitutes a diverse group of malignancies arising in the biliary tract.1,2 Incidence (0.3–6 per 100,000 inhabitants per year) and mortality (1–6 per 100,000 inhabitants per year, globally) of CCA have increased worldwide over the past decade, accounting for ~ 15% of all primary liver cancers and ~ 3% of gastrointestinal malignancies.1,3,4 Despite recent advances in clinical treatment, prognosis of patients with CCA remains poor with 5-year survival ranging from 7 to 20%.2 Unfortunately, patients undergoing curative-intent resection still suffer from a high incidence of recurrence, which compromises long-term survival outcomes.5,6,7,8
CCAs arise from different anatomical sites and are generally divided into intrahepatic, perihilar, and distal CCA according to the staging of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) system.9 In the AJCC/UICC system, perihilar cholangiocarcinoma is defined as arising predominantly in the main lobar extrahepatic bile ducts distal to segmental bile ducts and proximal to the cystic duct.9 In turn, perihilar cholangiocarcinoma can include both hilar cholangiocarcinoma (extrahepatic) and intrahepatic cholangiocarcinoma (ICC) arising from the second order of bile ducts. In fact, the definition of perihilar cholangiocarcinoma remains somewhat controversial. According to the fifth edition of World Health Organization (WHO) classification of tumors of the digestive system, perihilar cholangiocarcinoma is defined as tumors located in the extrahepatic biliary tree proximal to the origin of the cystic duct (hilar cholangiocarcinoma).10 As such, “perihilar” typically means “extrahepatic” with perihilar cholangiocarcinoma arising from the common, left and/or right hepatic duct.10 In fact, “perihilar cholangiocarcinoma” is not a pathological entity but a practical disease category.11 In the National Comprehensive Cancer Network (NCCN) guidelines, cholangiocarcinoma was divided into intrahepatic, hilar (but not perihilar), and distal cholangiocarcinoma.
While ICC arises above from second-order bile ducts within the liver, hilar and distal CCA derive from extrahepatic bile ducts with the insertion of cystic duct being used as the anatomical landmark to differentiate these tumors. In contrast to hilar and distal CCAs, ICCs are more heterogeneous and are made up of tumors deriving from different anatomical sites within the liver.12 Because the clinical features and extent of surgery depend largely on the site of the tumor, it is critical to clarify the pathologic and biologic behavior of ICC based on different anatomic sites.13 Intrahepatic bile ducts are proximal to the right or left hepatic duct and are classified as intrahepatic large and small bile ducts.14 On the basis of pathological features and cell-of-origin, ICCs originating from large bile ducts with peribiliary glands have been defined as ICC-perihilar subtype, whereas tumors arising from small bile ducts or canals of Hering are categorized as ICC-peripheral subtype, exhibiting distinct morphology, histological, and molecular characterizations.11,15,16 Morphologically, ICC-perihilar subtype are characterized by a periductal-infiltrating or intraductal growth pattern, whereas ICC-peripheral subtype mainly have a mass-forming gross morphologic pattern.11 These two types of ICC (i.e., ICC-perihilar and ICC-peripheral) may be distinct in terms of their etiology, clinical presentation, and management, as well as need for posttreatment surveillance.
Some investigators have proposed that ICC-perihilar subtype should be categorized with hilar and distal CCA as carcinoma of the bile ducts, whereas ICC-peripheral subtype should be classified as a primary liver malignancy, such as hepatocellular carcinoma (HCC).17 To date, there has been a paucity of data to examine whether such a new classification system to define the subtypes of ICC is warranted from the perspective of surgical management or prognosis. Therefore, the objective of the current study was to define the clinical presentation, pathological characteristics, as well as survival among patients undergoing curative-intent surgery of ICC, hilar CCA, or HCC using large international multi-institutional databases. Specifically, we sought to examine subtyping of ICC based on anatomical origin of tumors and propose a modification of the current classification system.
