Abstract
Approximately 25% of those who are diagnosed with colorectal cancer will develop colorectal liver metastases (CRLM) as their illness advances. Despite major improvements in both diagnostic and treatment methods, the prognosis for patients with CRLM is still poor, with low survival rates. Accurate employment of imaging methods is critical in identifying the most effective treatment approach for CRLM. Different imaging modalities are used to evaluate CRLM, including positron emission tomography (PET)/computed tomography (CT). Among the PET radiotracers, fluoro-18-deoxyglucose (18F-FDG), a glucose analog, is commonly used as the primary radiotracer in assessment of CRLM. As the importance of 18F-FDG-PET/CT continues to grow in assessment of CRLM, developing a comprehensive understanding of this subject becomes imperative for healthcare professionals from diverse disciplines. The primary aim of this article is to offer a simplified and comprehensive explanation of PET/CT in the evaluation of CRLM, with a deliberate effort to minimize the use of technical nuclear medicine terminology. This approach intends to provide various healthcare professionals and researchers with a thorough understanding of the subject matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 25% of the patients with colorectal cancer (CRC) will develop metastases specifically in the liver, known as colorectal liver metastases (CRLM) [1]. The molecular mechanisms underlying CRLM are intricate and multifaceted due to the involvement of various factors and processes in a complex cascade reaction [2]. Despite notable progress in diagnostic and therapeutic methods, the prognosis for patients with CRLM remains poor, with low survival rates [2]. The standard approaches for treating patients with CRLM involve curative resection, which refers to the surgical removal of the metastatic liver lesions, and chemotherapy [3]. Selected small CRLMs, when feasible, are also treated using percutaneous ablative techniques either as a standalone approach or in combination with resection [4]. Nonetheless, the feasibility of surgery as a treatment option for CRLM is limited to a mere 10–20% of cases, primarily due to factors like tumor size and location, presence of unresectable disease or extrahepatic disease, and the comorbidities of the patients [2, 5, 6]. Consequently, the 5-year survival rate for these patients is dishearteningly low, reaching as little as 30% [5, 6]. Moreover, patients who are deemed ineligible for surgery face an even bleaker outlook [2]. The management of CRLM patients relies on the evaluation of complex clinical, radiological, and biomarker data to determine the most appropriate course of action [1]. Imaging plays a crucial role in determining the most suitable treatment approach for CRLM. It is vital to have a clear understanding of the size, location, and vascular connections of the CRLM before devising a treatment plan and evaluating the response to neoadjuvant therapy [1]. Various imaging modalities are utilized in the assessment of CRLM, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)/CT. Among the PET radiotracers, fluoro-18-deoxyglucose (18F-FDG), a glucose analog, is widely employed as the primary radiotracer in clinical oncology [7]. 18F-FDG gains entry into cells through the membrane proteins glut-1 and glut-3, which are responsible for transporting glucose [7]. Following entry into the cell, 18F-FDG undergoes phosphorylation facilitated by the enzyme hexokinase. Unlike glucose, 18F-FDG-6-phosphate is unable to be further metabolized by glucose-6-phosphate isomerase, resulting in its entrapment within the tumor cell [7] (Fig. 1). To effectively interpret PET results, it is commonly necessary to combine the metabolic data obtained from PET with the anatomical information derived from CT scans [8]. By integrating this “metabolic signature” into the interpretation process, a more precise and comprehensive assessment of the disease can be achieved, offering valuable prognostic information and enhancing the characterization of the condition [8]. Currently, there is limited clinical evidence demonstrating the substantial impact of 18F-FDG PET/CT on the pre-operative clinical management of localized non-metastatic colorectal cancer [9]. Nevertheless, 18F-FDG PET/CT is widely recognized for its high accuracy and sensitivity in detecting CRLM, particularly those larger than 10 mm in size [10]. Liver metastases of a small size (< 10 mm) and those originating from certain mucinous adenocarcinomas may not be effectively detected or identified using 18F-FDG PET/CT [11,12,13]. The focus of this article is to provide an extensive review of the role of PET/CT in the evaluation of CRLM (Fig. 2). The article emphasizes the use of 18F-FDG as the primary PET radiotracer in clinical oncology.
Simplified mechanism of tumor visualization on FDG-PET scans. FDG is taken up by cells and converted into FDG-6- phosphate through phosphorylation. FDG-6-phosphate becomes trapped within cancer cells as a polar metabolite due to its inability to be metabolized. This characteristic forms the foundation for visualizing tumors on FDG-PET scans (reference of the figure’s legend: Wong et al. (2016). Chapter 11—Nuclear Medicine. Clinical Radiation Oncology (Fourth Edition)). The schematic part of this figure was created by the author using BioRender.com. The PET and PET/CT images obtained from PMID: 20237041
18F-FDG PET/CT for detection of CRLM
18F-FDG PET/CT has demonstrated high levels of precision and sensitivity in identifying liver metastases originating from various types of primary cancers (Fig. 3). In a prospective trial involving 45 patients with suspected liver metastases from various malignancies, 18F-FDG PET/CT showed superior performance to contrast-enhanced CT (CECT) in detecting CRLM [14] (Fig. 3). The authors discovered that CECT had a sensitivity of 87.9% and specificity of 16.7% in detecting hepatic metastases, whereas 18F-FDG PET/CT demonstrated a sensitivity of 97% and specificity of 75% for the same purpose [14]. Some studies compared the accuracy of 18F-FDG PET/CT and MRI for detection of CRLM and concluded that there was no significant difference between the two imaging modalities for showing liver metastases. Tahtabesi et al. [15], analyzed a group of 42 patients with primary colorectal, stomach, or pancreatic biliary system malignancies that had spread to the liver. The study found no statistically significant distinction between the number of liver metastases detected by MRI and 18F-FDG PET/CT (with respective average counts of 7.55 ± 7.96 and 6.36 ± 7.28; p = 0.11) [15]. In another study, Yang et al. examined a group of thirty consecutive patients who had known or suspected metastatic lesions and underwent scanning using both MRI and PET [16]. The reference standards for evaluation were histopathology and/or clinical outcome, as well as further cross-sectional imaging during follow-up. A total of 30 patients were enrolled in the trial; 16 had liver metastases that were found to be positive based on histology and/or clinical outcomes, and 14 had none [16]. On MRI, the sensitivity, specificity, and positive and negative predictive values were determined to be 85.7%, 100%, 100%, and 89%, respectively. In comparison, 18F-FDG PET-PET exhibited values of 71%, 93.7%, 90.9%, and 79% for sensitivity, specificity, positive predictive value, and negative predictive value, respectively [16]. The authors concluded that there was no statistically significant difference in the detection of liver metastases between MRI and 18F-FDG-PET; however, they mentioned MRI offers advantages in terms of spatial resolution and the ability to characterize the lesions [16]. The objective of another study was to evaluate and compare the precision of 18F-FDG PET/CT and 18F-FDG PET/MRI in detecting liver metastases [17]. Compared to PET/CT, PET/MRI demonstrated superior accuracy (PET/CT: 82.4%; PET/MRI: 96.1%; p < 0.001), sensitivity (67.8% vs. 92.2%, p < 0.01), and negative predictive value (82.0% vs. 95.1%, p < 0.05) [17].
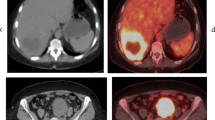
Liver metastatic deposit in a fatty liver, which is challenging to visualize on CT. a The unenhanced CT scan shows widespread fatty infiltration of the liver, with liver attenuation values significantly lower than those of the spleen. b On contrast-enhanced CT imaging, a very subtle lesion (indicated by an arrow) is observed in segment 7. c The 18F-FDG-PET scan demonstrates intense radiotracer uptake (arrow) in the right lobe of the liver. d Increased 18F-FDG uptake (arrow) on PET, when compared with the fused PET/CT image, correlates with the subtle liver lesion, indicating a hepatic metastasis. PMID: 22312527; under CC BY license
In some studies MRI appeared to be superior to PET/CT for detection of CRLM. Sivesgaard et al. conducted a study with the objective of evaluating the diagnostic precision of CECT, MRI, and 18F-FDG PET/CT in detecting CRLM in patients who were potential candidates for hepatic resection and/or local ablation [18]. A total of 260 CRLMs were confirmed and included in the analysis [18]. The readers of MRI demonstrated notably higher per-lesion sensitivity compared to contrast-enhanced CT (69.1% and 62.3%) and the reader pairs using PET/CT (72.0% and 72.1%) (p < 0.001) [18]. Between the various techniques, no discernible changes in per-lesion specificity were found. However, the area under the receiver operating characteristic curve (AUC-ROC) values for the MRI reader pairs were substantially higher (0.92 and 0.88 vs. 0.83 and 0.84, respectively) than those for the PET/CT reader pairs (p < 0.001) [18]. A meta-analysis involved the assessment of twelve prospective studies, which encompassed a total of 536 patients with CRLMs, totaling 1335 lesions [19]. When considering individual lesions, the sensitivity rates were determined to be 86% for contrast-enhanced ultrasound (CEUS), 84% for multidetector computed tomography (MDCT), 89% for MRI, and 62% for 18F-FDG PET/CT [19]. When evaluated on a per-patient basis, the sensitivity and specificity values for CEUS were 80% and 97% respectively, for MDCT they were 87% and 95%, for MRI they were 87% and 94%, and for 18F-FDG PET/CT they were 96% and 97% respectively [19]. The per-patient sensitivities of MRI and MDCT were comparable. However, MRI exhibited higher sensitivity compared to CEUS, MDCT, and 18F-FDG PET/CT for lesions smaller than 10 mm, as well as for lesions measuring at least 10 mm in size [19]. Based on their findings, the authors reached the conclusion that MRI is the preferred imaging technique for the assessment of CRLMs [19].
