Abstract
The ionic conductivity and dielectric properties of the solid nanocomposite polymer electrolytes formed by dispersing a low particle-sized TiO2 ceramic filler in a poly (ethylene oxide) (PEO)-AgNO3 matrix are presented and discussed. The solid nanocomposite polymer electrolytes are prepared by hot press method. The optimum conducting solid polymer electrolyte of polymer PEO and salt AgNO3 is used as host matrix and TiO2 as filler. From the filler concentration-dependent conductivity study, the maximum ionic conductivity at room temperature is obtained for 10 wt% of TiO2. The real part of impedance (Z′) and imaginary part of impedance (Z″) are analyzed using an LCR meter. The dielectric properties of the highest conducting solid polymer electrolyte are analyzed using dielectric permittivity (ε′), dielectric loss (ε″), loss tangent (tan δ), real part of the electric modulus (M′), and imaginary part of the electric modulus (M″). It is observed that the dielectric constant (ε′) increases sharply towards the lower frequencies due to the electrode polarization effect. The maxima of the loss tangent (tan δ) shift towards higher frequencies with increasing temperature. The peaks observed in the imaginary part of the electric modulus (M″) due to conductivity relaxation shows that the material is ionic conductor. The enhancement in ionic conductivity is observed when nanosized TiO2 is added into the solid polymer electrolyte.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes (SPE) have received considerable attention for the last few decades due to its potential applications in solid-state batteries, fuel cells, supercapacitors, sensors, etc. [1, 2]. Solid polymer electrolytes are synthesized dissolving the alkali metal salt in host polymer [3]. These electrolytes have many attractive advantages over its liquid counterparts such as they are leakage free, safe, light weight, and easy to synthesize in desirable thickness and area [4]. Poly (ethylene oxide) (PEO)-based solid polymer electrolyte is the most widely investigated system as PEO has low lattice energy, high solvating power for alkali metal salts, good electrochemical stability, and suitable structure to support fast ion transport [5]. PEO contains Lewis base ether oxygen, which coordinates with the cations and thus help to dissolve the salts [6, 7]. The main drawback of PEO-based solid polymer electrolyte is its low ionic conductivity at room temperature due to the presence of high crystalline phase concentration below the melting temperature which does not satisfy the general requirements to use in batteries or in other practical electrochemical devices [8, 9]. It has been revealed that the pure PEO has a crystalline phase and an amorphous phase with dissolved salt. The ion conduction in PEO takes place primarily in the amorphous phase [10] and the phase diagram is affected by many factors, such as the concentration of salt, salt species, preparation method, and temperature. In order to enhance their ionic conductivity and to improve their thermal, mechanical, and electrochemical properties, several modifications in the structure of these polymer electrolytes have been realized [11]. Various techniques have been developed to reduce the crystallinity of PEO and enhance the ionic conductivity at room temperature. The most common approach has been the addition of liquid plasticizers such as ethylene carbonate (EC), propylene carbonate (PC), and dibutyl phthalate (DBP) [12, 13]. However, incorporation of liquid plasticizers leads the deterioration of the electrolytes’ mechanical properties and increases its reactivity towards the metal anode. Therefore, the liquid plasticizer-added polymeric electrolytes are not promising for use in metal batteries because of their limited processability and high reactivity, both of them resulting in serious problems in terms of battery manufacturing, cyclability, and safety. Thus, the ideal achievement in electrolytes would be the enhancement of the low-temperature ionic conductivity by modifications which avoid any liquid contamination [14]. To overcome these problems, a technique developed for solid polymer electrolyte is the dispersion of nanosized ceramic filler particles such as SiO2, TiO2, Al2O3, Fe2O3, SnO2, ZnO, etc. into the polymer electrolyte system [15, 16]. The enhancement in ionic conductivity with the addition of ceramic fillers has been reported by many authors. Wieczorek et al. suggested an active role of the surface groups of the ceramic particles in modification of local structure and substantial increase in ionic conductivity at room temperature [17, 18].
In the present work, study on ionic conductivity and dielectric properties of PEO-based solid nanocomposite polymer electrolyte dispersed with nanosized TiO2 is reported. Enhancement in ionic conductivity and dielectric properties is observed with the addition of TiO2 nanofiller in the highest conducting solid polymer electrolyte 90PEO:10AgNO3.