Methods
Study Cohort and Data Collection
Patients who underwent curative-intent resection (R0/R1) for intrahepatic CCA between January 2000 and August 2020 were identified from a database of 15 hepatobiliary centers in North America, Europe, Australia, and Asia (Beaujon Hospital, Clichy, France; Curry Cabral Hospital, Lisbon, Portugal; Eastern Hepatobiliary Surgery Hospital, Shanghai, China; Erasmus University Medical Centre, Rotterdam, Netherlands; Emory University, Atlanta, GA; Fundeni Clinical Institute, Bucharest, Romania; Johns Hopkins Hospital, Baltimore, MD; University of Ottawa, Ottawa, ON, Canada; Royal Prince Alfred Hospital, University of Sydney, Sydney, Australia; Ospedale San Raffaele, Milan, Italy; Stanford University, Stanford, CA; University of Virginia, Charlottesville, VA; University of Verona, School of Medicine, Verona, Italy; Yokohama City University School of Medicine, Yokohama, Japan; the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China). Patients who underwent curative resection of hilar CCA between the years 2000 and 2020 were identified from a multi-institutional database that included ten hepatobiliary centers (Ohio State University Wexner Medical Center, Johns Hopkins University, Stanford University, University of Wisconsin, University of Washington, Vanderbilt University, New York University, Louisville University, Wake Forest University, and Emory University). In addition, patients who underwent curative liver resection for HCC between 2000 and 2020 were identified from 11 international centers from America, Europe, Australia and Asia (The Ohio State University Wexner Medical Center, Columbus, OH, USA; The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China; University of Verona, Verona, Italy; Hopedale San Raffaele, Milano, Italy; Curry Cabral Hospital, Lisbon, Portugal; APHP, Beaumont Hospital, Clichy, France; Westhead Hospital, Sydney, Australia; Stanford University, Stanford, CA, USA; Funding Clinical Institute, Bucharest, Romania; University of Ottawa, Ottawa, Canada; The University of Sydney, School of Medicine, Sydney, Australia).
Data on demographics, liver function, primary tumor characteristics, details of the initial surgical procedure, biochemical labs, imaging, pathologic findings, as well as treatment details of recurrent disease were collected by using standardized datasheets at each institution. Surgical specimens were reviewed by expert hepatic pathologists. Tumor-related characteristics, including maximal tumor diameter, number, location, tumor morphology, major vascular invasion, histological grade, microvascular invasion, perineural invasion, and lymph node status, were evaluated based on final pathology.
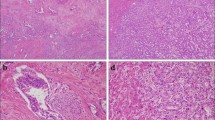
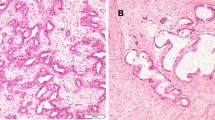
According to tumor morphology on macroscopic examination, intrahepatic CCA was classified into mass-forming, periductal-infiltrating, and intraductal-growing subtype.18 Tumors with both periductal-infiltrating and mass-forming features were collectively recorded as periductal-infiltrating subtype. ICC with morphology of periductal-infiltrating or intraductal-growing pattern was defined as ICC-perihilar subtype, while mass-forming pattern was categorized as ICC-peripheral subtype (Fig. 1).
Patients were regularly followed after surgery with ultrasound, abdominal computed tomography, and/or MRI scanning. Overall survival (OS) was calculated from the date of surgery and censored at the date of death or last follow-up. Disease-free survival (DFS) was defined as time duration from the date of surgery to tumor recurrence.
Statistical Analysis
Numerical variables were expressed as median values with interquartile ranges (IQR) and compared with Student t-test or Mann-Whitney U test between the two groups. Nominal variables were expressed as number and percentages and compared with chi-squared test or Fisher’s exact test. Kaplan-Meier curves were used to estimate median survival; the log-rank test was used to assess differences in DFS and OS. Because baseline patient characteristics with various tumor types were different, propensity score matching (PSM) was used to mitigate selection bias. Specifically, variables potentially affecting long-term outcomes were utilized in the propensity score. Propensity score analysis with 1:1 matching was performed without replacement using a caliper with a width 0.1 of the standard deviation to generate matched pairs of the patients. In all analyses, two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed by using SPSS version 22.0 (IBM SPSS Inc., Chicago, IL).
