Abstract
Solid nanocomposite polymer electrolytes based on polyethylene oxide (PEO), AgNO3 as salt, and nanosized Fe2O3 (less than 50 nm size) as filler are prepared by hot press method. In (100-x) PEO:xAgNO3 system (where x = 5 ≤ x ≤ 50 wt%), the solid polymer electrolyte 90PEO:10AgNO3 gives highest ionic conductivity. This composition is further used as host matrix and Fe2O3 as filler for the preparation of solid nanocomposite polymer electrolytes (100-x) (90PEO:10AgNO3):xFe2O3 (where x = 5 ≤ x ≤ 30 wt%). The real impedance (Z') and imaginary impedance (Z") of the samples are analysed using LCR meter. The maximum ionic conductivity is observed for 10 wt% of filler Fe2O3. The optimum conducting composition 90(90PEO:10AgNO3):10Fe2O3 is used for further study. The dielectric response of the samples is analysed using dielectric constant (ε'), dielectric loss (ε"), loss tangent (tanδ), and real and imaginary part of electric modulus (M' and M"). The ionic conductivity and dielectric response of the solid nanocomposite polymer electrolytes are studied within the frequency range of 100 Hz–5 MHz and within the temperature range of 300–323 K. It is observed that the dielectric constant rises sharply towards low frequencies due to electrode polarization effects. The maxima of the loss tangent (tan δ) shift towards higher frequencies with increasing temperature. The enhancement in ionic conductivity is observed when nanosized Fe2O3 filler is added into the solid polymer electrolyte.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The solid polymer electrolytes (SPEs) are emerging as a new class of technologically important materials suitable to use for compact, light weight, high energy density energy storage devices [1–4]. The solid polymer electrolyte is defined as water-free system with an ion conducting phase formed by dissolved salts in a host polymer matrix [5–7]. The advantages of solid electrolyte in devices are that there is no need of separator as a component and there won’t be any leakage which is the advantage over liquid electrolyte [8]. In the current work, polyethylene oxide (PEO) based on high molecular weight is used as polymer backbone with AgNO3 salt to provide sufficiently high ionic conductivity [9, 10]. However, the ionic conductivities of PEO-based polymer electrolytes are still very low due to its largely crystalline in nature [4, 11]. Pure PEO has crystalline phase and an amorphous phase with dissolved salt. It has been revealed that the ion conduction takes place primarily in the amorphous phase [12, 13], and the phase diagram is affected by many factors, such as the salt species, preparation method, concentration, and temperature. In order to enhance their ionic conductivity and to improve their thermal, mechanical, and electrochemical properties, several modifications in the structure of these polymer electrolytes have been realized [12]. To enhance the ionic conductivity of solid polymer electrolytes, numerous research works were carried out over a decade. In this content, the incorporation of various fillers like Al2O3, CeO2, TiO2, and SiO2 into SPE was reported [14–19]. These additives have interaction between surface groups on the polymer chain which reduces the degree of PEO crystallinity [2] and enhances the transport properties, the resistance to crystallization, and the stability of the electrode-electrolyte interface of the PEO-based electrolyte [13]. PEO is widely used for the preparation of solid nanocomposite polymer electrolytes due to its ability to solvate a wide variety of salts through interaction of its ether oxygens with cations [20], and also PEO structure supports fast ion transport. Unfortunately, the working temperature of widely studied PEO-based polymer electrolytes is usually limited within 100 °C due to low melting point (65 °C) of PEO, still they attracted immense interest because of their high chemical stability, leak-proof, flexibility, low cost, and high ionic conductivity. Various methods have been applied to maintain their flexibility and mechanical stability over a wide temperature range [21].
In the current work, solid polymer electrolyte (90PEO:10AgNO3), which is identified as the optimum conducting composition in polymer electrolytes (100-x) PEO:xAgNO3, is taken as host matrix and nanosized Fe2O3 (<50 nm) as a filler for the preparation of solid nanocomposite polymer electrolytes (100-x) (90PEO:10AgNO3):xFe2O3. These electrolytes are synthesized by hot press method. The highest conducting composition is obtained for 10 wt% Fe2O3. The aims of this study were to use Fe2O3 as filler and to investigate its effect on ionic conductivity and dielectric properties of the solid polymer electrolyte since Fe2O3 as filler in polymer electrolyte is not much investigated.
