Abstract
Background
Current management of malignant gastric outlet obstruction (GOO) includes surgical diversion or enteral stent placement for unresectable cancer. We analyzed the long-term results, predictive factors of outcomes, and complications associated with enteral stents with focus on their management.
Methods
Between 1997 and 2007, 46 patients with malignant GOO underwent placement of self-expandable metal stents (SEMS) for palliation. Patients were captured prospectively after 2001 and followed until complication or death. Patency, management of complications, and long-term survival were analyzed.
Results
Forty-six patients had a mean survival of 152 ± 235 days and a mean SEMS patency rate of 111 ± 220 days. SEMS patency rates of 98%, 74%, and 57% at 1, 3, and 6 months were seen. Thirteen patients presented with obstruction and included two SEMS migration, two early occlusion, one fracture, four malignant ingrowth, and four with delayed clinical failure. Interventions included seven endoscopic revisions with three SEMS replacements. Six had percutaneous endoscopic gastrostomy with jejunal arm placed. Two patients eventually underwent surgical bypass. Two patients required surgery for complications including delayed duodenal perforation and aortoenteric fistula.
Conclusions
SEMS effectively palliate gastric outlet obstructions that result from upper gastrointestinal malignancies. Their benefits offset potential complications or malfunctions, when a pluridisciplinary approach is adopted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients presenting with gastric outlet obstruction, upper gastrointestinal (GI) malignancies are the source in up to 39% of cases.1 Surgical resection with curative intent is the standard of care in patients who lack significant comorbidities.2 In unresectable patients, gastrojejunostomy remains the standard of care if a surgical intervention is to be undertaken. In those patients with significant comorbidities, the morbidity rate approaches 40%,3 encouraging alternatives to surgery. In patients who are not surgical candidates, enteral stenting offers an attractive option.4 Self-expandable metal stents (SEMS) have proven themselves to be a safe5 and relatively cost-effective6 alternative to surgical palliation allowing the patient to be discharged and start PO intake earlier.7–8 However, most series are retrospective and underscore the magnitude of potential complications. Our aim is to analyze our 10-year experience using enteral stents and pluridisciplinary management of all possible complications or malfunctions.
Materials and Methods
Patients
Between 1997 and 2007, 46 patients with malignant gastric outlet obstruction (GOO) underwent SEMS placement (Table 1). All patients were not surgical candidates for curative resection based on staging or comorbidities. Twelve patients had previously undergone attempted curative resection with disease recurrence. Follow-up 24 h post-procedure included phone contact by an endoscopy nurse. The patients were evaluated in clinic when enrolled in a chemo-radiation protocol with laboratories, performed every 2 months until death. Clinical response to SEMS placement, procedure-related morbidity, and overall patient survival were captured. Data were collected prospectively (43 patients) starting in 2001. Patients before this date (three patients) were captured retrospectively. The study was approved by our Institutional Review Board; all patients provided written consent for their procedures.
Enteral Stent Insertion and Deployment
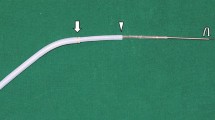
After endoscopic access was obtained proximal to the stricture, using fluoroscopy the length of each stricture was determined, and balloon dilation was performed at the discretion of the operator (Fig. 1). The SEMS delivery system was advanced proximal to the stricture over a guidewire where the SEMS was partially deployed (Figs. 2 and 3) and centered across the stricture before full expansion (Figs. 4). In case a biliary stent needed to be placed, this was typically inserted before the deployment of the enteral stent. All procedures were performed by dedicated pancreatico-biliary endoscopists.
Forty patients (87%) had a Wallstent (Boston Scientific, Natick, MA, USA) placed. Four had an Alimaxx (Alveolus, Charlotte, NC, USA), and two had a Bard (Bard, Tempe, AZ, USA) stent placed.