Results
Baseline Characteristics
A total of 1264 patients underwent curative-intent resection in the ICC cohort. Median patient age was 60 years (interquartile range [IQR], 51–69), and 707 patients (56.0%) were male. Most patients had unifocal disease (n = 1046, 83.5%) with a median tumor size of 6.0 cm (IQR, 4.0–8.5). Perineural invasion was present in 219 (17.3%) tumors, whereas microvascular invasion was noted in 354 (28.0%) patients. Roughly one-half (n = 572, 45.3%) of patients had a lymphadenectomy; 42.8% (245/572) of these patients had lymph node metastasis (LNM). Among patients in the hilar CCA cohort, 257 patients underwent a curative-intent resection. Median age was 67 years (IQR, 58–73), and 151 (58.8%) patients were male. On pathological examination, perineural and microvascular invasion were present in 173 (67.3%) and 83 (32.3%) patients, respectively. Roughly one-third of patients (n = 96, 37.4%) had LNM (Table 1). Among the 1004 patients who underwent curative-intent resection for HCC, median age of patients was 62 years (IQR, 53–71), and the majority (n = 788, 78.5%) was male. Most HCC patients (n = 885, 85.1%) had unifocal disease with a median tumor size of 5.0 cm (IQR, 3.0–8.5). On histopathological examination, 589 (58.7%) patients had microvascular invasion (Table 2).
Classification of ICC into ICC-Perihilar and ICC-Peripheral Subtype
Among patients with ICC, 1066 (84.3%) were defined as having ICC-peripheral subtype (mass-forming pattern), whereas 198 (15.7%) were classified as ICC-perihilar subtype (periductal-infiltrating pattern: n = 162, 12.8%; intraductal growth pattern: n = 36, 2.8%; Fig. 1). ICC-peripheral subtype was more likely to be associated with viral hepatitis (ICC-peripheral 21.2% vs. ICC-perihilar 10.1%, p = 0.011) and liver cirrhosis compared with ICC-perihilar subtype (ICC-peripheral 11.4% vs. ICC-perihilar 3.5%, p = 0.009; Table 1). In contrast, ICC-perihilar subtype was associated with a higher proportion of lymphadenectomy (ICC-perihilar 69.2% vs. ICC-peripheral 41.7%, p < 0.001) and more aggressive tumor characteristics compared with ICC-peripheral subtype, including a higher incidence of LNM (ICC-perihilar 52.6% vs. ICC-peripheral 39.0%, p = 0.005), macrovascular (ICC-perihilar 21.2% vs. ICC-peripheral 11.6%, p < 0.001) and microvascular invasion (ICC-perihilar 34.3% vs. ICC-peripheral 26.8%, p = 0.035), perineural invasion (ICC-perihilar 27.3% vs. ICC-peripheral 15.5%, p < 0.001), as well as R1 margin status (ICC-perihilar 20.7% vs. ICC-peripheral 11.3%, p < 0.001). Of note, patients with ICC-perihilar subtype had a worse OS (median: ICC-perihilar 19.8 months vs. ICC-peripheral 37.1 months; p < 0.001; Fig. 2a) and DFS (median: ICC-perihilar 12.8 months vs. ICC-peripheral 15.2 months; p = 0.019; Fig. 2b) versus patients with ICC-peripheral subtype. While survival estimates for patients with ICC-peripheral subtype trended with the survival outcomes of patients with HCC, individuals with ICC-perihilar subtype had long-term outcomes more comparable to patients with hilar CCA (Fig. 2).