Experimental
Pure PEO (MW 6 × 105, Aldrich, USA), fine powder of AgNO3 (AR grade purity 99.9 %, Aldrich, USA), and nanosized Fe2O3 (<50 nm, Aldrich, USA) are used for the preparation of solid nanocomposite polymer electrolytes. The solid polymer electrolytes (100-x) PEO:xAgNO3 where 05 ≤ x ≤ 50 (in wt%) are prepared by hot press method. The materials are mixed for 20 min and then heated for 20 min at 70 °C (~melting point of PEO). Slurry is obtained, and then it is pressed between two stainless steel blocks in about 2 t pressure. The detailed and advantages related to hot pressing technique over solution cast method have been given elsewhere [22]. The thickness of the samples was 0.066, 0.067, 0.065, 0.068, 0.058, 0.063, and 0.061 cm corresponding to 5, 10, 15, 20, 30, 40, and 50 wt% of AgNO3. The area of each samples was 1.327 cm2. LCR-meter (HIOKI 3532–50 LCR HiTester, Japan) is used for impedance spectroscopy study within a temperature range 300–323 K and frequency range 100 Hz–5 MHz. Two silver electrodes are used while taking observations. From the salt concentration-dependent conductivity studies of these samples at room temperature, the optimum conducting composition is identified. The optimum conducting composition is further used as host matrix and nanosized Fe2O3 (<50 nm) as filler for the preparation of polymer nanocomposite electrolytes (100-x) (90PEO:10AgNO3):xFe2O3 (where x = 5 ≤ x ≤ 30 wt%). The solid nanocomposite polymer electrolytes are prepared by hot press method. The thickness of the samples was 0.075, 0.078, 0.079, 0.072, 0.071, and 0.066 cm corresponding to 5, 10, 15, 20, 25, and 30 wt% of Fe2O3 filler. The area of each samples was 1.327 cm2. The highest conducting composition 90(90PEO:10AgNO3):10 Fe2O3 is obtain after filler-dependent ionic conductivity studies.
The dielectric constant (ε’) and dielectric loss (ε”) are determined using relation:
where C p is the parallel capacitance of the sample, t is the thickness of the sample, ε 0 is the permittivity of free space, σ is ionic conductivity, ω is the angular frequency (=2πf), and A is the area of the sample [23].
Real electric modulus M′ and imaginary electric modulus M″ are given [23–25] as:
Result and discussion
Complex impedance analysis
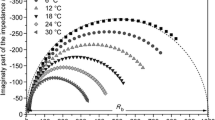
Electrochemical impedance spectroscopy (EIS) technique is commonly used in analysis of electrolytes where ionic conduction strongly predominates [26]. The complex impedance plot of solid polymer electrolyte (100-x) PEO:xAgNO3 at various concentration of salt AgNO3 is shown in Figs. 1 and 2. The complex impedance plot of the nanocomposite polymer electrolytes (100-x) (90PEO:10AgNO3):xFe2O3 at various concentrations of Fe2O3 as filler is shown in Fig. 3a and of the optimum conducting composition 90(90PEO:10AgNO3):10Fe2O3 at different temperatures is shown in Fig. 3b. Typical plot consists of the high frequency distorted semicircle representing the parallel combination of bulk resistance and capacitance which could be due to the bulk conductivity of the solid polymer electrolytes [5, 27] and the low frequency inclined spike (non-vertical) like region attributed to the ion diffusion in polymer electrolyte [28]. The low frequency tail indicates the capacitive nature of the interface, and the absence of electronic conductivity while the distorted semicircle is represents the grain boundary effects in the sample as reported by Scrosati et al. [5]. The intercepts of semicircles on real axis (x-axis) give the value of bulk resistance (R b) of the electrolytes.