Recently, PET/MRI has emerged as a suggested imaging modality for detecting CRLM, offering a combination of MRI’s excellent sensitivity with the metabolic information provided by PET. Additionally, the use of MRI-specific contrast agents can further enhance CRLM detection sensitivity. This can be particularly promising for the detection of small CRLMs, which can be a significant limitation when relying solely on PET for CRLM assessment. However, the main challenge lies in the limited availability of PET/MRI compared to other imaging modalities such as PET, PET/CT, CECT and MRI.
Dual time point imaging (early and delayed PET) for increasing the detection rate of CRLM
Dual Time Point Imaging (DTPI), which primarily used to differentiate between inflammatory and malignant lesions, relies on the observation that the uptake of 18F-FDG increases in malignant tissues over time, peaking around 4 h after injection [20]. This phenomenon can be attributed to the higher expression levels of glucose transporter membrane proteins (GLUT) and higher ratios of hexokinase to 18F-FDG-6-phosphatase in malignant cells. These factors contribute to an increased accumulation rate of 18F-FDG in malignant tissues compared to non-malignant tissues [20]. In a study on CRLM, the initial whole-body scan for image acquisition commenced at an average time of 69 min (ranging from 55 to 110 min) after the injection of 18F-FDG. The mean duration between the administration of 18F-FDG and the subsequent delayed scan was 100 min (with a range of 85 to 166 min) [21]. Out of the 90 confirmed liver metastases identified in 34 patients, the initial scan accurately detected 53 lesions (59%). However, in the subsequent delayed scan, a higher number of lesions, 81 (90%), were correctly diagnosed (p < 0.001) [21]. The average standardized uptake values (SUV) in the initial scan and the second delayed scan were measured to be 6.59 g/mL and 8.09 g/mL, respectively (p < 0.001). Furthermore, the tumor-to-background ratio in the first scan was 2.0, whereas in the second delayed scan, it increased to 2.7 (p = 0.04). The DTP imaging of the liver revealed a noteworthy elevation in the dimensions of hypermetabolic lesions and the tumor-to-background ratio. However, it is important to note that while only the second scan revealed 30% of all confirmed liver lesions, 10% of malignant liver lesions could not be identified using 18F-FDG PET/CT [21].
Assessment of disease stage and modification of treatment approaches
The utilization of 18F-FDG PET/CT as an adjunctive staging technique has demonstrated a substantial impact on therapeutic decision-making in a range of 14–65% of patients with CRLM [22] (Fig. 4). It is particularly effective in identifying previously undetected extrahepatic disease in some cases, thereby leading to improved management strategies [23, 24]. A prospective study by Ruers et al. [12] exhibited a significant change in the clinical approach in 20% of patients (10 out of 51 patients) who were being evaluated as potential candidates for the surgical removal of CRLMs. This was primarily attributed to the detection of previously unidentified extrahepatic disease. In another investigation involving 102 patients with suspected or confirmed regional recurrence of colorectal cancer, 18F-FDG PET played a significant role in influencing management decisions in 59% of cases [25] (Fig. 5). The study’s findings demonstrated a substantial impact on treatment planning, primarily by preventing unnecessary surgery in patients with extensive disease [25]. In a meta-analysis, Huebner et al. reported that the pooled percentage of change in management of recurrent CRC by means of whole-body 18F-FDG PET was estimated to be 29% (with a 95% confidence level ranging from 25 to 34%) [26]. In another meta-analysis conducted by Wiering et al., the pooled change in management of CRLM was determined to be 32% with a range of 20% to 58% [27]. The findings from multiple studies have led to a widespread consensus regarding the valuable role of 18F-FDG PET/CT in the restaging of recurrent colorectal cancer [22]. The studies by Zhou et al. [28] and Grassetto et al. [29] are summarized in Table 1.
TNM stage of colorectal cancer by American Joint Committee on Cancer (AJCC) 8th edition. Stage IV: Any T or N stage with distant metastasis including liver (TNM1); Red arrow: the primary or metastatic tumors; yellow arrow: the metastatic lymph nodes. PMID: 36620584; under CC BY license. (Color figure online)
The medical team was considering performing surgery on a patient with a seemingly isolated lung metastasis. However, further evaluation using PET imaging revealed the presence of multiple liver metastases, which were not initially detected on CT scans. The upper panel of the image displays the lung lesion, while the lower panel highlights the additional liver metastases identified through PET imaging. Based on these findings, surgical intervention was avoided, and subsequent imaging confirmed the presence of metastases in the abdomen. PMID: 11937593, JNM, open access article
Prognostic significance
During the qualitative analysis of PET scans, 18F-FDG avid lesions are visually identified. Additionally, the level of 18F-FDG uptake is assessed through semi-quantitative measures using SUV [30]. SUV is a commonly used PET parameter to quantify the metabolic activity in lesions [31]. SUV is determined by dividing the concentration of radioactivity in the tissue by the administered dose, and it is further normalized by either body weight or lean body weight [31]. SUVmax, which refers to the highest SUV value within the tumor, is commonly used among different SUV measurements. Its widespread adoption in clinical oncology is attributed to its simplicity and the fact that it does not rely on observer interpretation [30, 31] (Fig. 6). In a comprehensive analysis of 15 studies involving 867 patients, Xia et al. demonstrated that PET/CT, specifically the assessment of metabolic response to therapy evaluated by the difference between baseline and follow-up SUVmax values, was a significant predictor of event-free survival (EFS) and overall survival (OS) [32]. The hazard ratio (HR) for EFS and OS were calculated as 0.45 (95% confidence interval [CI] 0.26–0.78) and 0.36 (95% CI 0.18–0.71), respectively [32]. Moreover, a high SUV observed on pre-treatment 18FDG PET/CT scans was significantly correlated with poorer OS, with a HR of 1.24 (95% CI 1.06–1.45) [32]. However, the analysis did not show a statistically significant association between post-treatment SUV and OS (HR 1.68 (95% [CI] 0.63–4.52)) [32]. The results of this meta-analysis provide support for the effectiveness of 18FDG PET/CT as a valuable tool in predicting survival outcomes for patients with liver metastases [32]. Based on the study’s quantitative analysis, the nonresponding group of patients with CRLM exhibited a 2.5-fold higher risk of death in OS and a 2.632-fold higher risk in EFS compared to the responding group. This suggests that due to the tumors hyperactive metabolism, which can be seen on the 18FDG PET/CT scans, these lesions may be more aggressive or invasive [32]. Compared to the low SUV group, the high SUV group showed a significant difference in OS. This significant association was observed irrespective of whether patients received curative surgery or chemotherapy [32]. The risk of death was seen to rise by a staggering 17% with every additional SUV unit [33]. Despite of the promising results, it should be noted that SUVmax only captures the uptake in a single voxel within the lesion, which may not fully represent the overall uptake of the tumor [34]. Moreover, SUVmax can be more susceptible to the influence of noise and motion artifacts, as it represents uptake in a limited and specific region [34]. Metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which are volumetric PET metrics, have emerged as potential solutions to overcome certain limitations associated with conventional PET metrics like SUVmax (Fig. 7). Grut et al. enrolled a total of 40 participants with CRLM in a study [35]. Individuals with a lower MTV experienced significantly longer OS (p < 0.001), disease-free survival (DFS) (p < 0.001), and post-recurrence survival (p = 0.006) compared to those with higher MTV values [35] (Fig. 8). Moreover, participants with higher MTV had elevated levels of carcinoembryonic antigen, a greater number of liver metastases, larger size of the largest liver metastasis, more advanced N-stage, increased number of chemotherapy cycles, and a higher incidence of disease progression at the time of liver transplantation when compared to individuals with lower MTV values [35] (Fig. 8). In light of these data, a poor prognosis is suggested to be indicated by increased glucose metabolism in liver metastases.