Indication for SEMS Placement
Indication for SEMS insertion included obstructive symptoms in the setting of unresectable or recurrent malignancy. The majority of patients had pancreatic adenocarcinoma (23 patients, 50%) or gastric cancer (eight patients, 17%; Table 1). Poor generalized medical status was defined as an American Society of Anesthesiologist score of 3 or greater and was present in 84%.
Serum albumin was used as a marker of patient’s nutritional status, with a mean of 3.2 ± 0.7 mg/dl.
Definition of Events
Successful SEMS placement was defined as deployment of the SEMS across the stricture with patency visualized both endoscopically and fluoroscopically. Clinical success was defined as relief of obstructive symptoms and ability to take oral intake within 24 h of SEMS placement independent of SEMS patency on imaging or endoscopic evaluation.
Complications were stratified as early (occurring ≤30 days of SEMS placement) and late (occurring >30 days following SEMS placement). Patency was defined as the period of time between SEMS insertion and repeat intervention or death. Repeat intervention was defined as any procedure to improve obstructive symptoms after initial SEMS placement. This included balloon dilation, placement of additional SEMS, percutaneous endoscopic gastrostomy with jejunal arm (PEGJ), or surgical intervention. Patients who had a PEGJ placed prior to or concurrently with stent placement were not defined as post-procedure complications.
Statistical Methods
The composite primary end point was stent malfunction requiring reintervention or death. Patient survival and SEMS patency were calculated using Kaplan–Meier estimates with censoring at end of follow-up. Univariate and multivariate logistic regression were used to determine if there were independent variables predicting combined mortality or stent failure using the method of maximum likelihood estimates. Factors were included in the multivariate analysis if they were established risk factors for mortality based on the literature or were significant in the univariate analysis to a level of 0.20. Data manipulation and analyses were performed with SAS©, version 9.1 (Cary, NC, USA) and Graphpad Prism©, version 4.0 (San Diego, CA, USA). The level of type 1 error for statistical significance was assumed to be less than or equal to 0.05. All statistical tests were two-sided.
Results
SEMS were successfully placed in all patients. No patient died as a direct result of SEMS placement, and all causes of death were related to progression of disease. There were no occurrences of perforation at stent placement. Forty-two patients (91%) had a clinical response to SEMS placement. Four patients failed to resolve their obstructive symptoms despite confirmed endoscopic patency; these patients were treated with PEGJ placement. In patients with an initial clinical response, a total of seven complications were recorded, five early and two late. Four patients (9%) had local tumor ingrowth through the mesh of the stent leading to secondary obstruction that was treated with either endoscopic intervention (three patients) or surgery (one patient). These were not included as complications, since they were resultant from progression of the primary disease.
Patients with Previous Surgical Interventions
Twelve patients who underwent attempts at curative surgical resection were treated with SEMS after tumor recurrence or progression. An additional four patients had laparoscopic evaluation for potential resection aborted in the setting of metastatic disease. Thirty patients did not undergo attempts at primary resection or surgical staging secondary to either medical comorbidities or tumor burden. Five patients included in this study had jejunal SEMS placement after previous pancreaticoduodenectomy (two patients) or gastrectomy (three patients).
Chemotherapy and Radiation
All patients were offered chemoradiation, with a total of 27 patients (57%) enrolled in an institution-specific protocol, based on their oncologist’s recommendation for their specific tumor type.
Early Complications (≤30 days)
Early complications related to SEMS placement included stent migration in two patients, managed by removal of the original SEMS and replacement with a new SEMS. Two patients who had initially responded to SEMS placement developed delayed-onset obstructive symptoms with endoscopically patent SEMS. Each was managed by PEGJ placement for decompression with the presumption that disease distal to the SEMS, in the setting of carcinomatosis, was the etiology. One patient developed stent fracture managed by stent removal with dilation (Table 2).
