Abstract
Polymer blended films of polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):lithium perchlorate (LiClO4) embedded with silver (Ag) nanofiller in different concentrations have been synthesized by a solution casting method. The semi-crystalline nature of these polymer films has been confirmed from their X-ray diffraction (XRD) profiles. Fourier transform infrared spectroscopy (FTIR) and Raman analysis confirmed the complex formation of the polymer with dopant ions. Dispersed Ag nanofiller size evaluation study has been done using transmission electron microscopy (TEM) analysis. It was observed that the conductivity increases when increasing the Ag nanofiller concentration. On the addition of Ag nanofiller to the polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):Li+ electrolyte system, it was found to result in the enhancement of ionic conductivity. The maximum ionic conductivity has been set up to be 1.14 × 10−5 S cm−1 at the optimized concentration of 4 wt% Ag nanofiller-embedded (45 wt%) polyethylene oxide (PEO) + (45 wt%) polyvinyl pyrrolidone (PVP):(10 wt%) Li+ polymer electrolyte nanocomposite at room temperature. Polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):Li+ +Ag nanofiller (4 wt%) cell exhibited better performance in terms of cell parameters. This is ascribed to the presence of flexible matrix and high ionic conductivity. The applicability of the present 4 wt% Ag nanofiller-dispersed polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):Li+ polymer electrolyte system could be suggested as a potential candidate for solid-state battery applications. Dielectric constants and dielectric loss behaviours have been studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Remarkable research efforts in solid polymer electrolytes have been motivated by their numerous potential applications in various electrochemical devices, such as secondary batteries, super capacitors, fuel cells and sensors [1]. Polyethylene oxide (PEO) is a familiar host polymer for solid polymer electrolytic applications due to the benefit structure of PEO. It can support the faster ionic mobility. The centre focused requirement for a high conductivity of the polymer electrolyte seems to be the facile dissolution of the cation by the coordination group of the polymer. The polymer that contains a Lewis base, usually etheric oxygen, can serve to coordinate the cations, thus promotes the dissolution of the salt. Polyethylene oxide is the semi-crystalline polymer; it possesses both amorphous and crystalline phases at ambient temperature. As the PEO is a linear polymer, the regularity of the structure of the unit allows a high degree of crystallinity [2].
The PEO chemical structure consists of the sequential oxyethylene group –CH2-CH2-O- that has a series of polar group –O- which can associate with metal cations. Moreover, PEO can solvate a wide variety of salts even at very high salt concentrations. However, PEO itself only has C-C, C-O, C-H bonds; the reactivity is exceedingly low; because of this, it is stabilized chemically and electrochemically [3]. Various methods and procedures have been made to reduce the crystallinity of the PEO-based electrolyte while maintaining their required flexibility and mechanical stability. One of the most successful approaches has been made on the preparation of polymer blends, which offers ease of preparation and easy control of physical properties within the compositional regime. Blended polymer electrolyte systems obtained by the mixture of PEO with different polymers give rise to the reduced crystalline phase of the polymer. These could improve flexible amorphous content, thus exhibiting higher ionic conductivity in comparison with pure PEO complex polymer electrolytes. Blend-based polymer materials which exhibit a wide range of properties are suitable to design various solid-state ionic devices [4]. Polyvinyl pyrrolidone (PVP) was chosen as a secondary polymer for making the blended polymer along with PEO for the present polymer host material. PVP is a special conjugate polymer because of its high amorphous nature, which can permit faster ionic mobility, and it is easily soluble in water as PEO. Thus, it is preferable to avoid phase separation in the blended polymer. The pyrrolidone group of the PVP is well known to form various salt complexes with many inorganic salts. Another significant advantage of the PVP is that it can be thermally cross-linked, resulting in suitable thermal stability, and improves the mechanical strength of the blended polymer material [5].