The schematic figure on the left side illustrates the quantitative parameters used in PET imaging. The magnified transverse image of the tumor (outlined in purple) displays the radiotracer uptake of 18F-FDG. Within the black square, the red spot represents the maximum standardized uptake value (SUVmax). The black circle represents the region of interest (ROI), while SUVpeak is the average SUV obtained from a 1 mL sphere within the tumor. On the right side, there is a representation of the receiver operating characteristic (ROC) curve, which evaluates the use of SUVmax in predicting disease progression of individual CRLMs. According to the curve, an SUVmax of 4.4 demonstrates a sensitivity of 70% and specificity of 71% in predicting progressive disease of the individual lesion. The area under the ROC curve (AUC) is 0.734 (with a 95% confidence interval of 0.602–0.865, p = 0.004), indicating the diagnostic performance of SUVmax in this context. The left image obtained from PMID: 36620584, The right image obtained from PMID: 30064385; under CC BY licences. (Color figure online)
In a patient with colorectal liver metastases, the 18F-FDG-PET scan reveals the presence of multiple active lesions in the liver (A). Using an iterative reconstruction algorithm, the 18F-FDG-avid lesions in the liver are semi-automatically segmented (B). The quantitative measurements of these lesions include SUVmean: 10.2, partial volume corrected SUVmean (pvcSUVmean): 15.3, SUVmax: 18.5, MTV: 50.5, TLG: 516.3, and pvcTLG: 772.6. The schematic part of this figure was created by the author using BioRender.com. PET images obtained from PMID: 31772823; open access article
Left side of images including maximum intensity projection, 18F-FDG-PET, CT, and fused 18F-FDG-PET/CT that were obtained from a CRLM patient with a low metabolic tumor volume (MTV) of 41.10 cm3. Remarkably, despite experiencing pulmonary relapse, the patient remains alive nearly 14 years after undergoing liver transplantation. Right side of images including maximum intensity projection, 18F-FDG-PET, CT, and fused 18F-FDG-PET/CT, were obtained from a CRLM patient with a high MTV of 194.35 cm3. Unfortunately, the patient experienced multiple site recurrence just 3 months after undergoing liver transplantation and passed away only 14 months after the procedure. PMID: 36241941; under CC BY license
Some studies have not shown a significant correlation between SUV and prognosis. For instance, Zalom et al. aimed to assess the effectiveness of 18F-FDG PET/CT in predicting the outcome of 31 patients who underwent 90Y radioembolization (RE) treatment for metastatic liver tumors [36]. Patients who developed new lesions in areas outside the liver after treatment had notably shorter survival times compared to those who did not experience such lesions. However, according to the Cox proportional hazard model, the levels of SUV before and after treatment were not found to be significant factors for predicting OS [36]. Another noteworthy point was the variations in the cutoff values utilized to distinguish between high and low SUV PET results, with thresholds ranging from 2.85 to 20 [36]. Higashi et al. [37] and Vansteenkiste et al. [38] observerd that SUVs can be dichotomized using a variety of thresholds and provide statistically discriminative log-rank probability values; this can imply that the correlation between an SUV’s prognosis and its dichotomization could be more progressive in nature rather than reliant on a single threshold [32]. In another words, this can imply that higher SUV values could be associated with a poorer prognosis in a continuous manner, rather than being determined by a specific cutoff point [32]. In addition, there has been a scarcity of research examining the predictive significance of post-treatment PET parameters for prediction of survival, and the majority of these studies have reported a lack of significant p-values [32]. The absence of statistical significance in this regard could be attributed to the limited number of studies that has conducted so far [32].
Response to Neoadjuvant Chemotherapy
Evaluating the probability of treatment response plays a vital role in the management of neoadjuvant chemotherapy for CRLM. It assists in determining the optimal timing for local curative treatment and monitoring the early response to treatment [39]. By offering insights into the metabolic processes occurring within the body, PET serves as a valuable modality for assessing and understanding this scenario. Several assessment criteria for PET/CT imaging were examined to measure treatment response in chemotherapy for CRLM [39]. Furthermore, a diverse array of chemotherapy drugs was utilized in these investigations. Burger et al. [40] examined a group of 69 patients who underwent neoadjuvant chemotherapy, and subsequently underwent an 18F-FDG PET/CT scan between 2 and 7 weeks prior to their scheduled surgery (within 8 weeks after chemotherapy). The difference between the SUV and the histological tumor regression grade (TRG) was examined before and after treatment. [40]. TRG 1–3 indicated the absence of viable tumor cells or the presence of a maximum of 50% tumor cells, while TRG 4–5 indicated the presence of tumor cells in 50–100% of the histological specimen [40]. A statistically significant correlation between SUV and TRG was observed with an Area Under the Curve (AUC) value of 0.773 [40]. It was also possible to distinguish between respondents (TRG 1–3) and non-responders (TRG 4–5) by determining an ideal cut-off point of 41% ΔSUV [40]. The studies by Lubezky et al. [41] and Garcia Vincente et al. [42] are summarized in Table 2.
Some studies have reported unfavorable outcomes regarding the use of 18F-FDG PET in this context, indicating that it may not be a promising approach. Tan et al. conducted a study involving 14 patients who underwent neoadjuvant chemotherapy, aiming to directly compare the metabolic response assessed on 18F-FDG-PET/CT with the pathologic response observed after surgical resection [43]. Twenty-nine lesions out of the 34 that showed a full metabolic response on 18F-FDG-PET/CT (85%) were found to have viable tumor cells upon pathology assessment [43]. The authors therefore concluded that achieving a complete metabolic response on 18F-FDG-PET following neoadjuvant chemotherapy cannot be considered a reliable indicator of complete pathologic response [43]. The study by Bacigalupo et al. is summarized in Table 2 [44].
PET quantitative parameters also showed some promise in assessment of response to neoadjuvant chemotherapy in CRLM. As previously discussed in this review article, common quantitative PET parameters, SUVmax, MTV, and TLG, have shown predictive significance and may serve as possible prognostic markers to predict long-term outcomes in CRLM patients. Lastoria et al. [45] examined a total of 33 patients who were subjected to imaging before chemotherapy and after one cycle of neoadjuvant chemotherapy. When compared to the response evaluation criteria in solid tumors (RECIST) criteria that rely on CT imaging, both SUVmax and TLG exhibited enhanced predictive abilities in estimating PFS and OS [45]. The authors of another study investigated the prognostic efficacy of 18F-FDG PET-CT and dynamic contrast-enhanced MR imaging (DCE-MR imaging) before and after the completion of neoadjuvant treatment in a prospective study. The pre-treatment parameters observed on DCE-MR did not demonstrate any predictive value for OS and PFS [46]. There was no significant difference in the pre-treatment SUVmax between individuals who responded to treatment and those who did not. However, a decrease in SUVmax during the follow-up period was linked to a higher likelihood of experiencing improved PFS [46]. The studies by Mertens et al. [47] and Nishioka et al. [48] are summarized in Table 2.
Evaluating response to palliative chemotherapy
Monitoring the response to palliative chemotherapy is crucial as it allows for the identification of patients who were initially considered unresectable but may exhibit a positive response to chemotherapy [39]. Such patients can potentially become eligible for curative treatments aimed at local disease control [39]. In addition, response monitoring of palliative chemotherapy plays a valuable role in determining the most appropriate chemotherapeutic agent for individual patients [39]. Timely evaluation of treatment response allows physicians to make early decisions, such as switching to an alternative chemotherapy regimen or temporarily suspending chemotherapy if necessary [39]. In a prospective phase II trial, 61 patients receiving cetuximab and irinotecan for palliative care had their response assessed using positron emission tomography response criteria in solid tumors (PERCIST) criteria on 18F-FDG PET/CT and RECIST criteria on CECT [49]. After every four cycles of chemotherapy, imaging scans were performed immediately before the start of the treatment. Both CECT and 18F-FDG PET/CT examinations indicate that none of the patients experienced a full recovery. The HR for OS was found to be higher in responders (partial response/partial metabolic response) compared to non-responders (progressive disease/partial metabolic response) when evaluated using CT scans, as opposed to 18F-FDG PET/CT evaluation [49]. Among patients with KRAS mutations, none demonstrated a partial response, but 44% showed a partial metabolic response. In conclusion, there was a lack of agreement between morphologic and metabolic response, primarily due to a significant portion of patients transitioning from stable disease based on CT evaluation to partial metabolic response when assessed using 18F-FDG PET/CT [49].
Quantitative parameters derived from PET imaging have been employed not only in neoadjuvant chemotherapy but also in the context of palliative chemotherapy. Heijmen et al. [50] evaluated the effectiveness of 18F-FDG PET/CT imaging in predicting the response to systemic treatment in 39 patients, 35 of whom received palliative chemotherapy. Before and after three cycles of chemotherapy, the SUVmax and TLG on 18F-FDG PET/CT images were measured. It was observed that higher SUVmax and TLG values prior to treatment were associated with a shorter OS [50]. Furthermore, a decrease in SUVmax was observed after one week of chemotherapy, indicating a potential positive response to treatment [50]. The studies by Chiu KWH et al. [51] and Hyun Kim et al. [52] are summarized in Table 3.