Long-Term Complications (>30 days)
There were no migrated or fractured SEMS beyond 30 days. Long-term complications include duodenal perforation 35 days after stenting, requiring emergent surgical repair with closure and Graham patch. One patient who had previously undergone a pancreaticoduodenectomy developed an aortoenteric fistula from stent erosion that presented as an upper GI bleed 12 months after initial stent placement. She was treated with an endovascular aortic stent followed by interval resection with definitive repair (Table 2).
Local Tumor Recurrence
Four patients were found to have occluded SEMS at 14, 62, 64, and 75 days post-stenting. These obstructions were consistent with local tumor ingrowth and progression of the primary disease process. These were treated, respectively, with repeat SEMS placement, argon plasma coagulation application, balloon dilation, and surgical bypass.
Stent Patency, Multivariate Analysis, and Patient Survival
Mean survival was 152 days (range, 13–1,411 days). Mean stent patency was 111 days (range, 3–1,411 days). Kaplan–Meier survival analysis showed a patency rate of 98%, 74%, 57%, and 58% at 1, 3, 6, and 12 months, respectively (Fig. 5).
Multivariate analysis failed to identify any factor predictive of survival. Factors analyzed included age, gender, serum albumin as a marker for nutritional status, or treatment with chemoradiation. (Table 3)
Overall, if migration, fracture, tumor ingrowth, erosion, and perforation are taken into account, the global long-term patency rate obtained is 76%. This rate does not include the four patients (9%) that did not gain a clinical response from SEMS placement despite the stent being patent endoscopically and radiographically.
Discussion
Gastric outlet obstruction is a common cause of preterminal morbidity, leading to a progressive deterioration in quality of life in patients with advanced upper GI malignancies. Up to 39% of patients with GOO will have a malignant etiology, commonly unresectable pancreatic cancer.1 Surgical palliation has been the accepted standard for treatment of these patients for many years.2 Singh et al.9 describe a retrospective review of 340 patients undergoing either curative resection, palliative surgery, or neither for pancreatic adenocarcinoma. Seventy patients underwent gastrojejunostomy, 20 prophylactically and 50 therapeutically for GOO. Of those who did not undergo bypass, 25% required a later repeat surgical intervention for gastrojejunostomy. They report morbidity rates greater than 30% in this patient population. Patient comorbidities and time to recovery/discharge after palliative surgery have promoted tertiary-care centers proficient in interventional endoscopy to use enteral stenting as an alternative. For over a decade, SEMS has been used as a minimally invasive technique for palliative treatment of patients with malignant gastric outlet obstruction.10–11
A comprehensive review of 32 case series, including 606 patients unable to take oral intake, reported successful stent deployment in 97% of patient and oral intake possible in all cases, with 87% of cases capable of eating at least a soft mechanical diet.12 Well-described complications of enteral stent placement include tumor overgrowth, obstruction, and stent migration.13 Graber et al.14 have published results from a prospective multicenter trial demonstrating a mortality rate of 9.8% due to stent complications with 25% developing SEMS occlusion secondary to local tumor ingrowth and disease progression with a subpopulation requiring surgical intervention for hemorrhage and perforation.
Our study confirms previous data in the literature. We show a 100% success rate in SEMS deployment with a 91% clinical success rate. Four patients had lack of improvement within the first week after stent placement. In these patients, peritoneal carcinomatosis with multi-level obstruction or autonomic infiltration with dysmotility was presumed responsible for symptoms persistence.
In our study population, seven patients (14%) had a PEGJ placed prior to SEMS insertion, and an additional four patients (9%) had a PEGJ placed concomitantly with SEMS placement for malnutrition. These patients had no additional evidence of stent dysfunction.
The data for this study were collected in a prospective manner starting in 2001 (43 patients, 93%) with the establishment of an enteral stent database; therefore, we were able to collect known complications more accurately compared to retrospective studies. Our data shows that seven (17%) of 42 patients with clinical response to SEMS placement had a complication related to enteral stenting. This rate does not include the four patients (9%) that developed local tumor recurrence requiring reintervention. In this setting, the reported complication rate is less than those of other published studies likely because all stent placement and treatment were performed at a tertiary-care center by dedicated interventional endoscopists. This trend of specialists having a lower complication rate has been reported in other fields,15 and a similar trend would be predicted in our study.