Experimental details
Pure PEO (MW 6 × 105, Aldrich, USA); fine powder of AgNO3 (AR grade purity 99.9%, Aldrich, USA); and nanosized filler TiO2 (<100 nm, Aldrich, USA) are used for the preparation of solid nanocomposite polymer electrolytes. The solid polymer electrolyte 90PEO:10AgNO3 which is identified as maximum conducting composition [19] is taken as host matrix and different wt% of nanosized TiO2 (<100 nm) as filler for the preparation of solid polymer nanocomposite electrolytes. All the electrolytes are prepared by hot press method. The hot press method has many advantages over the traditional solution cast method [20]. In hot press method, all the materials in appropriate wt% are mixed for 20 min. This mixture is then heated for 20 min at about 70 °C (~melting point of PEO), a slurry is obtained, then it is pressed between two stainless steel block in about 2 t pressure, and thin films are prepared. The thickness of the samples was 0.078, 0.079, 0.072, 0.065, 0.070, and 0.067 cm, corresponding to 5, 10, 15, 20, 25, and 30 wt% of TiO2, respectively. The area of each sample was 1.327 cm2. LCR meter (HIOKI 3532-50 LCR HiTester, Japan) is used for impedance spectroscopy study within a broad frequency range of 100 Hz–5 MHz and temperature range of 300–323 K. Two silver metal pellets are used as electrode in Ag│polymer electrolyte│Ag cell for all the electrochemical characterization of the polymer electrolytes. The area of each silver electrode was 1.327 cm2. The highest conducting composition 90 (90PEO:10AgNO3):TiO2 is obtained after filler-dependent ionic conductivity studies.
Results and discussion
X-ray diffraction analysis
The structure of the polymer electrolytes is investigated by XRD analysis. The XRD patterns of pure PEO, the optimum conducting solid polymer electrolyte 90PEO:10AgNO3 and solid nanocomposite polymer electrolyte 90 (90PEO:10AgNO3):TiO2 are shown in Fig. 1. The XRD pattern of pure PEO shows the intense and sharp diffraction peaks at 2θ values of ~19° and 23°. It is clear that the intensity of the diffraction peaks substantially decreases with the addition of AgNO3 salt into polymer due to transformation of crystalline phase into amorphous phase of PEO. The XRD pattern of solid nanocomposite polymer electrolyte shows that with the addition of TiO2 filler, the intensity of peak further decreases. The decrease in intensity of the peaks confirms the complexation of salt and filler with polymer [21, 22]. The enhancement in ionic conductivity of solid nanocomposite polymer electrolyte can be explained on the basis of the relative decrease in PEO crystallinity with the addition of nanofiller TiO2. The appearance of new peaks with the addition of filler in the polymer-salt complex is clearly seen in the XRD pattern which confirms the formation of nanocomposite polymer electrolyte having multiphase characteristics [23].
FTIR analysis
The complexation of salt AgNO3 and filler TiO2 with polymer PEO is also confirmed by FTIR spectra. The FTIR spectra of (a) pure PEO, (b) 90PEO:10AgNO3, and (c) 90 (90PEO:10AgNO3):10TiO2 are shown in Fig. 2. In the spectra of pure PEO, the C–O–C stretching mode is divided into three peaks at 1155, 1084, and 1063 cm−1 and the peaks corresponding to CH2 bending vibrations at 1344 and 1360 cm−1 which confirmed the existence of PEO crystallinity. The appearance of the peaks at 844, 964, and 2893 cm−1 are assigned to CH2 wagging, CH2 twisting, and CH2 stretching modes, respectively [24]. The broad band between the 2947 and 2805 cm−1 and two narrow band between 2739 and 2693 cm−1 are inherent band of asymmetric C–H stretching of PEO [25]. The shifting of peak from 1155 cm−1 (pure PEO) to lower wave number 1145 cm−1 (PEO-salt complex) and peak from 964 cm−1 (pure PEO) to 962 cm−1 (PEO-salt complex) confirms the complexation of salt in polymer [26]. With the addition of filler TiO2 in PEO-salt complex, some peaks around 600–750 cm−1 corresponding to pure PEO almost disappeared, which indicates the complexation of filler into PEO-salt matrix [4].
SEM analysis
The scanning electron microscope (SEM) is widely used to analyze the surface morphology of the polymer electrolytes. The SEM micrographs of solid polymer electrolyte 90PEO:10AgNO3 and solid nanocomposite polymer electrolyte 90 (90PEO:10AgNO3):TiO2 samples are shown in Fig. 3a, b and c, d, respectively, at two different magnifications. The sample images are also shown as an inset in the corresponding SEM micrograph.
The surface morphology and crystallinity of the polymer electrolyte plays an important role on the corresponding ionic conductivity and polymer electrolyte with smooth surface has better ionic conductivity [27]. The SEM micrograph for solid polymer electrolyte shows a rough surface which has several crystalline domains. It is clear from the figure that after the addition of filler TiO2, the surface morphology of the polymer-salt matrix is changed from rough to smooth which suggests the uniform distribution of filler in polymer-salt matrix. The smooth surface morphology of nanocomposite polymer electrolyte is closely related to the reduction in the PEO crystalline nature [28] which helps in ion transport in polymer electrolyte in order to improve ionic conductivity [29].