Similar to neoadjuvant chemotherapy, some studies have reported less favorable outcomes when utilizing PET parameters to assess the effectiveness of palliative chemotherapy. Nemeth et al. [53] conducted a prospective study to investigate the relationship between metabolic changes observed on 18F-FDG PET/CT scans and PFS in 53 patients after two cycles of combined chemotherapy. Among the individuals included in the study, 10 patients underwent neoadjuvant chemotherapy before undergoing liver resection, whereas 43 patients received palliative chemotherapy. The assessment of metabolic response was performed using adapted European Organization for Research and Treatment of Cancer (EORTC criteria). Neither SUVmax and TLG nor the changes (Δ) in SUVmax and TLG were predictive of PFS or OS [53]. The study by Correa-Gallego et al. is summarized in Table 3 [54].
18F-FDG PET/CT prior to surgical intervention
Compelling evidence supporting the significance of pre-operative staging with 18F-FDG PET/CT arises from studies that demonstrate its clinical impact on the selection of patients for treatment of solitary hepatic metastasis [55]. One study [22] analyzed 100 individuals with CRC who underwent 18F-FDG PET pre-operative staging. Research findings suggest that the median 5-year OS rate after surgically removing CRLM varies between 12 and 41%. Typically, the median value reported using conventional imaging techniques is around 30% [55]. In another study, Fernandez et al. [56] showed that the use of 18F-FDG PET had an excellent 5-year OS rates for patients who have undergone surgery to remove liver metastases from colorectal cancer. 18F-FDG PET identified a distinct group of patients for whom the grade of the tumor is a highly significant factor in predicting their prognosis [56]. Ruers et al. conducted a study with 150 patients selected for surgical treatment to explore an alternative method of assessing the significance of pre-operative staging for hepatic metastasis resection in colorectal cancer [57]. These patients were randomly assigned to two groups: one group receiving CT imaging alone (n = 75) and the other group receiving both CT and 18F-FDG PET imaging (n = 75). The results revealed that the CT-only group had 34 cases (45%) of futile operations, while the group with 18F-FDG PET imaging had 21 cases (28%). In favor of incorporating 18F-FDG PET imaging, the study found a relative risk reduction of 38% (95% CI 4–60%; p = 0.042) for a futile operation [57]. Nevertheless, some institutions advocate employing MRI with liver-specific contrast agents for pre-surgical evaluation. For example, in a study the investigators assessed the utility of gadoxetic acid-enhanced liver MRI in pre-operative staging of colorectal cancer and its potential impact on the management of liver metastasis [58]. The findings revealed that gadoxetic acid-enhanced liver MRI detected more metastatic nodules in comparison to PET/CT, particularly for small nodules (< 2 cm). The discovery of these additional nodules prompted changes in the management plan for 43.8% (7/16) of the patients [58].
Evaluation of post-operative performance
The primary objectives of post-treatment diagnostic follow-up for CRLM are to detect residual tumors, monitor local tumor progression, detect newly developed metastases within the liver and identify any presence of disease outside the liver at an early stage [39]. According to the European Society for Medical Oncology (ESMO) guidelines, CECT is the frequently used imaging technique for the purpose of identifying new intrahepatic metastases and detecting any presence of disease outside the liver [39]. Although MRI may offer advantages in identifying early local tumor progression, it is comparatively less effective than CECT in detecting extrahepatic disease [39]. Using 18F-FDG PET/CT imaging has also provided valuable results by combining both anatomical and metabolic imaging modalities [59, 60]. A total of 107 patients who experienced recurrent CRLM after liver resection were evaluated by Vigano et al. [61] to compare the efficacy of 18F-FDG PET/CT versus CT or MR imaging. The sensitivity of CT for detecting local liver recurrences was 100%, while MR imaging exhibited a sensitivity of 96.7%. 18F-FDG PET/CT showed a comparable sensitivity of 96.7% for this purpose. In comparison to CT or MR imaging, 18F-FDG-PET-CT revealed an additional 24 malignant sites outside the liver. The 18F-FDG PET/CT results were used to change the treatment plan for 16 patients. Furthermore, in 15 patients, surgery was avoided due to the identification of extrahepatic disease solely detected through.18F-FDG PET/CT [61]
PET/CT in interventional oncology (ablation)
18F-FDG PET/CT before and after ablation
Limited colorectal liver disease can be effectively treated and potentially cured through surgical resection, leading to improved long-term survival rates in carefully chosen patients [62]. For some patients with CRLM, image-guided percutaneous ablation therapies have emerged as a promising and secure alternative [62]. Ablation induces localized tissue destruction and has progressively demonstrated long-lasting elimination of tumors [62]. Percutaneous thermal ablation (TA) such as radiofrequency or microwave ablation exhibits good rates of local tumor control in patients with minor liver volume disease who can be treated with adequate margins, with up to 55% survival at 5 years [63]. Despite the potential advantages that ablation offers, the limited utilization of TA for treating CRLM can be attributed to earlier reports indicating high rates of local tumor progression (LTP) [64]. An ideal ablation zone (AZ) should extend beyond the borders of the CRLM with minimum ablation margins (MM) of at least 10 mm [4]. This is based on the fact that most intrahepatic micro metastases are normally located 10 mm or less from the CRLM’s edge [4]. When local cure is the goal of the ablation, a minimum margin of 5 mm is regarded as the absolute minimum required [4]. In this situation, PET imaging can be helpful because it can offer useful details for the precise characterization of the targeted tumor and its borders. In a recent study, Zirakchian Zadeh et al. analyzed 190 CRLMs from 125 participants who were enrolled in two prospective clinical trials that utilized PET/CT-guided TA [4]. The CRLMs were categorized based on their visibility on pre-TA CT imaging, including detectable, non-detectable, and those with poor conspicuity. Additionally, the CRLMs were categorized based on their detectability and 18F-FDG-avidity on PET/CT imaging following the initial dose. Using a 3D volumetric approach, the study assessed the ablation margins surrounding the targeted CRLMs. The findings revealed that out of 190 CRLMs, 129 (67.9%) were detectable based on CT imaging alone, while 61 CRLMs (32.1%) were either undetectable or had poor conspicuity, making it difficult to accurately visualize and target them using CT alone [4] (Fig. 9). Consequently, in these tumors (32.1%), CT alone was not sufficient to define the requisite 5- and 10-mm margins. Only 4 CRLMs (2.1%) remained undetected or displayed poor 18F-FDG avidity when intra-procedural PET/CT images were obtained and evaluated (PET/CT fusion) [4] (Fig. 9). The study’s findings led the authors to conclude that incorporating PET imaging alongside non-contrast CT enhanced the detection of CRLMs for the purpose of ablation targeting. In addition, by employing this combined approach, the need for multiple intravenous contrast injections before and during the ablation process is eliminated since 18F-FDG is utilized to specifically target CRLMs [4].
Zirakchian Zadeh et al. conducted an analysis of 190 CRLMs from 125 participants enrolled in two prospective PET/CT guided ablation clinical trials. Out of the total 190 CRLMs that were analyzed, 129 CRLMs (67.9%) were visible and detectable using CT imaging alone. However, 61 CRLMs (32.1%), were either undetectable or showed poor conspicuity on CT, making it challenging to accurately identify and target them. Consequently, it was not possible to determine the theoretical 5- and 10-mm margins for these tumors using CT alone. When intra-procedural 18F-FDG-PET/CT images were acquired and examined (fused PET/CT), only 4 CRLMs (2.1%) remained undetectable or exhibited low 18F-FDG avidity. The right image is showing the example of poor performance of CT in detecting CRLMs before ablation. The below imaging is an example of a CRLM with low 18F-FDG avidity and 5 − and 10 − mm margins assessment for the target CRLM; under CC BY license
The utility of 18F-FDG PET in assessing treatment response following ablation is also evident. A study by Veit et al. [65] demonstrated that in post-RFA surveillance, 18F-FDG PET/CT is more accurate than CECT (65% vs. 44%). Sahin et al.’s retrospective cohort analysis investigated [66] the performance of 18F-FDG PET in 134 patients who underwent laparoscopic TA. In the study, subsequent post-ablation follow-up 18F-FDG PET/CT scans were completed in 82 patients with a total of 180 lesions at the surgeon’s or oncologist’s discretion. The timing of these scans varied. Among these patients, 72% had rising serum CEA levels. The results revealed that follow-up 18F-FDG PET/CT outperformed CECT in 11 out of 51 patients (22%) in terms of diagnosing local recurrence [66]. However, in 2 out of 51 patients (4%), follow-up 18F-FDG PET/CT was found to be inferior to CECT in detecting local recurrence [66]. The studies by Veit et al. [65], Cornelis and colleagues [67], Nielsen et al. [68] Kuehl et al. [69] and Liu et al. [70] are summarized in Table 4.