In Fig. 1, it can be seen that initially the value of the bulk resistance (R b) decreases on increasing the wt% of AgNO3 and R b is minimum for 10 wt%. This could be interpreted, based on the previous works [29, 30], in a way that the number of charge carriers and the mobility of ions are higher and hence the obtained lower bulk resistance R b for 10 wt% of AgNO3 and on further increase in wt% of AgNO3, the bulk resistance increases which is mainly due to ion–ion interaction leads to aggregation of ions species so that ion clusters will form hence the number of charge carrier decreases [31]. Solid polymer electrolyte films beyond 50 wt% appeared brittle and less stable [32]. In Fig. 3a, it can be seen that initially the value of the bulk resistance R b decreases with increase in wt% of Fe2O3 and reaches at minimum for 10 wt% of Fe2O3. On further increase in wt% of Fe2O3, now R b increases up to 20 wt% of Fe2O3 and again decreases at 25 wt% which is explained on the basis of two percolation models, suggested by Laxmi and Chandra [33]. After 25 wt% of Fe2O3, now bulk resistance increases. The increase in bulk resistance is mainly due to ion–ion interaction leads to aggregation of ions species so that ion clusters will form, hence the number of charge carrier decreases [31]. The minimum bulk resistance at 10 wt% of Fe2O3 indicates that the number of charge carriers and the mobility of ions are higher for 10 wt% of Fe2O3. Figure 3b shows the variation of complex impedance on increasing temperature for optimum conducting solid nanocomposite polymer electrolyte. It is clear that, with increase in temperature, the intersection of the impedance plot on the real axis (Z') shifts towards the origin reflecting a decrease in the bulk resistance R b of the sample [2].
Figure 4a shows the variation of real part of impedance (Z') with frequency at different temperatures. The values of Z' decrease with rise in frequency and temperature. The magnitude of Z' decreases on increasing temperature at the lower frequencies which merges in the higher frequency region irrespective of temperature. This nature may be due to the release of space charge. The reduction in barrier properties of the materials with rise in temperature may be a responsible factor for enhancement of ionic conductivity of the materials at higher frequencies [34, 35]. In higher frequency region, complete merger of Z' plot above a certain fixed frequency is observed. At high frequency Z', value of each temperature coincides implying that the interfacial polarization is removed. The shift in Z' plateau indicates the existence of frequency relaxation process in the material. The curves display single relaxation process and indicate the increase in ionic conductivity with increase in temperature and frequency [35].
Figure 4b (inset) shows the variation of imaginary part of impedance (Z") with frequency at the different temperatures. The magnitude of Z" is observed to be high in intensity at a lower frequency and the typical peak like curve decreases in intensity as the temperature increases. The broadening of the peaks suggests that there is a spread of relaxation times. The significant widening of peaks (in the x-axis of Fig. 4b) on increasing temperature suggests the presence of a temperature-dependent relaxation process in the material. The relaxation species may be possibly due to ions at low temperature and the presence of defects at higher temperature. The shift of the peak (maximum Z") to lower frequency is observed for higher temperatures. The observed difference in peak positions explains the increase in conductivity with the increase in temperature and frequency due to formation of extra conductive pathway for Fe2O3 added nanocomposite polymer electrolyte [2, 35].
Ionic conductivity and activation energy measurements
The variation in ionic conductivity as a function of AgNO3 wt% is shown in Fig. 2. It is clear from figure that the ionic conductivity increases with increase in salt concentration due to increase in charge carrier and it reaches at maximum for 10 wt%. On further increase in salt concentration, ionic conductivity decreases because of ion aggregation and again increases after 20 wt% of AgNO3. The ionic conductivity reaches at maximum value again for 40 wt% of AgNO3 but the value is less than that of 10 wt% of AgNO3. The ionic conductivity as a function of filler concentration shows a double peak which is explained by double percolation model. Such a peak is reported for many solid polymer electrolytes [32]. The maximum ionic conductivity value in (100-x)PEO:xAgNO3 is obtain for the electrolyte composition 90PEO:10AgNO3 (x = 10 wt%) and is σ ~ 4.5 × 10−7 S/cm which is an slightly higher than the reported value by S Chandra et al. using solution cast method [36] but is slightly lower than reported by Agrawal et al. [37]. The ionic conductivity of (100-x) (90PEO:10AgNO3):xFe2O3 as a function of wt% of Fe2O3 is shown in Fig. 5. It can be seen that the nanocomposite polymer electrolyte with 5 wt% of Fe2O3 exhibits the lowest ionic conductivity (σ ~ 5.2 × 10−7 S/cm). In increase in wt% of Fe2O3, the ionic conductivity increases and reaches at maximum for 10 wt% of Fe2O3 (σ ~ 2.2 × 10−6 S/cm). The increase in conductivity could be due to the presence of filler facilitating the path for ionic transport and polymer segmental motion [2, 38]. On further increase in filler concentration, conductivity then decreases, this could be explained in a way that excessive fillers in the solid polymer electrolyte may lead to ion pairs and ion aggregation inhibiting the ionic conduction and slowing its mobility [39, 40] in an amorphous phase. Again, small increase in ionic conductivity is observed for 25 wt% of Fe2O3. The existence of two conductivity maxima has been observed in the majority of nanocomposite polymer electrolyte films reported in the past and has been explained on the basis of two percolation model, suggested by Laxmi and Chandra [33]. It has been proposed that the two kinds of conductivity maxima are possibly due to transport mechanism operative in these systems. Accordingly, the first maxima is possibly due to the dissociation of ion aggregates and/or undissociated salt which result into the generation of free ion carriers as a consequence of addition of nano-sized Fe2O3 particles. The second conductivity maxima as well as the conductivity variation around this ratio are related to the well-known 2-phase composite effect and can be explained on the basis of space-charge and/or percolation model [41].