In recent years Li-ion battery is one of the most essential power sources because of its excellent properties such as higher energy density, shape and design flexibility, lower self-discharge rate, no memory effect and long lifespan compared to other secondary batteries. High lithium ion conductivity solid electrolytes are attractive for all non-flammable solid-state batteries and have potential applications as a separator for the water-stable lithium–air rechargeable batteries. Actually, in recent years, a special attention has been focussed on the lithium-ion batteries for their use in electrical vehicles as a power sources. Lithium ion conducting solid polymer electrolytes have received a great deal of interest in the last two decades due to their growing demand for lithium-ion batteries in several applications. These range from potential portable electronic devices such as mobile phones, computers, cameras and electro-optical devices to hybrid electric vehicles. Lithium polymer batteries have drawn much attention due to their wide variety of advantages such as the absence of electrolyte leakage, light weight, ease of fabrication, flexible geometry and improved safety [6]. Hence, lithium salt has been chosen as a dopant to present blended polymer complex electrolytes. Despite the blended polymer films containing some conducting ions like lithium, there is no significant ionic conductivity that might be achieved until now. To overcome this problem, we can add some nanofillers to the polymer matrix [7].
The polymer electrolytes used in ionic devices had some primary problems, and queries are still unsolved. The problems, such as low cation transference numbers at ambient temperature, low ionic conductivity and the formation of resistive layers of the electrode–electrolyte interface, are crucial. To avoid these problems, various modifiers of polymer salt complexes have been explored. The primitive approach of the composite polymer electrolyte formation is the incorporation of nanofiller into the polymer salt matrix. The major role of nanofiller in the solid polymer electrolyte system is crucial as it serves as an agent to improve the conductivity of the electrolyte. Two kinds of nanofillers have been introduced into the polymer electrolyte system earlier; they are organic and inorganic molecular nanofillers. But the disadvantages of the organic molecular fillers such as propylene carbonate (PC) and ethylene carbonate (EC) are more expensive compared to inorganic molecular fillers. The major goal of the researchers has been directed towards improving the ionic conductivity at ambient temperature, while retaining the mechanical properties and stability towards metallic anodes by the incorporation of nanostructured inorganic fillers like SiO2, TiO2 and Al2O3 and also some metal nanoparticles like silver (Ag) nanofiller, Fe nanofiller, Cu-Sn nanofiller, etc., in the polymer electrolytic systems. Sometimes, this type of nanocomposite polymer electrolytes have been shown that nanosized metal or alkali metal nanofiller incorporated into the polymer salt matrix, which can act as solid plasticizers inhibiting crystallization kinetics and promoting the retention of the amorphous phase down to subambient temperature. Polymer composites can be made stronger, stiffer and electronically conductive by the incorporation of various additives like nanofillers. Most of these modifications are made by the addition of inorganic fillers to the polymer. These fillers, present in varying degrees, also affect the basic mechanical properties of the polymer [8]. These synthesized nanocomposite polymer electrolytes could have been shown improvised ionic conductivity and enhanced thermal and mechanical properties also [9]. Moreover, they possess better electrochemical stability towards a metal anode and an enhanced cation transport numbers [10]. The enhancement in ionic conductivity by the addition of nanofiller has been generally explained in terms of the disruption of crystallization of the polymer host matrix. There are numerous reports on the silver nanofiller-dispersed polymer electrolytes and their electrical properties [11]. In our present work, Ag nanofiller has been used for the first time to improve the electrical properties of the PEO + PVP:Li+ blended polymer for solid-state electrolyte battery applications.
Experimental studies
Silver nitrate was purchased from Sigma-Aldrich, India, and was used without further purification. The glassware was washed with concentrated nitric acid, later on thoroughly washed with double distilled water and dried in hot air oven. Abrus precatorius leaves were collected from S.V.U. Hostel garden, Tirupati, Andhra Pradesh, India. The leaves were washed thoroughly thrice with distilled water and shade dried for 10 days. The fine powder was obtained from the dried leaves by using kitchen blender. The leaf powder was sterilized at 121 °C for 5 min. Five grams of powder was taken into a 250-ml conical flask and 100 ml of sterile distilled water and boiled for 15 min at 100 °C. Then, the leaf extract was collected in a separate conical flask by a standard filtration method. Twenty milliliters of 1 × 10−3 M aqueous solution of silver nitrate was taken in an Erlenmeyer flask, and 2 ml of leaf extract was added to it at room temperature (RT). After 10 min, the solution turns yellow to yellow–red to dark brown indicating the formation of silver nanoparticles, which seem like as earlier report [12]. Later on, the dark brown colour solution is heated at 100 °C above, and silver nanoparticles remain in a glass beaker. The particle size of the synthesized pure silver nanoparticles are found to be ∼70, It was confirmed from the TEM analysis.