Quantitative 18F-FDG PET parameters in evaluation of ablation
Some studies used PET quantitative parameters in assessment of ablation. In a recent study, Zirakchian Zadeh et al. enrolled a group of 46 patients with a total of 55 CRLMs [71]. To assess the metabolic characteristics of each CRLM, measurements such as TLG and MTV were obtained using different PET segmentation methods applied to pre-ablation 18F-FDG PET scans. Based on the results the author concluded predicting local tumor progression in patients with CRLM who undergo microwave ablation can be achieved by analyzing volumetric PET parameters derived from immediate pre-ablation 18F-FDG PET scans [71] (Fig. 10). In another study by Cornelis et al. [72], they assessed the utility of immediate 18F-FDG PET/CT scans following TA in 39 patients who underwent 62 ablation procedures using the split-dose approach. The study aimed to determine the correlation between SUV ratios obtained from post-ablation 18F-FDG PET/CT scans and histopathological analysis of biopsied samples [72]. The PET/CT imaging process yielded SUVs, and the ratios of SUVs were computed using three-dimensional regions of interest positioned within the AZ as well as the adjacent normal liver tissue. The study found that the tumors with local recurrence had considerably higher SUV ratios than the tumors that responded well to treatment [72].
Zirakchian Zadeh et al. employed gradient-based techniques that utilize the image gradient between higher SUV values within tumors and lower SUV values in surrounding non-tumor tissues to delineate tumor boundaries. Additionally, Kaplan–Meier estimators were employed to assess survival based on volumetric PET metrics obtained from the gradient-based methodology, yielding significant log-rank p-values (< 0.001); https://doi.org/10.1007/s00270-023-03470-6; reused with permission
Utility of 18F-FDG PET/CT before and after radioembolization (RE)
Prognostic stratification
Several PET parameters, including SUVmax, MTV, and TLG appear to hold predictive significance in patients with CRLM undergoing RE treatment. Seraj et al. conducted a study with the objective of evaluating the prognostic significance of pre-treatment 18F-FDG PET in patients with CRLM who underwent Yttrium 90 (90Y) radioembolization [73]. The study focused on assessing global disease measures as potential prognostic indicators [73]. Active malignant liver lesions were identified and segmented using an adaptive thresholding method on PET scans. There was no observed correlation between pre-treatment conventional 18F-FDG PET parameters and PFS or OS. Pre-treatment volumetric characteristics, however, were found to be significant predictors of PFS and OS in the univariate Cox regression analyses [73]. In a similar study, researchers conducted a retrospective analysis involving 49 patients who had a total of 119 target CRLMs aiming to evaluate post-treatment outcomes [74]. The findings revealed that response assessment based on MTV and TLG exhibited a statistically significant correlation with the prediction of OS. On the other hand, response measured by SUVmax and SUVpeak, as well as the absence of disease progression according to RECIST criteria, did not demonstrate a significant association with prolonged OS [74]. The studies by Fendler WP, et al. [75], and Soydal et al. [76] are summarized in Table 5.
Imaging response criteria
The evaluation of imaging response criteria has been a common practice in assessing patients with CRLM. Zerizer et al. [77] conducted a study in 25 patients with 121 CRLM who underwent treatment with Yttrium-90 (90Y) RE comparing Choi, RECIST, and EORTC PET criteria. The researchers examined the correlation between imaging parameters and changes in tumor markers, as well as the 2-year PFS rates [77]. The findings revealed that a higher number of patients showed a partial response to therapy based on PET criteria as opposed to RECIST and Choi criteria. Additionally, a strong predictor of PFS was found to be the metabolic response seen on 18F-FDG PET/CT imaging, which demonstrated a substantial link with the normalization of tumor markers [77]. In another study, twenty-five individuals with 46 target lesions were included in a retrospective analysis [78]. Choi criteria, EORTC PET criteria, tumor attenuation criteria, and RECIST 1.1 criteria for evaluating treatment response and prediction of hepatic PFS after RE were used. The study revealed a statistically significant relationship between changes in SUVmax and changes in tumor attenuation, expressed as Hounsfield units [78]. Furthermore, Choi criteria, tumor attenuation, and EORTC PET assessments were found to be reliable indicators of hepatic PFS [78]. The studies by Sabet et al. [79], Jongen JM, et al. [80] and Sager et al. [81] are summarized in Table 5.
Counting microspheres and measuring activity in biopsy specimens
Naydenov et al., recently investigated a total of 86 core biopsy specimens obtained from 18 CRLMs immediately after trans-arterial RE (TARE) using either resin or glass microspheres [82]. Throughout the procedure, real-time 90Y PET/CT guidance was used. A high-resolution micro-CT scanner was also used to see the microspheres in some of the specimens, allowing the calibration of autoradiography images or direct quantification of 90Y activity [82]. The average doses delivered to the specimens were calculated by analyzing the activity concentrations measured in the specimens and the PET/CT scan data obtained at the biopsy needle tip’s location for all CRLMs. The average measured 90Y activity concentrations in the CRLM specimens at the time of infusion were determined to be 2.4 ± 4.0 MBq/mL. The biopsies exhibited higher levels of activity heterogeneity in comparison to PET imaging. The study’s findings lead the authors to draw the conclusion that it is both safe and practical to count microspheres and measure activity in biopsy specimens collected during TARE [82]. This method provides a high spatial resolution method for identifying the administered activity and its distribution inside the treated and biopsied liver tissue. The authors mentioned that a more precise correlation between histopathologic alterations and absorbed dose in the studied specimens can be accomplished by combining this method with 90Y PET/CT imaging [82].
Unexplained elevation of carcinoembryonic antigen (CEA) levels
Around 66% of individuals who experience a recurrence of CRC exhibit elevated levels of CEA in their blood. This elevation in CEA levels has been associated with a median period of 3–9 months as an advanced warning of the recurrence compared to anatomic imaging modalities [22]. Therefore, regular monitoring of serum CEA levels every 2–3 months for a minimum duration of 2 years following surgery has been recommended [83,84,85,86]. Implementing a rigorous follow-up protocol following primary curative treatment has the potential to identify a higher number of cancer relapses that can be effectively treated through curative resection [22]. Two separate meta-analyses have demonstrated that intensive follow-up strategies lead to improved OS and a reduction in absolute mortality by 9–13% [87, 88]. Although heightened levels of CEA in the blood can serve as a sign of recurrence, they do not offer insights into the precise location of the recurrence [22]. This poses a clinical dilemma for patients who exhibit rising serum CEA levels but do not show any detectable disease on morphological imaging. In certain instances, it has been observed that CEA can become positive following a PET scan. It is crucial to highlight that atypical serum CEA levels can also be detected in several non-malignant conditions, such as liver diseases, bowel diseases, smoking, and renal failure [83]. The issue with falsely elevated serum CEA levels is that it can result in unnecessary imaging procedures or even surgeries, which can lead to potential complications [22]. Numerous studies have shown the effectiveness of 18F-FDG PET in assessment of patients who experience an increase in serum CEA levels but do not have detectable lesions using conventional imaging methods [25, 89,90,91,92,93]. 18F-FDG PET has demonstrated a sensitivity ranging from 79 to 100% in detecting recurrence in patients without symptoms but with rising serum CEA levels and no aberrant findings on conventional diagnostic testing [22]. A suggested threshold of 10 ng/ml for serum CEA has been advised as a marker for 18F-FDG PET, with a reported specificity ranging from 70 to 84% in relation to tumor recurrence. The specificity has been reported to be between 50 and 83%, while the overall accuracy falls within the range of 74–93% [22]. Liu et al. [94] reported a significant difference in cumulative survival between patients with unexplained serum CEA levels exceeding 25 ng/ml and those with levels below 25 ng/ml. In another study, a second look laparotomy was performed on 28 patients who had rising serum CEA levels, and negative imaging results [95]. Biopsy confirmation of recurrence was obtained in 94% of the patients, and out of these recurrences, 38% were deemed unresectable [95]. PET scans accurately predicted unresectable disease in 90% of cases, while CEA scintigraphy scans did not provide any successful predictions. Regarding resectable disease, PET scans correctly predicted it in 81% of cases, whereas CEA scintigraphy scans had a significantly lower accuracy of only 13% [95]. SimÓ et al. showed among a cohort of 58 patients who exhibited an unexplained rise in serum CEA, 34 individuals (59%) experienced a change in their management plan following 18F-FDG PET. This included 18 patients (31%) who proceeded with curative resection and 16 patients (28%) who were recommended systemic chemotherapy as part of their treatment strategy [96] The authors concluded that 18F-FDG PET can be suggested as a suitable option for patients who show an inexplicable increase in serum CEA levels following primary curative treatment for colorectal carcinoma, as long as they are deemed medically suitable for salvage surgery [96].