Survival Outcomes for ICC-Perihilar Subtype Versus Hilar CCA
Compared with hilar CCA, ICC-perihilar subtype was associated with a higher incidence of LNM (ICC-perihilar 52.6% vs. hilar CCA 37.4%, p = 0.004), yet lower frequency of perineural invasion (ICC-perihilar 27.3% vs. hilar CCA 67.3%, p < 0.001) and R1 margin status (ICC-perihilar 20.7% vs. hilar CCA 34.6%, p = 0.001; Table 1). Of note, patients with ICC-perihilar subtype had comparable OS (median: ICC-perihilar 19.8 months vs. hilar CCA 21.4 months; p = 0.581; Fig. 2a) and DFS (median: ICC-perihilar 12.8 months vs. hilar CCA 16.8 months; p = 0.140; Fig. 2b) similar to individuals with hilar CCA. After propensity-scoring matching, 67 pairs of patients were assessed (Supplementary Table 1). Of note, patients with ICC-perihilar subtype persisted in having a comparable OS (median: ICC-perihilar 32.3 months vs. hilar CCA 22.1 months; p = 0.197; Fig. 3a) and DFS (median: ICC-perihilar 17.3 months vs. hilar CCA 19.5 months; p = 0.545; Fig. 3b) compared with patients who had hilar CCA.
Survival Outcomes for ICC-Peripheral Subtype Versus HCC
ICC-peripheral subtype was more likely to be multiple (multiple tumors, ICC-peripheral 15.7% vs. HCC 11.9%, p = 0.01) and larger versus HCC tumors (≥5 cm, ICC-peripheral 65.8% vs. HCC 47.8%, p < 0.001). In addition, compared with HCC, ICC-peripheral subtype was associated with a higher incidence of macrovascular vascular invasion (ICC-peripheral 11.6% vs. HCC 5.4%, p < 0.001), yet a lower incidence of microvascular invasion (ICC-peripheral 26.8% vs. HCC 58.7%, p < 0.001; Table 2). Patients with ICC-peripheral subtype had a shorter OS (median: ICC-peripheral 37.1 months vs. HCC 74.3 months; p < 0.001; Fig. 2a) and DFS (median: ICC-peripheral 15.2 months vs. HCC 45.5 months; p < 0.001; Fig. 2b) versus patients with HCC. Following PSM, among 468 pairs of matched patients (Supplementary Table 2), individuals with ICC-peripheral subtype still had an unfavorable OS (median: ICC-peripheral 40.0 months vs. HCC 66.3 months; p < 0.001; Fig. 4a) and DFS (median: ICC-peripheral 14.0 months vs. HCC 32.9 months; p < 0.001; Fig. 4b) compared with patients who had HCC. Of note, among patients with no LNM, the prognosis of patients with ICC-peripheral subtype (n = 124) was more comparable with the outcomes of patients with HCC (n = 453) (OS: median: ICC-peripheral 66.3 months vs. HCC 73.6 months, p = 0.251; DFS: median: ICC-peripheral 23.5 months vs. HCC 35.7 months, p = 0.044; Fig. 4c, d).
Discussion
CCAs can arise at any anatomic location within the biliary ducts, yet have different etiologies, as well as unique genomic, molecular, pathologic, and clinical features.13,19,20 Traditionally, CCAs have been divided into intrahepatic and extrahepatic carcinoma (i.e., hilar and distal CCA) according to the site of origin. This categorization has been widely accepted as each anatomic subtype often is treated with a different surgical strategy. For example, while distal CCAs are treated with a Whipple procedure, surgery for hilar CCA often requires hepatectomy concomitant with bile duct resection and biliary anastomosis. For intrahepatic CCA, peripheral tumors within the liver are treated with hepatic resection like HCC, whereas treatment of ICC close to hilum is similar to hilar CCA. While often managed differently, the subtypes of ICC have not been clearly delineated relative to long-term prognosis following curative-intent resection. To this point, ICC arising from intrahepatic large bile ducts (ICC-perihilar type) may have a more aggressive tumor behavior than tumors originating from the small bile ducts (ICC-peripheral type).11,15 The current study was important, because we specifically examined ICC subtypes relative to hilar CCA and HCC. Interestingly, there were distinct tumor characteristics, as well as long-term outcomes, among patients who had ICC-perihilar versus ICC-peripheral subtype. While OS and DFS among patients with ICC-perihilar subtype was comparable to patients with hilar CCA, prognosis of individuals with ICC-peripheral subtype was worse than individuals with HCC, yet more comparable when examining only patients with no LNM. In turn, the data suggest that ICC-peripheral subtype is more akin to “true” primary liver cancers, while ICC-perihilar subtype should be classified more as a type of perihilar cholangiocarcinoma based on differences in presentation, surgical management, and prognosis (Fig. 1).