DSC analysis
The DSC thermograms of solid polymer electrolyte 90PEO:10AgNO3 and solid nanocomposite polymer electrolyte 90 (90PEO:10AgNO3):10TiO2 is shown in Fig. 4. The sharp endothermic peaks indicate the melting temperature (T m ) of solid polymer electrolyte 90PEO:10AgNO3 and solid nanocomposite polymer electrolyte 90 (90PEO:10AgNO3):10TiO2. There is a reduction in melting temperature (T m ) when nanofiller is added to the solid polymer electrolyte. The reduction in the melting temperature (T m ) is due to the enhancement of polymer segmental motion as well as increase in the amorphous nature induced by the filler-polymer interaction of polymer electrolyte. Hence, the movement of ions in the polymer matrix is increased, resulting in the increase of conductivity [30]. This is supported by obtained value of the ionic conductivity of solid nanocomposite polymer electrolyte. Thus, DSC results confirm the decrease in crystallinity or the increase in flexibility of the polymer chains [26].
TGA analysis
Thermogravimetric analysis (TGA) is the widely used method for thermal characterization of the materials. It is based on the measurement of mass loss of the material with increasing temperature in a controlled atmosphere. The TGA curves of pure PEO, 90PEO:10AgNO3, and 90(90PEO:10AgNO3):TiO2 are shown in Fig. 5. For pure PEO, the initial weight loss of about 2.8% up to 150 °C is observed due to complete dehydration. The weight loss gradually increases up to 12% at 280 °C. The weight loss of about 80% is observed from 280 to 400 °C which shows the decomposition of the material. There is no major weight loss observed for PEO pure after 400 °C. Hence, a total weight loss of pure PEO is about 92%. The thermal stability of PEO has been enhanced slightly, when it is complexed with AgNO3 salt. For 90PEO:10AgNO3 polymer electrolyte, the initial weight loss of about 2% up to 150 °C is observed due to complete removal of moisture. The remarkable weight loss is observed after 315 °C. The total weight loss of about 87% is observed for 90PEO:10AgNO3 polymer electrolyte. From the TGA curve of nanocomposite polymer electrolyte 90 (90PEO:10AgNO3):TiO2 the remarkable weight loss is observed between 310 and 450 °C and a total weight loss of about 84% is obtained. It is clear that the addition of salt and filler in polymer enhances the thermal stability of the material.
Complex impedance analysis
The complex impedance plot (Nyquist plot) of solid nanocomposite polymer electrolytes with different wt% of TiO2 at temperature 300 K is shown in Fig. 6. The plot consists of two well-defined regions, the compressed semicircle at higher frequency and spike at lower frequency. The high frequency semicircle is due to the parallel combination of bulk resistance (R b ) and the bulk capacitance while the spike at lower frequency is due to ion diffusion in polymer electrolyte [31, 32]. The intercept of the semicircle on x-axis gives the value of bulk resistance (R b ). It is clear from the figure that, as the wt% of TiO2 increases, the bulk resistance decreases and it is the minimum for 10 wt% of TiO2. On further increase in filler wt%, now bulk resistance increases and it again attain the low value at 25 wt%. With again increase in filler wt%, now bulk resistance increases. This is in accordance with the double percolation model suggested by Chandra and Laxmi [33]. The minimum value of bulk resistance for 10 wt% of TiO2 shows that the charge carriers and mobility of ions is higher at this wt%. The increase in bulk resistance with increase in filler wt% is due to the ion-ion aggregation [34].
Figure 7 shows the complex impedance plots of polymer electrolytes corresponding to 5, 10, 15, 20, 25, and 30 wt% of TiO2 at a temperature range of 300–323 K. It is clear that the intercepts of the semicircle on the real axis shift towards the origin with increase in temperature of each composition. Hence, the bulk resistance decreases with an increase in temperature for all the composition. This is because the number of charge carrier increases with increase in temperature [34].
The variation of the real part of impedance (Z′) with frequency of highest conducting polymer electrolyte is shown in Fig. 8a. The real impedance Z′ decreases with increase in frequency and temperature. The magnitude of Z′ decreases with increase in temperature at lower frequencies which merges at higher frequency region. This is due to the release of space charge. The responsible factor for enhancement in ionic conductivity with increase in temperature is the reduction of barrier potential of the electrolyte with increase in temperature [35]. The complete merger of all the Z′ spectra is observed above a particular frequency which is due to the removal of the interfacial polarization at higher frequencies. The shifting of Z′ plateau indicates the presence of frequency relaxation process in the material [34].