Presence of kirsten rat sarcoma viral oncogene homologue (KRAS) mutation
Patients who have undergone surgical removal of CRLM and possess the KRAS mutation have been linked to decreased OS rates and a shorter period until the recurrence of the disease. A study involving 97 patients revealed that KRAS mutation emerged as a significant prognostic factor for the development of new liver metastases (P = 0.037) and peritoneal metastases (P = 0.015) based on multivariate analysis [97]. Additionally, the presence of KRAS mutation was identified as a significant prognostic factor for LTP following RFA of CRLM with margins between 1 and 5 mm. The statistical analysis revealed a significant association (P = 0.018), with an LTP rate of 80% (12 out of 15 cases) in patients with KRAS mutation, whereas the LTP rate was 41% (11 out of 27 cases) for those without the KRAS mutation (wild type) [97]. Several studies also have indicated a relationship between KRAS mutation and the uptake of 18F-FDG. In a recent investigation involving 23 patients who underwent PET/CT guided biopsies, a correlation was discovered between the standardized uptake value peak (SUVpeak) of 18F-FDG and the standardized uptake value lean body mass peak (SULpeak) and KRAS missense mutation in CRLM [98]. In a separate study, a retrospective analysis was performed on 55 CRLM that were detected using 18F-FDG PET/CT before undergoing surgical resection [99]. Upon analyzing the 55 tumors, no substantial correlation was found between SUVmax and KRAS status. However, when focusing solely on tumors larger than 10 mm to mitigate the partial-volume effect, a bias that affects the evaluation of small lesions in PET imaging, it was observed that the group with KRAS mutations exhibited significantly higher SUVmax values compared to the group with wild-type KRAS (8.3 ± 4.1 vs. 5.7 ± 2.4, respectively; P = 0.03) [99] (Fig. 11). Additionally, a multivariate analysis showed that SUVmax was statistically correlated with KRAS mutations (p = 0.04) [99].
a 78-year-old male patient presented with a liver metastasis measuring 23 mm in diameter. The metastasis had a mutated KRAS status, and on PET/CT scans, there was a notable and intense accumulation of 18F-FDG within the liver tumor (arrow), with a maximum standardized uptake value (SUVmax) of 8.3. b 61-year-old male patient also had a single liver metastasis measuring 27 mm in diameter. This metastasis, however, had a wild-type KRAS status. On PET/CT scans, there was a moderate accumulation of 18F-FDG in the tumor (arrow), with an SUVmax of 4.5. c Analysis of the SUVmax based on the KRAS status revealed that, among all liver tumors (n = 55), there was no significant difference in SUVmax between the mutated KRAS group and the wild-type KRAS group (6.3 ± 4.2 and 5.4 ± 2.6, respectively; P = 0.84). d However, among metastatic tumors larger than 10 mm (n = 42), the SUVmax was significantly higher in the mutated KRAS group compared to the wild-type KRAS group (8.3 ± 4.1 and 5.7 ± 2.4, respectively; P = 0.03). https://doi.org/10.2967/jnumed.115.160614; open access article
Novel PET radiotracers
The uptake of 18F-FDG can be increased not only in cancerous diseases but also in various non-cancerous conditions such as infections and inflammatory processes [100]. This heightened uptake can lead to false positive results, emphasizing the importance of considering other PET radiotracers in these cases. In recent years, several new PET tracers have been investigated for their potential use in patients with CRLM, yielding diverse outcomes. Recent research has highlighted the promising role of fibroblast-activation-protein inhibitors (FAPI) as a new PET tracer in the detection of various solid tumors. These studies have shown substantial potential for FAPI in both pre-clinical and clinical investigations [101]. In general, FAPI radiotracers have demonstrated a high sensitivity in detecting liver lesions, including both primary tumors and metastases (Figs. 12 and 13). The studies about the application of novel PET radiotracers are summarized in Table 6 [102,103,104,105,106].
Figure a is showing the fundamental mechanism behind Fibroblast Activation Protein Inhibitor (FAPI) PET. FAPI is employed to specifically focus on the group of cells located in the supportive tissue surrounding the tumor, known as cancer-associated fibroblasts. b Several published studies have compared the diagnostic performance of FAPI-PET and 18F-FDG -PET in detecting various types of cancer, particularly primary tumors. In general, FAPI radiotracers have shown a good sensitivity for detection of liver lesions, both primary and metastatic; https://doi.org/10.1148/radiol.220749, under CC BY license
A comparison was made between eight patients who had various types of tumors. These patients underwent both 68 Ga-FAPI PET and 18F-FDG PET imaging within a timeframe of less than one week. Primary tumors were represented by solid arrows, while metastasis lesions were indicated by dotted arrows. Overall, FAPI radiotracers have demonstrated a high level of sensitivity in detecting liver lesions, including both primary and metastatic ones. NPC stands for nasopharyngeal carcinoma. PMID: 35198057, open access
Radiomics
Radiomics analysis measures the assessment of multiple and imperceptible molecular characteristics that exist in diagnostic and therapeutic images [107]. The utilization of 18F-FDG PET/CT radiomics enables the detection of diverse disorders in a non-invasive and efficient manner [107]. By employing machine learning techniques, radiomics incorporates all available clinical and imaging features, assisting clinicians in making personalized treatment decisions and predicting patient outcomes [22]. Over the past few years, numerous studies have emerged with a particular emphasis on radiomics in metastatic colorectal cancer. However, only a limited number of these studies have investigated radiomic features in PET, and none of them specifically explored the application of radiomics in evaluating treatment response following local therapy [108, 109]. Ninety-nine individuals who had palliative chemotherapy participated in a retrospective analysis. The analysis of radiomic features obtained from PET imaging was the main focus of the work, with particular attention paid to three local intensity features, four morphological features, two intensity histogram features, and one intensity-volume histogram feature. These characteristics were linked with changes in lesion anatomy, treatment response, PFS, and OS [108]. The researchers discovered that the effects of treatment and future survival were inversely related to tumor volume, tumor heterogeneity, and non-sphericity. In another study, 52 patients with CRLM were examined to determine the viability of employing radiomic PET features as a predictive model. In the trial, a total of 41 radiomic characteristics were investigated [109]. According to the authors’ conclusions, the incorporation of one or more radiomic features along with SUV measures in a multivariate analysis can lead to a notable enhancement in prognostic accuracy.
PET/CT limitation
In general, achieving thorough patient preparation for PET/CT examination, which includes diet and activity restrictions, managing blood glucose levels in diabetic patients, and taking into account the impact of medications [110], can be challenging in certain patients. Additionally, PET’s limitation in low spatial resolution hampers the detection of small CRLM, an area where anatomical imaging like MRI excels. Moreover, advanced PET devices with new features, such as time of flight, have the potential to improve imaging accuracy. However, standardizing results across institutions might prove challenging due to variations introduced by these techniques. As discussed earlier in this manuscript, the non-specific nature of 18F-FDG underscores the necessity for more specific radiotracers. Furthermore, 18F-FDG PET/CT demonstrated a significant rate of false negative results in individuals with mucinous CRLM. [111]. Therefore, in cases where patients have a low percentage of viable tumor cells after chemotherapy, negative 18F-FDG PET/CT results should be interpreted with caution [111]
Conclusions
18F-FDG PET/CT has proven effective in detecting CRLM, but its low spatial resolution hinders detecting small lesions. It excels in disease stage assessment, treatment adjustments, prognostic stratification and monitoring responses to both neoadjuvant and palliative chemotherapy. Though some guidelines favor CECT before surgery for CRLM, PET/CT has shown equivalent efficacy. It is beneficial in ablation procedures and locating tumors when CEA levels spike. New concepts such as novel PET radiotracers and radiomics require more investigations. The primary limitations of 18F-FDG PET/CT are its low PET resolution, the non-specific attributes of the 18F-FDG radiotracer, and difficulties in identifying mucinous metastasis. Summary statement of this review article is summarized in Table 7.