The temperature dependence of the ionic conductivity of the samples shows the conductivity increases with temperature, and it follows a near Arrhenius-type relation. Figure 6 represents ionic conductivity versus inverse temperature plot (Arrhenius plot). The correlation coefficient of Arrhenius plots corresponding to 5, 10, 15, 20, 25, and 30 wt% of Fe2O3 is 1, 1, 1, 0.98, 1, and 1, respectively. Through the conductivity versus 1/T plot, the activation energies are calculated and the obtained values are plotted in Fig. 7. The migration of ion depends mainly on the segmental movement of the polymer chain (PEO) in the amorphous region of the polymer [2]. The activation energy varies between 0.52 and 0.325 eV with the increase in Fe2O3 filler from 5 to 30 wt% of Fe2O3. The lowest activation energy found to be 0.325 eV corresponding to 10 wt% of Fe2O3. It is also generally believed that the content of the amorphosity in the polymer host will directly correlate the lowest amount of activation energy (Ea) that required for the defect formation and migration of the Ag ion. Therefore, initially when the concentration of filler increases, the Ea decreased and conductivity increased with the increase of Fe2O3 filler and the optimum value of ionic conductivity is found to be at 10 wt% implying that the Ag ions to migrate from one to another site require lower energy of 0.325 eV. On further increase in filler content, now activation energy increases up to 0.48 eV. The increase in activation energy is due to ion aggregation. For 25 wt% of Fe2O3, activation energy again attains lower value of 0.36 eV. This is explained on the basis of two percolation model, suggested by Laxmi and Chandra [33]. The lowest activation energy at 10 wt% of Fe2O3 suggests the high mobility of ions present in the sample. This effect is due to well-known mechanism that the addition of filler increases the conductivity through inhibiting the crystallization of PEO chains in the host polymer while providing the conducive pathway for Ag ions to travel at the surface of the Fe2O3 through Lewis acid base interactions between the filler and the polymer [42, 43]. The results show that Ea values are inversely proportional to σ values. It is obvious from the ionic conductivity and activation energy studies that 90 (90PEO:10AgNO3):10Fe2O3 (filler content of 10 wt%) shows the best performance and hence it is chosen for further analysis.
Dielectric constant and dielectric loss analysis
Figure 8 shows the frequency dependence of the dielectric constant (ε') and dielectric loss (ε") (inset), at various temperatures corresponding to 90 (90PEO:10AgNO3):10Fe2O3 polymer nanocomposite electrolyte. It can be seen that ε' and ε" show similar behavior with frequency at different temperatures. The absence of relaxation peaks in both the figures indicates that the increase in conductivity was primarily due to an increase in the number density of mobile ions [44]. The dielectric constant is higher at lower frequencies, and it decrease continuously with increasing frequency and reaches a constant value. A rapid decrease in dielectric constant may be noticed above a particular frequency. The higher value of ε' at lower frequencies is due to the orientation of polar groups, ions, and space charges polarization at these frequencies [45]. However, in the high frequency range, the dipoles are unable to align themselves in the direction of the applied field, thus they oscillating begins to lag behind and hence the values of the dielectric constant decreases [46]. In a similar way, the dielectric loss ε" is higher at lower frequencies due to polarization effects on the electrode/electrolyte interface and free charge motion in the system [47], and it is lower at higher frequencies. This is due to that at lower frequencies, there was enough time for the charges to build up at the interface before the electric field was reversed and contributed to a large apparent value of ε". With increasing frequency, there was no time for the buildup of charges at the interface, but only for the buildup of charges at the boundaries of conducting species in the material and at the ends of conducting paths [47, 48]. It can be seen from figure that both ε' and ε" increase with temperature due to the increase in concentration of free ions. However, increase in temperature increases the degree of redissociation process of ion pairs and asserts the increase in concentration of free charge carriers at the interface. High temperature may prevent the ion aggregation and ion clusters in the polymer matrix and thus increases the equivalent capacitance by forming space charge region, exhibiting high dielectric constant [23].