SEMS placement has become a preferred palliative tool in most tertiary-care centers since it is more cost effective than palliative surgery6 and permits earlier discharge, with faster return to PO intake and less postoperative recovery time.16
In our case series, we reviewed the long-term results and complications associated with enteral stents with special consideration given to their management, outlining the excellent collaboration between the GI and surgical communities. It also shows no statistically significant correlation between survival and any of our independent variables including age, gender, albumin, and chemoradiation therapy.
Conclusions
In conclusion, SEMS offer an efficacious palliation of malignant gastric outlet obstruction in patients. Our series underlines the need to establish a pluridisciplinary approach involving interventional radiologists, endoscopists, and surgeons alike in order to successfully manage any complications or failures associated with SEMS.
References
Awan A, Johnston DE, Jamal MM. Gastric outlet obstruction with benign endoscopic biopsy should be further explored for malignancy. Gastrointest Endosc 1998;48(5):497–500.
Fisher WE, Andersen DK, Bell RH Jr, Saluja AK, Brunicardi FC. Chapter 32: The Pancreas. Schwartz’s Principles of Surgery. Eighth edition, (October 14, 2004).
Medina-Franco H, Abarca-Pérez L, España-Gómez N, Salgado-Nesme N, Ortiz-López LJ, García-Alvarez MN. Morbidity-associated factors after gastrojejunostomy for malignant gastric outlet obstruction. Am Surg. 2007;73(9):871–875.
Graber I, Dumas R, Filoche B, Boyer J, Coumaros D, Lamouliatte H, Legoux JL, Napoleon B, Ponchon T, Societe Francaise d’Endoscopie Digestive. The efficacy and safety of duodenal stenting: a prospective multicenter study. Endoscopy 2007;39(9):784–787.
Maetani I, Tada T, Ukita T et al. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy 2004;36:73–78.
Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg 2004;28:812–817.
Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol 2007;7:18.
Espinel J, Sanz O, Vivas S, Jorquera F, Muñoz F, Olcoz JL, Pinedo E. Malignant gastrointestinal obstruction: endoscopic stenting versus surgical palliation. Surg Endosc 2006;20(7):1083–1087.
Singh SM, Longmire WP Jr, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg 1990;212(2):132–139.
Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 2001;344:1681–1687.
Baron TH, Harewood GC. Enteral self-expandable stents. Gastrointest Endosc 2003;58:421–433.
Dormann A, Meisner S, Verin N et al. Self expainding metal stents for gastroduodenal malignancies: systemic review of their clinical effectiveness. Endoscopy 2004;36:543–550.
Mauro MA, Koecher RE, Baron TH. Advances in gastrointestinal intervention. The treatment of gastroduodenal and colorectal obstructions with metallic stents. Radiology 2000;215:659–669.
Graber L, Dumas R, Foliche B et al. The efficacy and safety of duodenal stenting: a prospective multicenter study. Endoscopy 2007;39:784–787.
Beecherl EE, Shires GT, Shires GT. Treatment of Post-pancreaticoduodenectomy Complications. Curr Treat Options Gastroenterol 2004;7(5):365–370.
Mittal A, Windsor J, Woodfield J et al. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg 2004;91:205–209.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at Digestive Disease Week/SSAT, May 2008, San Diego, California.
Rights and permissions
About this article
Cite this article
Phillips, M.S., Gosain, S., Bonatti, H. et al. Enteral Stents for Malignancy: A Report of 46 Consecutive Cases over 10 years, with Critical Review of Complications. J Gastrointest Surg 12, 2045–2050 (2008). https://doi.org/10.1007/s11605-008-0598-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0598-4