References
Martin J et al (2020) Colorectal liver metastases: current management and future perspectives. World J Clin Oncol 11(10):761–808
Zhou H et al (2022) Colorectal liver metastasis: molecular mechanism and interventional therapy. Signal Transduct Target Ther 7(1):70
Akgül Ö et al (2014) Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 20(20):6113
Zirakchian Zadeh M et al (2022) Real-time split-dose PET/CT-guided ablation improves colorectal liver metastasis detection and ablation zone margin assessments without the need for repeated contrast injection. Cancers (Basel) 14(24):6253
Bandar A, Hussain M, Kim NK (2017) Current status and future perspectives on treatment of liver metastasis in colorectal cancer. Oncol Rep 37(5):2553–2564
Takahashi H, Berber E (2020) Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr 9(1):49
Schirrmeister H, Arslandemir C (2010) Chapter 24—diagnosis of skeletal metastases in malignant extraskeletal cancers. In: Heymann D (ed) Bone cancer. Academic Press, San Diego, pp 283–294
Hofman MS, Hicks RJ (2016) How we read oncologic FDG PET/CT. Cancer Imaging 16(1):35
Cipe G et al (2013) Routine use of positron-emission tomography/computed tomography for staging of primary colorectal cancer: does it affect clinical management? World J Surg Oncol 11(1):1–8
Deng J, Tang J, Shen N (2014) Meta-analysis of diagnosis of liver metastatic cancers: comparison of 18 FDG PET-CT and gadolinium-enhanced MRI. J Med Imaging Radiat Oncol 58(5):532–537
Rohren EM et al (2002) The role of F-18 FDG positron emission tomography in preoperative assessment of the liver in patients being considered for curative resection of hepatic metastases from colorectal cancer. Clin Nucl Med 27(8):550–555
Ruers T et al (2002) Value of positron emission tomography with [F-18] fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol 20(2):388–395
Sahani DV et al (2005) Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium–enhanced liver MRI and whole-body FDG PET. Am J Roentgenol 185(1):239–246
D’souza MM et al (2009) Prospective evaluation of CECT and 18F-FDG-PET/CT in detection of hepatic metastases. Nucl Med Commun 30(2):117–125
Tahtabasi M, Erturk SM, Basak M (2021) Comparison of MRI and 18F-FDG PET/CT in the liver metastases of gastrointestinal and pancreaticobiliary tumors. Sisli Etfal Hastan Tip Bul 55(1):12–17
Yang M et al (2003) Comparison of MR and PET imaging for the evaluation of liver metastases. J Magn Reson Imaging 17(3):343–349
Beiderwellen K et al (2015) Accuracy of [18F]FDG PET/MRI for the detection of liver metastases. PLoS ONE 10(9):e0137285
Sivesgaard K et al (2018) Diagnostic accuracy of CE-CT, MRI and FDG PET/CT for detecting colorectal cancer liver metastases in patients considered eligible for hepatic resection and/or local ablation. Eur Radiol 28(11):4735–4747
Tsili AC et al (2020) Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: a meta-analysis. Acta Radiol 62(3):302–312
Zirakchian Zadeh M et al (2020) Correlation of whole-bone marrow dual-time-point (18)F-FDG, as measured by a CT-based method of PET/CT quantification, with response to treatment in newly diagnosed multiple myeloma patients. Am J Nucl Med Mol Imaging 10(5):257–264
Dirisamer A et al (2008) Dual-time-point FDG-PET/CT for the detection of hepatic metastases. Mol Imaging Biol 10(6):335–340
de Geus-Oei LF et al (2006) FDG-PET in colorectal cancer. Cancer Imaging 6(Special Issue A):S71-81
Staib L et al (2000) Is 18F-fluorodeoxyglucose positron emission tomography in recurrent colorectal cancer a contribution to surgical decision making? Am J Surg 180(1):1–5
Imdahl A et al (2000) Impact of 18 F-FDG-positron emission tomography for decision making in colorectal cancer recurrences. Langenbecks Arch Surg 385:129–134
Kalff V et al (2002) The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med 43(4):492–499
Huebner RH et al (2000) A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 41(7):1177–1189
Wiering B et al (2005) The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 104(12):2658–2670
Zhou N et al (2021) The Value of (18)F-FDG PET/CT and abdominal PET/MRI as a one-stop protocol in patients with potentially resectable colorectal liver metastases. Front Oncol 11:714948
Grassetto G et al (2010) Additional value of FDG-PET/CT in management of “solitary” liver metastases: preliminary results of a prospective multicenter study. Mol Imag Biol 12:139–144
Zirakchian Zadeh M et al (2022) A review of different methods used for quantification and assessment of FDG-PET/CT in multiple myeloma. Nucl Med Commun 43(4):378–391
Im HJ et al (2018) Current methods to define metabolic tumor volume in positron emission tomography: which one is better? Nucl Med Mol Imaging 52(1):5–15
Xia Q et al (2015) Prognostic significance of 18FDG PET/CT in colorectal cancer patients with liver metastases: a meta-analysis. Cancer Imaging 15(1):19
de Geus-Oei LF et al (2006) FDG-PET for prediction of survival of patients with metastatic colorectal carcinoma. Ann Oncol 17(11):1650–1655
Zwezerijnen GJC et al (2023) Reproducibility of [18F]FDG PET/CT liver SUV as reference or normalisation factor. Eur J Nucl Med Mol Imaging 50(2):486–493
Grut H et al (2022) Metabolic tumor volume predicts long-term survival after transplantation for unresectable colorectal liver metastases: 15 years of experience from the SECA study. Ann Nucl Med 36(12):1073–1081
Zalom M et al (2012) FDG PET/CT as a prognostic test After 90Y radioembolization in patients with metastatic hepatic disease. Clin Nucl Med 37(9):862
Higashi K et al (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43(1):39–45
Vansteenkiste JF et al (1999) Prognostic importance of the standardized uptake value on 18F-fluoro-2-deoxy-glucose–positron emission tomography scan in non–small-cell lung cancer: an analysis of 125 cases. J Clin Oncol 17(10):3201–3206
Bijlstra OD et al (2022) The value of (18)F-FDG-PET-CT imaging in treatment evaluation of colorectal liver metastases: a systematic review. Diagnostics (Basel) 12(3):715
Burger IA et al (2013) Correlation between therapy response assessment using FDG PET/CT and histopathologic tumor regression grade in hepatic metastasis of colorectal carcinoma after neoadjuvant therapy. Ann Nucl Med 27:177–183
Lubezky N et al (2007) The role and limitations of 18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 11:472–478
Vicente AMG et al (2013) Response assessment of colorectal liver metastases with contrast enhanced CT/18F-FDG PET. Eur J Radiol 82(6):e255–e261
Tan MC et al (2007) Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg 11:1112–1119
Bacigalupo L et al (2010) Assessment of liver metastases from colorectal adenocarcinoma following chemotherapy: SPIO-MRI versus FDG-PET/CT. Radiol Med (Torino) 115(7):1087–1100
Lastoria S et al (2013) Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab. J Nucl Med 54(12):2062–2069
De Bruyne S et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106(12):1926–1933
Mertens J et al (2013) Standardized added metabolic activity (SAM) IN 18F-FDG PET assessment of treatment response in colorectal liver metastases. Eur J Nucl Med Mol Imaging 40(8):1214–1222
Nishioka Y et al (2018) Fluorine-18-fluorodeoxyglucose positron emission tomography as an objective substitute for CT morphologic response criteria in patients undergoing chemotherapy for colorectal liver metastases. Abdom Radiol 43(5):1152–1158
Skougaard K et al (2014) CT versus FDG-PET/CT response evaluation in patients with metastatic colorectal cancer treated with irinotecan and cetuximab. Cancer Med 3(5):1294–1301
Heijmen L et al (2015) Multimodality imaging to predict response to systemic treatment in patients with advanced colorectal cancer. PLoS ONE 10(4):e0120823
Chiu KWH et al (2018) Long-term outcomes and recurrence pattern of 18F-FDG PET-CT complete metabolic response in the first-line treatment of metastatic colorectal cancer: a lesion-based and patient-based analysis. BMC Cancer 18(1):776
Kim DH et al (2012) Intermodality comparison between 3D perfusion CT and 18F-FDG PET/CT imaging for predicting early tumor response in patients with liver metastasis after chemotherapy: preliminary results of a prospective study. Eur J Radiol 81(11):3542–3550
Nemeth Z et al (2020) Metabolic parameters as predictors for progression free and overall survival of patients with metastatic colorectal cancer. Pathol Oncol Res 26(4):2683–2691
Correa-Gallego C et al (2015) Prospective evaluation of 18F-fluorodeoxyglucose positron emission tomography in patients receiving hepatic arterial and systemic chemotherapy for unresectable colorectal liver metastases. HPB (Oxford) 17(7):644–650
Sacks A et al (2011) Value of PET/CT in the management of liver metastases, part 1. Am J Roentgenol 197(2):W256–W259
Fernandez FG et al (2004) Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 240(3):438
Ruers TJ et al (2009) Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG PET: a randomized study. J Nucl Med 50(7):1036–1041
Oh JW et al (2016) Does the gadoxetic acid-enhanced liver MRI Impact on the treatment of patients with colorectal cancer? Comparison study with 18F-FDG PET/CT. Biomed Res Int 2016:8412071
Van Cutsem E et al (2016) Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers 8(9):81