Loss tangent analysis
Figure 9 shows the variation of loss tangent with frequency for polymer nanocomposite electrolyte 90(90PEO:10AgNO3):10Fe2O3 in temperature range 300–323 K. It is clearly seen from the figure that the loss tangent spectra consist of peaks but not so strong, at particular frequency. The peaks appearing at different temperatures correspond to the electrode polarization relaxation frequency. The peak frequency is inversely proportional to the relaxation time of the ions for the particular sample. The longer the relaxation time, the lower the conductivity since the relaxation time is given by τ = 1/ (2πf), where f is the frequency of the relaxation peak [49]. The peak of the spectra shifts towards the higher frequency as the temperature of the nanocomposite polymer electrolyte increases. Therefore, relaxation time decreases with increase of temperature which results in an increase in the ionic conductivity. The dielectric loss at lower frequencies characterized by high values of tan δ is due to the contribution of both ion jump and conduction or ion migration loss in addition to the polarization loss [49, 50]. The loss tangent shows a maximum at low frequency end in the high temperature region because of thermally generated defects in the sample [51].
Complex electric modulus analysis
Figure 10 shows the variation of real modulus (M') and imaginary modulus (M") (inset) with frequency at different temperature. It is clear that, though relaxation peaks are absent, M' increases abruptly at higher frequencies and at lower frequencies it approaches to zero. This long tail indicates the suppression of electrical double layer effect at the electrode–electrolyte interface [21, 52]. Electric moduli analysis facilitates the processes of identification and separation of polarization effect from the bulk relaxation phenomenon in the polymer electrolyte. In the real part of electric modulus (M'), all the curves demonstrate superposition features in the all temperature regime at high frequency and thus indicates that the modulus spectral formalisms exhibit temperature-independent properties, revealing the excellent electrochemical stability of polymer nanocomposite electrolyte [23].
In the imaginary part of the modulus (M"), the formation of loss peaks is clearly observed in the figure. The peaks in the M" show that the polymer electrolyte is ionic conductor [53, 54]. It is obvious that at lower frequencies M" exhibits low value, which might be due to the large value of capacitance associated with the electrode polarization effect, as a result of accumulation of a large amount of charge carriers at the electrode/electrolyte interface [55]. However, at high frequencies, well-defined peaks are observed. The broad and asymmetric peaks on both sides of the maxima predict the non-Debye behavior. The region on the left of the peak determines the range in which charge carriers are mobile over long distances, while region to the right is where carriers are confined to potential wells being mobile over short distance [55, 56]. The position of the peak shifts toward the higher frequency end as the temperature increases. As the temperature increases, the movement of the charge carriers becomes faster due to an increase in the flexibility of the material, leading to a decrease in the relaxation time. Hence, a shift is observed in the M" peak toward the higher frequency side. This behavior suggests that the relaxation is thermally activated in which hopping process of charge carriers dominate intrinsically [57, 58]. The peaks are broader as compared to that predicted by Debye for relaxation phenomenon and are significantly asymmetric. The relaxation time τ associated with each peak is determined from the frequency at which M"max occurs [58].