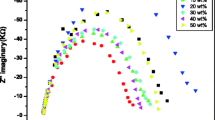
Figure 8b (inset) shows the variation of the imaginary part of impedance Z″ with frequency at different temperatures. The magnitude of Z″ is the maximum at a particular frequency showing a peak-like curve at all temperatures. The position of peaks shifts towards a higher frequency side with an increase in temperature. The broadening of peaks with increase in temperature suggests that there is a spread of relaxation time [36]. The significant widening of peaks (in the x-axis of Fig. 8b) on increasing temperature suggests the presence of a temperature-dependent relaxation process in the material. The relaxation species may be possibly due to ions at low temperature and the presence of defects at higher temperature. The decrease in peak position with increase in temperature is due to the formation of extra conductive pathways for ions in polymer electrolyte [19].
Ionic conductivity and activation energy measurements
The bulk resistance R b obtained from the complex impedance plot is used to determine the ionic conductivity of the solid nanocomposite polymer electrolytes. The ionic conductivity is calculated using the relation:
where l is the thickness of the sample, R b is the bulk resistance of the sample, and A is the area of the sample.
The obtained values of ionic conductivities of polymer electrolytes corresponding to 5, 10, 15, 20, 25, and 30 wt% of TiO2 are shown in Fig. 9. It can be seen that the ionic conductivity increases on increasing the wt% of TiO2. The highest value of ionic conductivity (σ ~ 1.1 × 10−6 S/cm) is obtained for 10 wt% of TiO2. On further increase in filler wt% the decrease in ionic conductivity is observed. The increase in conductivity could be due to the presence of filler facilitating the path for ionic transport and polymer segmental motion [37]. The decrease in ionic conductivity on increasing filler concentration could be explained in a way that excessive fillers in the solid polymer electrolyte may lead to ion pairs and ion aggregation inhibiting the ionic conduction and slowing its mobility [38, 39] in an amorphous phase. Again, small increase in ionic conductivity is observed for 25 wt% of TiO2. The existence of two conductivity maxima has been observed in many nanocomposite polymer electrolytes and has been explained on the basis of two percolation model which is suggested by Chandra and Laxmi [33]. It has been proposed that the two kinds of conductivity maxima are possibly due to transport mechanism operative in these systems. According to this model, the first maxima is possibly due to the dissociation of ion aggregates and/or undissociated salt, which result in the generation of free ion carriers as a consequence of the addition of nano-sized TiO2 particles. The second conductivity maxima as well as the conductivity variation are related to the well known two-phase composite effect which can be explained on the basis of space charge and/or percolation model [40]. The enhancement in ionic conductivity is observed when nanosized filler TiO2 is added to the optimum conducting solid polymer electrolyte 90 PEO:10AgNO3 which has ionic conductivity σ ~ 4.5 × 10−7 S/cm reported in our previous work [19].
Figure 10 shows the Arrhenius plot (log σ vs. 1000/T plot) of solid nanocomposite polymer electrolytes corresponding to 5, 10, 15, 20, 25, and 30 wt% of TiO2. The temperature dependence of the ionic conductivity of nanocomposite polymer electrolytes with different wt% of TiO2 shows that the ionic conductivity is thermally activated with increasing temperature. Therefore, conductivity increases with increase in temperature and follows Arrhenius relation. The activation energy (E a ) which is the combination of defect formation energy and migration energy is evaluated from the slop of the conductivity versus 1/T plot and the obtained values are plotted in Fig. 11. The lowest value of activation energy 0.33 eV is obtained for 10 wt% of TiO2, i.e., 90(90PEO:10AgNO3):10TiO2 which is the maximum conducting solid nanocomposite polymer electrolyte. On further increase in filler wt%, now activation energy increases. The maximum activation energy is 0.50 eV for 30 wt% of TiO2 which is due to the aggregation of ion as the filler content increases. Thus, the number of mobile charge carriers decreases. The minimum value of activation energy of 10 to percent of filler indicates that the mobility of ions is very high for this composition. This effect is due to the well-known mechanism that the addition of filler increases the conductivity by reducing the crystalline phase of PEO chains in the host polymer and hence providing the extra conducting pathway for Ag+ ions to travel on the surface of the TiO2 through Lewis acid base interactions between the filler and the polymer chains [41, 42].