Lin Y-M et al (2021) Image-guided ablation for colorectal liver metastasis: principles, current evidence, and the path forward. Cancers 13(16):3926
Viganò L et al (2017) Positron emission tomography-computed tomography for patients with recurrent colorectal liver metastases: impact on restaging and treatment planning. Ann Surg Oncol 24:1029–1036
Petre EN, Sofocleous C (2017) Thermal ablation in the management of colorectal cancer patients with oligometastatic liver disease. Visc Med 33(1):62–68
Solbiati L et al (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Shady W et al (2016) Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 278(2):601–611
Veit P et al (2006) Detection of residual tumor after radiofrequency ablation of liver metastasis with dual-modality PET/CT: initial results. Eur Radiol 16:80–87
Sahin DA et al (2012) The utility of PET/CT in the management of patients with colorectal liver metastases undergoing laparascopic radiofrequency thermal ablation. Ann Surg Oncol 19:850–855
Cornelis F et al (2016) 18F-FDG PET/CT is an immediate imaging biomarker of treatment success after liver metastasis ablation. J Nucl Med 57(7):1052–1057
Nielsen K et al (2013) PET-CT after radiofrequency ablation of colorectal liver metastases: suggestions for timing and image interpretation. Eur J Radiol 82(12):2169–2175
Kuehl H et al (2008) Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: initial results. Eur J Radiol 67(2):362–371
Liu Z-y et al (2010) Early PET/CT after radiofrequency ablation in colorectal cancer liver metastases: is it useful? Chin Med J 123(13):1690–1694
Zirakchian Zadeh M et al (2023) Gradient-based volumetric PET parameters on immediate pre-ablation FDG-PET predict local tumor progression in patients with colorectal liver metastasis treated by microwave ablation. CardioVasc Int Radiol. https://doi.org/10.1007/s00270-023-03470-6
Cornelis FH et al (2018) Immediate postablation 18F-FDG injection and corresponding SUV are surrogate biomarkers of local tumor progression after thermal ablation of colorectal carcinoma liver metastases. J Nucl Med 59(9):1360–1365
Seraj SM et al (2019) Pretreatment volumetric parameters of FDG-PET predict the survival after Yttrium-90 radio-embolization in metastatic liver disease. Am J Nucl Med Mol Imaging 9(5):248–254
Shady W et al (2016) Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after 90Y radioembolization of colorectal liver metastases: a comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol 85(6):1224–1231
Fendler WP et al (2013) Validation of several SUV-based parameters derived from 18F-FDG PET for prediction of survival after SIRT of hepatic metastases from colorectal cancer. J Nucl Med 54(8):1202–1208
Soydal C et al (2013) The prognostic value of quantitative parameters of 18F-FDG PET/CT in the evaluation of response to internal radiation therapy with yttrium-90 in patients with liver metastases of colorectal cancer. Nucl Med Commun 34(5):501–506
Zerizer I et al (2012) The role of early 18 F-FDG PET/CT in prediction of progression-free survival after 90 Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 39:1391–1399
Shady W et al (2016) Surrogate imaging biomarkers of response of colorectal liver metastases after salvage radioembolization using 90Y-loaded resin microspheres. AJR Am J Roentgenolo 207(3):661
Sabet A et al (2015) Early post-treatment FDG PET predicts survival after 90 Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging 42:370–376
Jongen JM et al (2018) Anatomic versus metabolic tumor response assessment after radioembolization treatment. J Vasc Interventional Radiol 29(2):244–532
Sager S et al (2019) Comparison of PERCIST and RECIST criteria for evaluation of therapy response after yttrium-90 microsphere therapy in patients with hepatocellular carcinoma and those with metastatic colorectal carcinoma. Nucl Med Commun 40(5):461–468
Naydenov N et al (2023) 90Y activity quantification in PET/CT-guided biopsy specimens from colorectal hepatic metastases immediately after trans-arterial radioembolization using micro-CT and autoradiography. J Vasc Interventional Radiol. https://doi.org/10.1016/j.jvir.2023.05.022
Liu F-Y et al (2005) Utility of 2-fluoro-2-deoxy-D-glucose positron emission tomography in managing patients of colorectal cancer with unexplained carcinoembryonic antigen elevation at different levels. Dis Colon Rectum 48:1900–1912
Tutt A et al (2004) The role of positron emission tomography in the management of colorectal cancer. Colorectal Dis 6(1):2–9
Bast RC Jr et al (2001) 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 19(6):1865–1878
Pfister DG, Benson AB III, Somerfield MR (2004) Surveillance strategies after curative treatment of colorectal cancer. New Engl J Med 350(23):2375–2382
Rosen M et al (1998) Follow-up of colorectal cancer: a meta-analysis. Dis Colon Rectum 41:1116–1126
Renehan AG et al (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 324(7341):813
Flamen P et al (1999) Additional value of whole-body positron emission tomography with fluorine-18–2-fluoro-2-deoxy-D-glucose in recurrent colorectal cancer. J Clin Oncol 17(3):894
Flanagan FL et al (1998) Utility of FDG-PET for investigating unexplained plasma CEA elevation in patients with colorectal cancer. Ann Surg 227(3):319
Maldonado A et al (2000) 16. FDG-PET in the detection of recurrence in colorectal cancer based on rising CEA level. Experience in 72 Patients. Clin Positron Imaging 3(4):170
Flamen P et al (2001) Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET). Eur J Cancer 37(7):862–869
Libutti SK et al (2001) A prospective study of 2-[18 F] fluoro-2-deoxy-D-glucose/positron emission tomography scan, 99m Tc-labeled arcitumomab (CEA-scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol 8:779–786
Liu F-Y et al (2005) Utility of 2-Fluoro-2-Deoxy-D-Glucose positron emission tomography in managing patients of colorectal cancer with unexplained carcinoembryonic antigen elevation at different levels. Dis Colon Rectum 48(10):1900–1912
Libutti SK et al (2001) A prospective study of 2-[18F] Fluoro-2-Deoxy-D-Glucose/Positron emission tomography scan, 99mTc-Labeled Arcitumomab (CEA-Scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol 8(10):779–786
SimÓ M et al (2002) FDG-PET improves the management of patients with suspected recurrence of colorectal cancer. Nucl Med Commun 23(10):975
Shady W et al (2017) Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget 8(39):66117–66127
Kirov A et al (2019) KRAS missense mutation effects on the 18F-FDG uptake of colorectal adenocarcinoma metastases in the liver. J Nucl Med 60(supplement 1):213
Kawada K et al (2015) Relationship between 18F-FDG PET/CT scans and KRAS mutations in metastatic colorectal cancer. J Nucl Med 56(9):1322–1327
Kung BT et al (2019) An update on the role of (18)F-FDG-PET/CT in major infectious and inflammatory diseases. Am J Nucl Med Mol Imaging 9(6):255–273
Siveke JT (2018) Fibroblast-activating protein: targeting the roots of the tumor microenvironment. J Nucl Med 59(9):1412–1414
Pang Y et al (2021) Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology 298(2):393–402
Şahin E et al (2021) Comparison of (68)Ga-DOTA-FAPI and (18)FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur J Radiol 142:109867
Cuda TJ et al (2020) PET Imaging quantifying (68)Ga-PSMA-11 uptake in metastatic colorectal cancer. J Nucl Med 61(11):1576–1579
Hong YS et al (2013) 3′-Deoxy-3′-18F-fluorothymidine PET for the early prediction of response to leucovorin, 5-fluorouracil, and oxaliplatin therapy in patients with metastatic colorectal cancer. J Nucl Med 54(8):1209–1216
Mogensen MB et al (2017) FLT-PET for early response evaluation of colorectal cancer patients with liver metastases: a prospective study. EJNMMI Res 7(1):56
Anan N, Zainon R, Tamal M (2022) A review on advances in (18)F-FDG PET/CT radiomics standardisation and application in lung disease management. Insights Imaging 13(1):22
van Helden EJ et al (2018) Radiomics analysis of pre-treatment [18F]FDG PET/CT for patients with metastatic colorectal cancer undergoing palliative systemic treatment. Eur J Nucl Med Mol Imaging 45(13):2307–2317
Rahmim A et al (2019) Prognostic modeling for patients with colorectal liver metastases incorporating FDG PET radiomic features. Eur J Radiol 113:101–109
Surasi DS et al (2014) 18F-FDG PET and PET/CT patient preparation: a review of the literature. J Nucl Med Technol 42(1):5–13
Borello A et al (2021) Diagnostic performance of the FDG-PET/CT in patients with resected mucinous colorectal liver metastases. Surgeon 19(5):e140–e145
Funding
There was no funding for this review article.
Author information
Authors and Affiliations
Contributions
MZZ wrote the main manuscript text and prepared the figures.
Corresponding author
Ethics declarations
Conflict of interest
MZZ declares that he has no conflict of interest for this publication.
Informed consent
N/A to this review article. Large Language Model (ChatGPT) used solely for enhancing the language accuracy of the manuscript, not for the design or creating of ideas.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zirakchian Zadeh, M. PET/CT in assessment of colorectal liver metastases: a comprehensive review with emphasis on 18F-FDG. Clin Exp Metastasis 40, 465–491 (2023). https://doi.org/10.1007/s10585-023-10231-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-023-10231-9