Abstract
Hepatoid adenocarcinoma of the stomach (HAS) is a rare distinct variant of gastric carcinoma with earlier metastases and worse prognosis compared to the more common intestinal types. It is often misdiagnosed as hepatocellular carcinoma, especially when primary HAS is insignificantly anatomically abnormal, produces high alpha-fetoprotein and develops early liver metastasis. In this case we show the significance of dual-time-point 18F-fluorodeoxyglucose positron emission tomography (PET)/computed tomography (CT) in accurately diagnosing and staging HAS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatoid adenocarcinoma is a rare primary extra hepatic adenocarcinoma with similar morphology, immunohistochemistry, and behavior to those of hepatocellular carcinoma, mostly characterizing with the high level of serum alpha-fetoprotein (AFP) [1]. It is aggressive with early metastases and poor prognosis [2]. Stomach and ovary are the most common sites in which the hepatoid adenocarcinomas have been diagnosed [3].
Hepatoid adenocarcinoma of the stomach (HAS) is a distinct variant of gastric carcinoma with worse prognosis compared to the more common types of gastric adenocarcinoma. HASis often misdiagnosed as hepatocellular carcinoma because it usually produces extremely high AFP and develops early liver metastasis [4]. To the best of our knowledge, there is no literature which reports the significance of dual-time-point 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) imaging in accurately diagnosing and staging HAS.
Case report
A 73-year-old man presented with upper abdominal pain for 2 years. The serum AFP level was extremely high (9,186 μg/l), but other tumor markers, including carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and CA 125, were all within the normal range. The primary hepatocellular carcinoma was concerned although there was no medical history of hepatitis or cirrhosis. Multi-phase contrast-enhanced computed tomography (CT) of the abdomen demonstrated multiple enlarged abdominal lymph nodes, but no lesion was found in the liver.
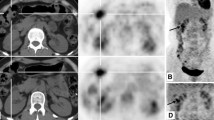
Whole body PET/CT (Discovery LS, GE Healthcare, Milwaukee, WI) scanning was performed 60 minutes after intravenous injection of 389 MBq (10.5 mCi) 18F-FDG to explore the primary tumor and evaluate the disease extent. The enlarged abdominal lymph nodes showed intense FDG accumulations at the hepatic portal (white arrows, Fig. 1a, b, g) and the mesenteric root (white arrowheads, Fig. 1c, d, g). No abnormal FDG uptake was found in the liver. In addition, a clearly intense focal FDG uptake was detected in the gastric antrum (red arrows, Fig. 1e, g) without significant anatomic abnormality in the CT images (Fig. 1f). Although the use of CT for accurate anatomic correlation of areas with increased FDG uptake on the PET/CT images leads to a significant reduction of false positive findings, small focal FDG uptake in the gastric antrum may still be the source of false positive reports, such as those caused by physiological accumulation. Therefore, a second PET/CT scan of the abdomen was carried out 1 h later to confirm the findings. The maximum standard uptake value (SUVmax) of the gastric antrum increased from 5.0 in first scan to 5.4 at the later time-point PET/CT image, and the SUVmax of abdominal lymph nodes increased from 7.8 and 5.1 to 9.7 and 6.8, respectively, suggesting primary gastric malignancy with loco-regional lymph node metastases.
Abdominal CT and PET/CT image of 73-year-old male patient with extremely high AFP-producing hepatoid adenocarcinoma of the stomach. a–d Transverse PET/CT fusion and CT images showing high FDG accumulation in the enlarged lymph nodes at the hepatic portal (white arrows) and the mesenteric root (white arrowheads). e, f Transverse PET/CT fusion and CT images clearly show small intense focal FDG uptake in the gastric antrum (red arrows). g Whole-body FDG PET left anterior oblique maximum intensity projection image showed that the hypermetabolic lesion in the gastric antrum (red arrow) was surrounded by several enlarged hypermetabolic lymph nodes (white arrow and arrowhead). No other abnormal FDG uptake was noticeable
Consequently, a gastroscopy was performed and a 2-cm ulcerating mass was found at the gastric antrum. A biopsy was performed and the diagnosis of HAS was confirmed with AFP-positive histopathological findings, as the AFP-positive signals were found in the tumor cell cytoplasm in the hepatoid differentiated region of the tumor tissue (Fig. 2a). The treatment was discussed and carried out by a multidiscipline team. The patient received two cycles of neoadjuvant chemotherapy with paclitaxel and tegafur and oteracil potassium sustained capsules (S-1) and radiotherapy to the primary cancer and regional lymph node (45 Gy). Then radical surgery was performed. Postoperative pathology found significant treatment reactions such as histocyte and lymphocyte infiltration. A few adenocarcinoma cells were found in the submucosa and muscularis mucosae (Fig. 2b). Metastases were found in 4 of 6 regional lymph nodes. The pathological stage was defined as T2N1M0. Six cycles of adjuvant chemotherapy with FOLFOX4 (oxaliplatin, leucovorin and 5-Fu) were administered. The patient was followed-up routinely and has survived for 16 months without local recurrence and distant metastasis till last follow-up.