Dielectric constant and dielectric loss analysis
The dielectric constant (ε′) represents the amount of dipole alignment in a given volume. The dielectric loss (ε″) represents the energy loss of ion motion and dipole alignment when the polarity of the electric field reverses rapidly, and it is related to the electrical conductivity of the materials [43]. The dielectric constant (ε′) and dielectric loss (ε″) is determined using the following formula:
where C p is the parallel capacitance of the sample, t is the thickness of the sample, ε 0 is the permittivity of free space, σ is ionic conductivity, ω is the angular frequency (=2πf), and A is the area of the sample. Figure 12a, b (inset) shows the variation of dielectric constant (ε′) and dielectric loss (ε″) with frequency, respectively. It is clear from the figure that both ε′ and ε″ shows similar behavior with frequency at various temperatures. The absence of relaxation peaks in both the figures indicates that the increase in conductivity was primarily due to increase in the number of mobile ions [44]. The dielectric constant ε′ is higher at lower frequencies. The higher value of ε′ at lower frequencies is due to the orientation of polar groups, ions, and space charge polarization at these frequencies [45]. The value of ε′ decreases continuously with increasing frequency and reaches a constant value which is due to the electrical relaxation or inability of dipoles to rotate rapidly hence leading to a lag between frequency of oscillating dipoles and applied field [46, 47]. Similarly, the dielectric loss ε″ due to electrode/electrolyte polarization and free charge motion in the system [48], and it attains lower values at higher frequencies. This is due to that at lower frequencies, there was enough time for the charges to build up at the interface before the reversal of electric field and contributed to a large apparent value of ε″. As the frequency increases, there was no time for the buildup of charges at the interface, but only for the buildup of charges at the boundaries of conducting species in the material and at the ends of conducting paths [48, 49]. It can be seen from figure that with increase in temperature both ε′ and ε″ increases due to the increase in the concentration of free ions. However, increase in temperature increases the degree of redissociation process of ion pairs and asserts the increase in the concentration of free charge carriers at the interface. High temperature may prevent the ion aggregation and ion clusters in the polymer matrix and thus increases the equivalent capacitance by forming space charge region, exhibiting high dielectric constant [19, 50].
Loss tangent analysis
The dielectric relaxation parameter of the solid nanocomposite polymer electrolytes can be obtained from the study of loss tangent (tan δ) as a function of frequency. The loss tangent or dissipation factor (tan δ) is the ratio of dielectric loss (ε″) to dielectric constant (ε′) of the material. It is given by
The loss tangent measures the ratio of the electric energy lost to the energy stored in an applied electric field. The variation of loss tangent with frequency is shown in Fig. 13. It is clear that tan δ increases with increase in frequency and reaches a maximum value at a particular frequency. With further increase in frequency, now it decreases rapidly. The peaks obtained at different temperature corresponds to the electrode polarization frequency. The relaxation time is given by τ = 1/(2πf) where f is the frequency corresponding to the relaxation peak. It is clear from the formula that the peak frequency is inversely proportional to relaxation time of the ions. The longer the relaxation time, the lower the ionic conductivity [51]. The change in peak positions is observed with increase in temperature. The peak position shifts towards the higher frequency with increase in temperature of the polymer electrolyte. This leads to a decrease in relaxation time, hence increase in ionic conductivity. The maximum value of loss tangent at the lower frequency end of the high temperature region is due to thermally generated defects in polymer electrolyte [52].
Electric modulus analysis
Further analysis of the dielectric behavior of the solid nanocomposite polymer electrolyte is done with the electric modulus formulation. Electric modulus spectra are a powerful tool to show the conductivity relaxation process and ion hopping mechanism in polymer electrolyte [53]. The variation of the real part of the modulus (M′) and imaginary part of the modulus (M″) with frequency at different temperatures is shown in Figs. 14 and 15, respectively. The values of M′ and M″ are obtained by the following formula:
It can be seen from Fig. 14 that the real modulus M′ increases with increase in frequency. At lower frequencies, the M′ value approaches to zero, which indicates that the electrode polarization is negligible. The appearance of the long tail in the low-frequency region is due to the capacitance effect at the electrodes [54]. In the spectra of M′, all the curves demonstrate superposition features in the all temperature regime at high frequency which shows that the modulus spectral formalisms exhibit temperature-independent properties. This indicates the excellent electrochemical stability of the material [50].
Figure 15 represents the variation of the imaginary part of electric modulus with frequency for the solid nanocomposite polymer electrolyte at temperature range 300–323 K. The imaginary modulus M″ increases on increasing frequency are related to the long-range random hopping of ions in the material which reaches to a maximum value at a particular frequency. The peaks observed at this frequency in imaginary modulus spectra are attributed to the ionic conductivity relaxation process in polymer electrolyte where long-range motion is restricted to caged motion. The peaks in M″ spectra show that the material is ionic conductor [55, 56]. The peaks are broader and asymmetric which predict the non-Debye behavior. Further increase in frequency, M″ decreases which shows that the charge carriers confined within the potential well being mobile over short distance [57, 58]. The peak position shifts towards higher frequency side as the temperature of the polymer electrolyte increases. This is because as the temperature increases, the movement of the charge carriers becomes faster due to an increase in the flexibility of the material, leading to a decrease in the relaxation time. Hence, a shift is observed in the M″ peak towards the higher frequency side with an increase in temperature. This behavior suggests that the relaxation is thermally activated in which hopping process of charge carriers dominate intrinsically [59, 60]. The peaks are broader and asymmetric as compared to that predicted by Debye for relaxation phenomenon. The frequency corresponding to M″max gives the relaxation time τ from the condition 2πf max τ = 1 [60, 61].