Based on the classification of the Liver Cancer Study Group of Japan, intrahepatic CCA is morphologically classified into three patterns: mass-forming, periductal-infiltrating, and intraductal-growth pattern.18,21 Different carcinogenesis and progression pathways may give rise to each morphological ICC subtype. For example, canals of Hering and interlobular bile ducts represent the cell-of-origin for peripheral small duct-derived carcinoma, namely the proposed ICC-peripheral subtype, which can develop into a mass-forming pattern in the background of chronic hepatitis and cirrhosis.22 In contrast, peribiliary glands are likely the origin of large duct-derived carcinoma corresponding to ICC-perihilar subtype and produce periductal-infiltrating tumors related to biliary inflammation.23 Of note, the intraductal-growth pattern, another type of ICC-perihilar subtype tumor, represents a distinct pathway from large bile ducts and often is associated with a more favorable prognosis. As such, even when ICC demonstrates similar gross features, there may be different pathways of carcinogenesis and tumor progression between perihilar large duct and peripheral small duct type intrahepatic CCA.11 ICC-perihilar subtype tumors are likely derived from the large bile duct and be composed of a large tubular component or papillary proliferation of tall columnar epithelium. As such, upregulation of some mucin core proteins (MUC), such as MUC2, MUC6, and MUC5AC, as well as S100P, have been utilized as diagnostic markers.16,22,24,25,26 In contrast, ICC-peripheral subtype tumors are derived from the small bile ducts originating from hepatic progenitor cells or transformation of ductular with no large glands/tall columnar cells.11 NCAM overexpression within a background of viral hepatitis may be related to carcinogenesis from hepatic progenitor cells.27,28 Isocitrate dehydrogenase (IDH) 1 or 2 mutations, FGFR2 fusions, and VEGF-A overexpression are frequently associated with ICC-peripheral subtype, whereas KRAS and TP53 genetic alterations are more common noted among ICC-perihilar subtype.16,29,30,31 Understanding the different mechanisms of carcinogenesis of ICC-perihilar versus ICC-peripheral subtype is important to understand and direct CCA treatment. In the current study, ICC-perihilar subtype was associated with more advanced tumor features, such as a higher frequency of LNM, perineural invasion, as well as macro- and microvascular invasion versus ICC-peripheral subtype. Perhaps not surprisingly, lymphatic channels and neural fibers are much more prevalent along the Glisson sheath, which may be more often associated with perihilar tumors. As such, patients with ICC-perihilar subtype tumors had a more unfavorable prognosis than patients with ICC-peripheral subtype tumors.