Conclusion
PEO-based silver ion conducting solid nanocomposite polymer electrolytes (100-x) (90PEO:10AgNO3):xFe2O3 with different wt% of nanosized filler Fe2O3 are prepared by hot press method and reported. The highest conductivity of σ ~2.2 × 10−6 S/cm was obtained for the composition 90(90PEO:10AgNO3):10Fe2O3 due to high mobility and high concentration of charge carriers at this composition. The activation energy of the highest conducting sample is calculated using the Arrhenius plot, and it has been found to be 0.325 eV. The dielectric response study of solid nanocomposite polymer electrolyte shows that the dielectric constant (ε') and dielectric loss (ε") increase enormously with decreasing the frequency, which is related to the accumulation of charge carriers at the electrode–electrolyte interface. The loss tangent (tanδ) shows a maximum at low frequency end in the high temperature region suggest the thermally generated defects in the sample. The real part of electric modulus (M') increases abruptly at higher frequencies and at lower frequencies it approaches to zero. The peaks observed in imaginary part of electric modulus (M") indicates that the solid nanocomposite polymer electrolyte is ionic conductor.
References
Jayathilaka PARD, Dissanayake MAKL, Albinsson I, Mellander BE (2002) Effect of nano-porous Al2O3 on thermal, dielectric and transport properties of the (PEO)9LiTFSI polymer electrolyte system. Electrochem Acta 47:3257–3268
Verma ML, Minakshi M, Singh NK (2014) Structural and electrochemical properties of nanocomposite polymer electrolyte for electrochemical devices. Ind Eng Chem Res 53:14993–15001
Rajendran S, Babu RS, Rani MU (2011) Effect of complexing salt on conductivity of PVC/PEO polymer blend electrolytes. Bull Mater Sci 7:1525–1530
Zhang X-W, Wang C, Appleby AJ, Little FE (2002) Characteristics of lithium-ion conducting composite polymer-glass secondary cell electrolytes. J Power Sources 112:209–215
Tominaga Y, Asai S, Sumita M, Panero S, Scrosati B (2005) A novel composite polymer electrolyte: effect of mesoporous SiO2 on ionic conduction in poly(ethylene oxide) –LiCF3SO3 complex. J Power Sources 146:402–405
Kim DG, Shim J, Lee JH, Kwon SJ, Baik JH, Lee JC (2013) Preparation of solid state composite electrolytes based on organic/inorganic hybrid star-shaped polymer and PEG functionalised POSS for all-solid-state lithium battery applications. Polymer 54:5812–5820
Itoh T, Fujita K, Inoue K, Iwama H, Kondoh K, Uno T, Kubo M (2013) Solid polymer electrolytes based on alternating copolymers of vinyl ethers with methoxy oligo(ethleneoxy)ethyl groups and vinylene carbonate. Electrochim Acta 112:221–229
Scrosati B (2000) Recent advances in lithium-ion battery materials. Electrochim Acta 45:2461–2466
Yap YL, You AH, Teo LL, Hanapei H (2013) Inorganic filler sizes effect on ionic conductivity in polyethylene oxide (PEO) composite polymer electrolyte. Int J Electrochem Soc 8:2154
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all solid-state battery applications: an overview. J Phys D Appl Phys 41:223001 (18pp)
Persi L, Croce F, Scrosati B, Plichta E, Hendrickson MA (2002) Poly(ethylene oxide)-based, nanocomposite electrolytes as improved separators for rechargeable lithium polymer batteries: the Li/LiMn3O6 case. J Electrochem Soc 149:212
Fahmi EM, Ahmad A, Nazeri NNM, Hamzah H, Razali H, Rahman MYA (2012) Effect of LiBF4 salt concentration on the properties of poly(Ethylene Oxide)-based composite polymer electrolyte. Int J Electrochem Sci 7:5798–5804
Johan MF, Ting LM (2011) Structural, thermal and electrical properties of nano manganese-composite polymer electrolytes int. J Electrochem Sci 6:4737–4748
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Sun JK, Moon HS, Kim JW, Park JW (2003) Role of functional nano-sized inorganic fillers in poly(ethylene) oxide-based polymer electrolytes. J Power Sources 117:124–130