Conclusion
The effect of nanosized filler TiO2 on ionic conductivity and dielectric properties of PEO-based polymer electrolyte prepared by hot press method is studied within the temperature range 300–323 K and reported. The ionic conductivity of highest conducting solid nanocomposite polymer electrolyte 90(90PEO:10AgNO3):10TiO2 is σ ~ 1.1 × 10−6 S/cm. The enhancement in ionic conductivity is observed when nanosized TiO2 is added into the solid polymer electrolyte 90PEO:10AgNO3 which has the ionic conductivity σ ~ 4.5 × 10−7 S/cm. The similar results were obtained with addition of Fe2O3 filler into solid polymer electrolyte 90PEO:10AgNO3. The highest conductivity of σ ~ 2.2 × 10−6 S/cm was observed for 10 wt% of Fe2O3, reported in our earlier work. The sharp decrease in dielectric constant (ε′) with increase in frequency is observed, which is due to the polarization effect at electrode-electrolyte interface. The spectra of loss tangent (tan δ) show the decrease in relaxation time (τ) with an increase in frequency and the peak position shifts towards the higher frequency with increase in temperature. The real part of the electric modulus (M′) increases sharply at higher frequency and approaches to zero at lower frequencies. The peaks in the spectra of the imaginary part of the electric modulus (M″) confirm the conductivity relaxation in the material which shows that the material is ionic conductor. The peak position shifts towards the higher frequency with increase in temperature.
References
Money BK, Hariharan K, Swenson J (2014) Relation between structural and conductivity relaxation in PEO and PEO based electrolytes. Solid State Ionics 262:785–789
Deraman SK, Mohamed NS, Subban RHY (2013) Conductivity and electrochemical studies on polymer electrolytes based on poly vinyl (chloride)—ammonium triflate-ionic liquid for proton battery. Int J Electrochem Sci 8:1459–1468
Agrawal RC, Pandey GP (2008) Solid polymer electrolyte: materials designing and all-solid-state battery applications: an overview. J Phys D Appl Phys 41:223001 (18 pp)
Kumar Y, Hashmi SA, Pandey GP (2011a) Lithium ion transport and ion–polymer interaction in PEO based polymer electrolyte plasticized with ionic liquid. Solid State Ionics 201:73–80
Noor SAM, Ahmad A, Talib IA, Rahman MYA (2010) Morphology, chemical interaction, and conductivityof a PEO-ENR50 based on solid polymer electrolyte. Ionics 16:161–170
Kim S, Park SJ (2009) Preparation and electrochemical properties of composite polymer electrolytes containing 1-ethyl-3-methylimidazolium tetrafluoroborate salts. Electrochim Acta 54:3775–3780
Kumar KN, Kang M, Sivaiah K, Ravi M, Ratnakaram YC (2016) Enhanced electrical properties of polyethylene oxide (PEO) + polyvinylpyrrolidone (PVP): Li+ blended polymer electrolyte films with addition of Ag nanofiller. Ionics 22:815–825
Liu HK, Wang GX, Guo ZP, Wang JZ, Konstantinov K (2007) The impact of nanomaterials on Li-ion rechargeable batteries. J New Mat Electrochem 10:101–104
Ghosh A, Kofinas P (2008) PEO based block copolymer as solid state lithium nattery electrolyte. ECS Trans 11:131–137
Watanabe M, Nagano S, Sanui K, Ogata N (1986) Ionic conductivity of network polymers from poly(ethylene oxide) containing lithium perchlorate. Polym J 18:809–817
Fahmi EM, Ahmad A, Nazeri NNM, Hamzah H, Razali H, Rahman MYA (2012) Effect of LiBF4 salt concentration on the properties of poly (ethylene oxide)-based composite polymer electrolyte. Int J Electrochem Sci 7:5798–5804
Xue Z, Dan He D, Xie X Poly (ethylene oxide)-based electrolytes for lithium-ion batteries. J of Mater Chem A 3:19218–19253
Fenton DE, Parker JM, Wright PV (1973) Complexes of alkali metal ions with poly (ethylene oxide). Polymer 14:589
Kumer B, Scanlon LG (1994) Polymer-ceramic composite electrolyte. J Power Sources 52:261–268
Kumar B, Rodrigues SJ, Koka S (2002) The crystalline to amorphous transition in PEO-based composite electrolytes: role of lithium salts. Electrochim Acta 47:4125–4131
Pitawala HMJC, Dissanayake MAKL, Seneviratne VA (2007) Combined effect of Al2O3 nano-fillers and EC plasticizer on ionic conductivity enhancement in the solid polymer electrolyte (PEO)9 LiTf. Solid State Ionics 178:885–888