The separation point between intrahepatic and extrahepatic cholangiocarcinoma is defined by the level of second-order bile ducts.32 Conventionally, the right and left hepatic ducts, the confluence, and the first to third branches are termed as hilar and perihilar bile ducts that are located intra- and extra-hepatically, respectively. The AJCC/UICC staging manual did not make a distinction relative to bile duct order location when classifying perihilar carcinoma, because resection of the hepatic duct bifurcation is required irrespective of whether there is an intrahepatic component.32 In contrast, the new edition of WHO tumor classification defined perihilar cholangiocarcinoma specifically as located in the extrahepatic biliary tree proximal to the origin of the cystic duct (hilar CCA), which is consistent with the definition of hilar CCA according to NCCN guidelines.10 In fact, hilar CCA should be considered as originating from the common hepatic duct, bifurcation, and left or right hepatic ducts. To this point, in the current study, patients with ICC-perihilar subtype had comparable OS (Fig. 2a) and DFS (Fig. 2b) to individuals with hilar CCA—even after propensity scoring matching (Fig. 3). In turn, ICC-perihilar subtype tumors should be considered distinct from ICC-peripheral subtype and be conceptualized as tumors more consistent with hilar CCA.
Interestingly, ICC-peripheral subtype and HCC shared similar risk factors, such as liver cirrhosis and chronic viral hepatitis. However, compared with HCC, patients with ICC-peripheral subtype were more likely to present with advanced stage disease, including multiple and larger tumors with a higher incidence of vascular invasion and LNM. As such, individuals with ICC-peripheral subtype had an unfavorable OS (Fig. 4a) and DFS (Fig. 4b) compared with patients who had HCC. Interestingly, among patients with no LNM, outcomes following resection of ICC-peripheral subtype were more comparable with HCC. The surgical management of primary liver cancers such as ICC-peripheral subtype and HCC typically differs. For example, resection of the primary mass with regional lymphadenectomy is the standard approach for patients ICC-peripheral subtype, whereas resection and ablation—as well as transplantation—with no lymphadenectomy are standard surgical managements options for HCC.33 In aggregate, the data demonstrate the heterogeneity in the prognosis of patients of ICC-peripheral versus ICC-perihilar subtype, as well as HCC. Among patients with no LNM, prognosis of patients with ICC-peripheral subtype was similar, however, to individuals undergoing resection of HCC.
Results from the current study should be interpreted in light of several limitations. Although the multi-institutional nature of the cohort increased sample size and generalization of the results, patient selection, surgical approach, as well as follow-up surveillance strategies may have varied among centers. All institutions included in the consortium were high-volume centers and followed standard treatment algorithms. Data related to neoadjuvant and adjuvant therapies, as well as targeted and immuno-therapy, were not included in the database. Moreover, all patients underwent curative-intent resection; as such patients who received palliative treatments or systemic chemotherapy for advanced disease were not included, and therefore, the data cannot be extrapolated to nonoperative candidates.
Conclusions
ICC-perihilar (periductal-infiltrating and intraductal-growing patterns) and ICC-peripheral (mass-forming pattern) subtype had distinct clinicopathological features and prognosis. Patients with ICC-perihilar subtype typically undergo the same surgical procedures and have similar survival outcomes as patients with hilar CCA, whereas patients with ICC-peripheral subtype undergo hepatic resection like HCC and had survival outcomes that were intermediate to patients with HCC and hilar CCA. As such, data from the current study suggest the reclassification of ICC, with the ICC-perihilar subtype being considered similar to hilar and distal CCA as carcinoma of the bile ducts, whereas ICC-peripheral subtype was more akin to a primary liver cancer like HCC. Future studies are needed to investigate the molecular and carcinogenic pathway differences among the varied ICC tumor subtypes.
References
Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88.
Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–44.
Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13:261–80.
Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–14.
Wei T, Zhang XF, He J, et al. Prognostic impact of perineural invasion in intrahepatic cholangiocarcinoma: multicentre study. Br J Surg. 2022;109:610–6.
Sahara K, Tsilimigras DI, Toyoda J, et al. Defining the risk of early recurrence following curative-intent resection for distal cholangiocarcinoma. Ann Surg Oncol. 2021;28:4205–13.
Zhang XF, Beal EW, Chakedis J, et al. Defining early recurrence of hilar cholangiocarcinoma after curative-intent surgery: a multi-institutional study from the US Extrahepatic Biliary Malignancy Consortium. World J Surg. 2018;42:2919–29.