Nan CW, Fan L, Yin Y, Cai Q (2003) Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys Rev Lett 91:104–108
Yuan A, Zhao J (2006) Composite alkaline polymer electrolytes and its application to nickelmetal hydride batteries. Electrochim Acta 51:2454–2462
Morita M, Noborio H, Yoshimoto N, Ishikawa M (2006) Ionic conductance of composite electrolytes based on network polymer with ceramic powder. Solid State Ionics 177:715–720
Dey A, Karan S, De SK (2008) Thermal and electric properties of CeO2 nanoparticles dispersed in polyethylene oxide: NH4ClO4 complex. Solid State Ionics 178:1963
Teran AA, Tang MH, Mullin SA, Balsara NP (2011) Effect of molecular weight on conductivity of polymer electrolytes. Solid State Ionics 203:18–21
Li YH, Wu XL, Kim JH et al (2013) A novel polymer electrolyte with improved high-temperaturetolerance up to 170 °C for high-temperature lithium-ion batteries. J Power Sources 244:234–239
Verma ML, Minakshi M, Singh NK (2014) Synthesis and characterization of solid polymer electrolyte based on activated carbon for solid state capacitor. Electrochim Acta 137:497–503
Liew CW, Ramesh S, Durairaj R (2012) Impact of low viscosity ionic liquid on PMMA–PVC–LiTFSI polymer electrolytes based on AC -impedance, dielectric behavior, and HATR–FTIR characteristics. J Mater Res 27:2996–3004
Othman L, Chew KW, Osman Z (2007) Impedance spectroscopy studies of poly(methyl methacrylate)-lithium salts polymer electrolyte systems. Ionics 13:337–342
Tripathi SK, Gupta A, Kumari M (2012) Studies on electrical conductivity and dielectric behaviour of PVdF–HFP–PMMA–NaI polymer blend electrolyte. Bull Mater Sci 35:969–975
Liu Y, Lee JY, Hong L (2002) Functionalised SiO2 in poly (ethylene oxide)-based polymer electrolytes. J Power Sources 109:507–514
Arbizzani C, Mastragostino M, Meneghello L (1996) Polymer-based redox supercapacitors: a comparative study. Electrochim Acta 41:21–26
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paper like polymer supercapacitors. Nano Lett 10:4025–4031
Tan CG, Siew WO, Pang WL, Osman Z, Chew KW (2007) The effects of ceramic fillers on the PMMA-based polymer electrolyte systems. Ionics 13:361–364
Scrosati B, Croce F, Persi L (2000) Impedance spectroscopy study of PEO-based nanocomposite based electrolytes. J Electrochem Soc 147:1718–1721
Ataollahi N, Ahmad A, Hamzah H, Rahman MYA, Mohamed NS (2013) Ionic conduction of blend poly (Vinylidene Fluoride-Hexafluoro Propylene) and poly (Methyl Methacrylate)-grafted natural rubber based solid polymer electrolyte. Int J Electrochem Sci 8:7875–7884
Agrawal RC, Mahipal YK (2011) Study of electrical and electrochemical behaviour on hot-press synthesized nano-composite polymer electrolyte (NCPE) membranes: [(70PEO: 30 KNO3) + x SiO2]. Int J Electrochem Sci 6:867–881
Laxmi N, Chandra S (2001) Proton conducting composites of heteropolyacid hydrates (phosphomolybdic and phosphotungstic acids) dispersed with insulating Al2O3. Phys Stat Solidi (a) 186:395
Xu D, Sridhar V, Mahapatra SP, Kim JK (2009) Dielectric properties of exfoliated graphite reinforced flouroelastomer composites. J Appl Polym Sci 111:1358–68
Al-Saleh MH, Al-Anid HK, Husain YA, El-Ghanem HM, Jawad S (2013) Impedance characteristics and conductivity of CNT/ABS nanocomposites. J Phys D Appl Phys 46:385305 (8pp)
Chandra S, Hashmi SA, Saleem M, Agrawal RC (1993) Investigations on poly ethylene oxide based polymer electrolyte complexed with AgNO3. Solid State Ionics 67:1–7
Agrawal RC, Chandra A, Bhatt A, Mahipal YK (2008) Investigations on ion transport properties of and battery discharge characteristic studies on hot-pressed Ag + −ion-conducting nano-composite polymer electrolytes:(1–x) [90PEO : 10AgNO3] : xSiO2. New J Phys 10:043023 (10pp)
Maier J (2004) Ionic transport in nano-sized systems. Solid State Ionics 175:7–12
Sharma JP, Sekhon SS (2007) Nanodispersed polymer gel electrolytes: conductivity modification with the addition of PMMA and fumed silica. Solid State Ionics 178:439–445