Scrosati B, Croce F, Persi L (2000) Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J. Electrochemical Society 147:1718–1721
Wieczorek W, Florjancyk Z, Stevens JR (1995) Composite polyether based solid electrolytes. Electrochim Acta 40:2251–2258
Verma ML, Sahu HD (2015) Ionic conductivity and dielectric behavior of PEO-based silver ion conducting nanocomposite polymer electrolytes. Ionics 21:3223–3231
Verma ML, Minakshi M, Singh NK (2014a) Synthesis and characterization of solid polymer electrolyte based on activated carbon for solid state capacitor. Electrochim Acta 137:497–503
Polu AR, Rhee HW (2016) Effect of TiO2 nanoparticles on structural, thermal, mechanical and ionic conductivity studies of PEO12–LiTDI solid polymer electrolyte. J Ind Eng Chem 37:347–353
Johan MR, Ting LM (2011) Structural, thermal and electrical properties of nano manganese-composite polymer electrolytes. Int J Electrochem Sci 6:4737–4748
Mohapatra SR, Thakur AK, Choudhary RNP (2008) Studies on PEO-based sodium ion conducting composite polymer films. Ionics 14:255–262
Polu AR, Rhee HW (2015) Nanocomposite solid polymer electrolytes based on poly(ethylene oxide)/POSS-PEG (n = 13.3) hybrid nanoparticles for lithium ion batteries. J Ind Eng Chem 31:323–329
Kumar KK, Ravi M, Pavani Y, Bhavani S et al (2011b) Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionicconductivity and optical energy band gaps. Physica B 406:1706–1712
Karuppasamy K, Antony R, Thanikaikarasan S et al (2013) Combined effect of nanochitosan and succinonitrile on structural, mechanical, thermal, and electrochemical properties of plasticized nanocomposite polymer electrolytes (PNCPE) for lithium batteries. Ionics 19:747–755
Angulakshmi N, Yoo DJ et al (2013) MgAl2SiO6-incorporated poly (ethylene oxide)-based electrolytes for all-solid-state lithium batteries. Ionics DOI. doi:10.1007/s11581-013-0985-z
Prabakaran P, Manimuthu RP, Gurusamy S (2016) Influence of barium titanate nanofiller on PEO/PVdF-HFP blend-based polymer electrolyte membrane for Li-battery applications. J Solid State Electrochem DOI. doi:10.1007/s10008-016-3477-z
Singh NK, Verma ML, Minakshi M (2015) PEO nanocomposite polymer electrolyte for solid state symmetric capacitors. Bull Mater Sci 38:1577–1588
Karuppasamy K, Thanikaikarasan S, Antony R et al (2012) Effect of nanochitosan on electrochemical, interfacial and thermal properties of composite solid polymer electrolytes. Ionics. doi:10.1007/s11581-012-0678-z
Genova FKM, Selvasekarapandian S, Karthikeyan S, Vijaya N et al (2015) Lithium ion-conducting blend polymer electrolyte based on PVA-PAN doped with lithium nitrate. Polym-Plast Technol Eng. doi:10.1080/03602559.2015.1050523
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible andall-solid-state paper like polymer supercapacitors. Nano Lett 10:4025–4031
Laxmi N, Chandra S (2001) Proton conducting composites of heteropolyacid hydrates (phosphomolybdic and phosphotungstic acids) dispersed with insulating Al2O3. Phys Status Solidi (a) 186:395
Verma ML, Minakshi M, Singh NK (2014b) Structural and electrochemical properties of nanocomposite polymer electrolyte for electrochemical devices. Ind Eng Chem Res. doi:10.1021/ie502615w
Xu D, Sridhar V, Mahapatra SP, Kim JK (2009) Dielectric properties of exfoliated graphite reinforced flouroelastomer composites. J Appl Polym Sci 111:1358–1368
Al-Saleh MH, Al-Anid HK, Husain YA, El-Ghanem HM, Jawad S (2013) Impedance characteristics and conductivity of CNT/ABS nanocomposites. J Phys D Appl Phys 46:385305 (8pp)
Maier J (2004) Ionic transport in nano-sized systems. Solid State Ionics 175:7–12
Sharma JP, Sekhon SS (2007) Nanodispersed polymer gel electrolytes: conductivity modification with the addition of PMMA and fumed silica. Solid State Ionics 178:439–445
Saikia D, Chen-Yang YW, Chen YT, Li YK, Lin SI (2009) LiNMR spectroscopy and ion conduction mechanism of composite gel polymer electrolyte: a comparative study with variation of salt and plasticizer with filler. Electrochim Acta 54:1218–1227
Agrawal RC, Gupta RK (1999) Superionic solids: composite electrolyte phase—an overview. J Mater Sci 34:1131–1162
Croce F, Persi L, Scrosati B, Serraino-Fiory F, Plichta E, Hendrickson MA (2001) Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim Acta 46:2457–2461