Komaya K, Ebata T, Shirai K, et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg. 2017;104:426–33.
Amin MB. American joint committee on cancer: AJCC cancer staging manual. 8th edn. New York: Springer; 2017.
Board WCoTE. WHO Classification of Tumours, 5th Edition. World Health Organization, 2019; 2019.
Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. J Hepatobiliary Pancreat Sci. 2015;22:94–100.
Zhang XF, Bagante F, Chen Q, et al. Perioperative and long-term outcome of intrahepatic cholangiocarcinoma involving the hepatic hilus after curative-intent resection: comparison with peripheral intrahepatic cholangiocarcinoma and hilar cholangiocarcinoma. Surgery. 2018;163:1114–20.
Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract Res Clin Gastroenterol. 2015;29:277–93.
Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–70.
Aishima S, Kuroda Y, Nishihara Y, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059–67.
Liau JY, Tsai JH, Yuan RH, Chang CN, Lee HJ, Jeng YM. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol. 2014;27:1163–73.
Chen MS. Thinking and suggestion on the definition, classification and Chinese nomenclature of carcinoma of the bile ducts. Zhonghua Wai Ke Za Zhi. 2022;60:351–5.
Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–91.
Kendall T, Verheij J, Gaudio E, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):7–18.
Deng M, Ran P, Chen L, et al. Proteogenomic characterization of cholangiocarcinoma. Hepatology. 2023;77:411–29.
Bagante F, Spolverato G, Weiss M, et al. Impact of morphological status on long-term outcome among patients undergoing liver surgery for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24:2491–501.
Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–88.
Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology. 2012;55:2041–2.
Suh KS, Chang SH, Lee HJ, Roh HR, Kim SH, Lee KU. Clinical outcomes and apomucin expression of intrahepatic cholangiocarcinoma according to gross morphology. J Am Coll Surg. 2002;195:782–9.
Aishima S, Fujita N, Mano Y, et al. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol. 2011;35:590–8.
Tsai JH, Huang WC, Kuo KT, Yuan RH, Chen YL, Jeng YM. S100P immunostaining identifies a subset of peripheral-type intrahepatic cholangiocarcinomas with morphological and molecular features similar to those of perihilar and extrahepatic cholangiocarcinomas. Histopathology. 2012;61:1106–16.
Kozaka K, Sasaki M, Fujii T, et al. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: “bile ductular carcinoma.” Histopathology. 2007;51:390–400.
Aishima S, Fujita N, Mano Y, et al. p62+ Hyaline inclusions in intrahepatic cholangiocarcinoma associated with viral hepatitis or alcoholic liver disease. Am J Clin Pathol. 2010;134:457–65.
Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–8.
Nichetti F, Niger M. Isocitrate dehydrogenase mutations in cholangiocarcinoma: Still a long road ahead. JCO Precis Oncol. 2022;6:e2200065.
Yu Z, Ni Q, Jia H, et al. Prognostic analysis of radical resection for iCCA(phl) and iCCA(pps): a retrospective cohort study. Front Oncol. 2022;12:992606.
DeOliveira ML, Clavien PA. A common language to describe perihilar cholangiocarcinoma. Br J Surg. 2012;99:885–6.
Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62.
Acknowledgment
T.W. and X.-F.Z. were supported by Shaanxi Science Foundation for Distinguished Young Scholars (2021JC-36), Shaanxi Innovative Research Team for Science and Technology (2022TD-53), and the “Young Talent Support Plan” of Xi’an Jiaotong University, China.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Disclosure
Dr. Guillaume Martel—speaker’s honorarium from Incyte Biosciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, T., Lu, J., Xiao, XL. et al. Classification of Intrahepatic Cholangiocarcinoma into Perihilar Versus Peripheral Subtype. Ann Surg Oncol 31, 1232–1242 (2024). https://doi.org/10.1245/s10434-023-14502-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14502-3