Histopathological diagnosis of the HAS. a AFP immunohistochemical staining showed strong signals of AFP (brown) in the cytoplasm of the tumor cells with “hepatocellular differentiation”. b Surgical pathological findings. A few adenocarcinoma cells were found in the submucosa and muscularis mucosae. (magnification ×200)
Discussion
HAS was first described in 1985, characterizing with hepatoid differentiation and extremely high serum AFP levels [5]. Since then, cases of hepatoid adenocarcinoma of the uterus, ovary, pancreas, lung, colon and rectum, kidney, urinary bladder, esophagus, papilla of Vater, gallbladder, adrenal gland, vagina, and testicle have been documented [1]. The incidence of HAS reported in the literature ranged from 1.3 to 15 % in all gastric malignancies [3, 6, 7]. All the reported cases have occurred in elder adults (mean age 63 years, range 44–87 years), with a 2:1 male predominance. Epigastric dull pain, abdominal distention, and melena are the most common symptoms, but none of these is specific.
HAS is a distinct variant of gastric carcinoma with earlier liver metastases and worse prognosis. The 5-year survival rate of HAS is only 11.9 %: much lower than that of other gastric carcinomas (38.2 %) [3]. Most of the tumors have spread beyond the stomach at surgical resection. Lymph nodes and liver are the most common sites of metastatic disease. The serum AFP levels are often significantly increased (mean level 51,130.1 μg/l, range 1–700,000 μg/l) in most HAS patients at the initial examination [7]. Since lymph node and liver metastases could be the first clinical manifestation, in combination with the high AFP level, HAS may be misdiagnosed as a primary hepatocellular carcinoma. CT is of little value for the diagnosis of HAS, especially when there is no significant anatomic abnormality at the primary tumor site, as shown in the presented case. However, it is useful for the detection of lymph node metastases. As shown at the present case, FDG PET/CT scan is valuable in diagnosis and staging for HAS.
Multiple reports have shown that dual-time-point FDG PET/CT has a potential role in differentiating malignant tumors from structures with physiological uptake and inflammatory accumulation [8–10]. Normally, FDG uptake in the malignant primary tumor and lymph node metastases increases from the first to the second scan, whereas the FDG uptake in infectious lesions is essentially stable over time or slightly declines, and structures with physiological uptake may disappear or show decreased FDG uptake.
In conclusion, it should be kept in mind that gastric focal FDG accumulation could be hepatoid adenocarcinoma of the stomach when the patient has multiple abdominal lymph node metastases and/or liver metastasis accompanied by a high AFP level. Dual-time-point FDG PET/CT imaging is necessary for further differentiating malignant tumors from physiological gastric activity or inflammatory processes. It should be the basis for successful management.
References
Jung JY, Kim YJ, Kim HM, Kim HJ, Park SW, Song SY, et al. Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver. 2010;4:98–102.
Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. Alpha-fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654–61.
Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827–35.
Pan JH, Dong MJ, Ouyang XB. A hepatoid adenocarcinoma of the stomach with liver metastasis mimicking hepatocellular carcinoma detected by F-18 FDG PET/CT imaging. Clin Nucl Med. 2011;36:1137–9.
Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer. 1985;56:840–8.
Inagawa S, Shimazaki J, Hori M, Yoshimi F, Adachi S, Kawamoto T, et al. Hepatoid adenocarcinoma of the stomach. Gastric Cancer. 2001;4:43–52.
Roberts CC, Colby TV, Batts KP. Carcinoma of the stomach with hepatocyte differentiation (hepatoid adenocarcinoma). Mayo Clin Proc. 1997;72:1154–60.
Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, et al. Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med. 1999;26:1345–8.
Xiu Y, Bhutani C, Dhurairaj T, Yu JQ, Dadparvar S, Reddy S, et al. Dual-time point FDGPET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med. 2007;32:101–5.
Ahmadzadehfar H, Sabet A, Nake K, Hinterthaner B, Biersack HJ, Ezziddin S. Dual-time F-18 FDG-PET/CT imaging for diagnosis of occult non-Hodgkin lymphoma in a patient with esophageal cancer. Clin Nucl Med. 2009;34:168–70.
Acknowledgements
The present study was supported in part by grants from the National Natural Science Foundation of China (81001004 and 81272502) and the Shandong Natural Science Foundation (ZR2010HL027).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sun, X., Li, Y., Dong, M. et al. Hepatoid adenocarcinoma of the stomach: dual-time-point 18F-FDG PET/CT findings. Jpn J Radiol 32, 721–724 (2014). https://doi.org/10.1007/s11604-014-0366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-014-0366-1