Saikia D, Chen-Yang YW, Chen YT, Li YK, Lin SI (2009) Li NMR spectroscopy and ion conduction mechanism of composite gel polymer electrolyte: a comparative study with variation of salt and plasticizer with filler. Electrochim Acta 54:1218–1227
Agrawal RC, Gupta RK (1999) Superionic solids: composite electrolyte phase—an overview. J Mater Sci 34:1131–1162
Croce F, Perse L, Scrosati B, Serraino-Fiory F, Plichta E, Hendrickson MA (2001) Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim Acta 46:2457–2461
Chung SH, Wang Y, Persi L, Croce F, Greenbaum SG, Scrosati B, Plichta E (2001) Enhancement of ion transport in polymer electrolytes by addition of nanoscale inorganic oxides. J Power Sources 97:644
Shyly PM, Karuppasamy K, Linda T, Thiravetyan P, Balakumar S, Sahaya Shajan X (2012) Ionic conductivity and dielectric studies of chitin nanofiber (CNF) incorporated PMMA based polymer electrolytes. IOSR J Appl Phys 1:47–51
Zamri SFM, Latif FA et al (2014) Ionic conductivity and dielectric properties of LiBF4 doped PMMA/ENR 50 filled acid modified SiO2 electrolytes. Procedia Technol 15:850–856
Reicha FM, El-Hiti M, El-Sonabati AZ, Diab MA (1991) Conducting polymers. V. Electrical conductivity of polymer complexes of bis-2,6-diaminopyridinesulphoxide-copper halides. J Phys D Appl Phys 24:369
Aziz SB (2013) Li + ion conduction mechanism in poly (e-caprolactone)-based polymer electrolyte. Iran Polym J 22:877–883
Nithya H, Selvasekarapandian S, Kumar DA et al (2011) Thermal and dielectric studies of polymer electrolyte based on P(ECH-EO). Mater Chem Phys 126:404–408
Choudhary S, Sengwa RJ (2011) Dielectric relaxation spectroscopy and ion conduction in poly (ethylene oxide)-blend salts-montmorillonite nanocomposite electrolytes. Indian J Pure Appl Phys 49:204–213
Hassan MF, Yusof SZM (2014) Poly (acrylamide-co-acrylic acid)-zinc acetate polymer electrolytes: Studies based on structural and morphology and electrical spectroscopy. Microsc Res 2:30–38
Kumar TV, Chary AS, Bhardwaj S, Awasthi AM, Reddy SN (2013) Dielectric relaxation, ionic conduction and Complex impedance studies on NaNO3 fast ion conductor. Int J Mater Sci Appl 2:173–178
Shastry MCR, Rao KJ (1991) Ac conductivity and dielectric relaxation studies in AgI-based fast ion conducting glasses. Solid State Ionics 44:187–198
Ramesh S, Owg Poh Ling (2010) Effect of ethylene carbonate on the ionic conduction in poly (vinylidine fluoride-hexafluorooropylene) based solid polymer electrolytes. Polym Chem 1:702–707
Ramesh S, Chai MF (2007) Conductivity, dielectric behavior and FTIR studies of high molecular weight poly (vinyl chloride)-lithumtriflate polymer electrolytes. Mater Sci Eng B 139:240–245
Aziz SB, Abidin ZHZ, Arof AK (2010) Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosansilver triflate electrolyte membrane. eXPRESS Polym Lett 4:300–310
Dutta A, Sinha TP, Jena P, Adak S (2008) Ac conductivity and dielectric relaxation in ionically conducting soda-lime-silicate glasses. J Non-Cryst Solids 354:3952–3957
Kumar M, Tiwari T, Chauhan JK, Srivastava N (2014) Erratum on ‘Understanding the ion dynamics and relaxation behavior from impedance spectroscopy of NaI doped Zwitterionic polymer system’. Mater Res Express 1:049601
Pradhan DK, Choudhary RNP, Samantaray BK (2009) Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater Chem Phys 115:557–561
Acknowledgments
We gratefully acknowledge the kind support of the management of Shri Shankaracharya Technical Campus (SSTC). Helpful discussions with Manickam Minakshi (School of Engineering and Information Technology, Murdoch University, Australia) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, M.L., Sahu, H.D. Ionic conductivity and dielectric behavior of PEO-based silver ion conducting nanocomposite polymer electrolytes. Ionics 21, 3223–3231 (2015). https://doi.org/10.1007/s11581-015-1517-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1517-9