Chung SH, Wang Y, Persi L, Croce F, Greenbaum SG, Scrosati B, Plichta E (2001) Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J Power Sources 97:644
Ravi M, Song S, Gu K, Tang J, Zhang Z (2015) Electrical properties of biodegradable poly(ε-caprolactone): lithium thiocyanate complexed polymer electrolyte films. Mater Sci Eng B 195:74–83
Shyly PM, Karuppasamy K, Linda T, Thiravetyan P, Balakumar S et al (2012) Ionic conductivity and dielectric studies of chitin nanofiber (CNF) incorporated PMMA based polymer electrolytes. IOSR J Appl Phys 1:47–51
Zamri SFM, Latif FA et al (2014) Ionic conductivity and dielectric properties of LiBF4 doped PMMA/ENR 50 filled acid modified SiO2 electrolytes. Procedia Technol 15:850–856
Tripathi SK, Gupta A, Jain A, Kumari M (2013) Electrochemical studies on nanocomposite polymer electrolytes. Indian J Pure & Appl Phys 51:358–361
Reicha FM, El-Hiti M, El-Sonabati AZ, Diab MA (1991) Conducting polymers. V. Electrical conductivity of polymer complexes of bis-2,6-diaminopyridinesulphoxide-copper halides. J. Phys. D. Appl Phys 24:369
Aziz SB (2013) Li+ ion conduction mechanism in poly (ecaprolactone)- based polymer electrolyte. Iran Polym J 22:877–883
Nithya H, Selvasekarapandian S, Kumar DA et al (2011) Thermal and dielectric studies of polymer electrolyte based on P(ECH-EO). Mater Chem Phys 126:404–408
Liew CW, Ramesh S, Durairaj R (2012) Impact of low viscosity ionic liquid on PMMA–PVC–LiTFSI polymer electrolytes based on AC -impedance, dielectric behavior, and HATR–FTIR characteristics. J Mater Res 27:2996–3004
Choudhary S, Sengwa RJ (2011) Dielectric relaxation spectroscopy and ion conduction in poly (ethylene oxide)-blend saltsmontmorillonite nanocomposite electrolytes. Indian J Pure Appl Phys 49:204–213
Kumar TV, Chary AS, Bhardwaj S, Awasthi AM, Reddy SN (2013) Dielectric relaxation, ionic conduction and complex impedance studies on NaNO3 fast ion conductor. Int J Mater Sci Appl 2:173–178
Gurusiddappa J, Madhuri W, Suvarna RP, Dasan KP (2016) Conductivity and dielectric behavior of polyethylene oxide-lithium perchlorate solid polymer electrolyte films. Indian Journal of Advances in Chemical Science 4:14–19
Sikkanthar S, Karthikeyan S, Selvasekarapandian S et al (2016) Structural, electrical conductivity, and transport analysis of PAN–NH4Cl polymer electrolyte system. Ionics 22:1085–1094
Kumar M, Srivastava N (2014) Conductivity and dielectric investigation of NH4I-doped synthesized polymer electrolyte system. Ionics. doi:10.1007/s11581-014-1294-x
Ramlli MA, Isa MIN (2014) Conductivity study of carboxyl methyl cellulose solid biopolymer electrolytes (SBE) doped with ammonium fluoride. Res J Recent Sci 3:59–66
Aziz SB, Abidin ZHZ, Arof AK (2010) Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosansilver triflate electrolyte membrane. Express Polym Lett 4:300–310
Dutta A, Sinha TP, Jena P, Adak S (2008) Ac conductivity and dielectric relaxation in ionically conducting soda-lime-silicate glasses. J Non-Cryst Solids 354:3952–3957
Kumar M, Tiwari T, Chauhan JK, Srivastava N (2014) Erratum on ‘Understanding the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped Zwitterionic polymer system’. Mater Res Express 1:049601
Pradhan DK, Choudhary RNP, Samantaray BK (2009) Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater Chem Phys 115:557–561
Raghu S, Archana K, Sharanappa C, Ganesh S, Devendrappa H (2015) The physical and chemical properties of gamma ray irradiated polymer electrolyte films. J. Non-Cryst Solids 426:55–62
Acknowledgments
We gratefully acknowledge the kind support of the management of Shri Shankaracharya Technical Campus (SSTC). Helpful discussions with Prof. R C Agrawal (School of Studies in Physics Pt. RSU, Raipur, Chattishgarh, India) and Dr. Manickam Minakshi (School of Engineering and Information Technology, Murdoch University, Australia) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, M.L., Sahu, H.D. Study on ionic conductivity and dielectric properties of PEO-based solid nanocomposite polymer electrolytes. Ionics 23, 2339–2350 (2017). https://doi.org/10.1007/s11581-017-2063-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2063-4