Blended polymer films were prepared by using a traditional solution casting method with triple distilled water. Initially, polyethylene oxide (PEO) (MW = 6 × 105) and polyvinylpyrrolidone (PVP) (MW = 13 × 105) were obtained from Sigma-Aldrich. Films (thickness ∼100 μm) of PEO + PVP blended polymers were doped with lithium. The precursor materials that are PEO, PVP and lithium perchlorate (LiClO4) salt were taken in weight percent ratio (45:45:10 wt %), and the triple distilled water taken as a solvent. PEO, PVP and dopant salts were dissolved in triple distilled water and stirred at RT (∼30 °C) for 10–12 h to get a homogeneous mixture. A separate solution of water with a certain concentration of Ag nanofiller (2, 4 and 8 wt%) was stirred for 1 h. Then, the two solutions were intermixed with each other, making a homogeneous mixture by using an ultra sonicator. In order to make a homogeneous mixture of the polymer composites, a high intensity ultrasonic processor (750 W, probe-type sonicator—Vibra Cell—VC 750, Cole-Parmer, USA) was used. It is working at 20 kHz with a 6.5 mm microtip, applying an amplitude of 30 % of the maximum power supplied by the instrument corresponding to 20 W and pulsed cycles of 5 s On and 5 s OFF for 15 min. Due to applying the low power in the order of 20 W, the polymer chains could not be affected. The solution was cast onto polypropylene dishes and allowed to evaporate slowly at room temperature. The dried composite polymer films were peeled off from the polypropylene dishes and analysed.
The XRD spectra profiles of PEO + PVP:Li+ blended polymer films were measured on SEIFERT 303 TT X-ray diffractometer with CuKα (line of 1.5405 Å), and it was operated at 40 kV voltage and 50 mA anode current. The FTIR spectra of Ag nanofiller-embedded PEO + PVP:Li+ polymer films were recorded using Thermo Nicolet IR200 spectrometer at room temperature in the wavenumber range of 3000–400 cm−1. Raman spectra of the prepared blend polymer films were carried out at room temperature in the wavenumber range of 1800–800 cm−1 using LabRAM HR 800 confocal Raman spectrometer, which has a Nd:YAG laser source (532.15 nm). The absorption spectra of Ag nanofiller-embedded PEO + PVP:Li+ films were measured on a PerkinElmer absorption spectrophotometer in the range of 250–850 nm. A Philips TECHNAI FE 12 transmission electron microscope (TEM) was used for particle size confirmation. The impedance measurements were performed using a computer-controlled phase sensitive multimeter (PSM 1700) in the frequency range of 1 kHz–1 MHz at RT. In this battery formation technique, the nanocomposite polymer samples were sandwiched between two lithium reversible electrodes.
Results and discussion
The measured XRD profiles of the films are shown in Fig. 1. PEO + PVP polymer blended films exhibit crystalline peaks of PEO, one peak with a maximum intensity at 19.2° (1 2 0), another intense peak at 23.6° (1 1 2) and relatively less intense peak at 27.1°. There is no well-defined sharp peak attributable to PVP; instead, a broad peak has been observed at 13°, which suggests an amorphous nature of PVP. The characteristic peaks of pure PEO + PVP complex show variation in intensity suggesting that the ordering of the PEO polymer crystallinity is disturbed due to the coordination interactions between the Li+ ions and etheric oxygens. These observations confirmed that the present polymer blend systems possess both crystalline and amorphous nature. However, with the addition of small amounts of lithium salt to the PEO + PVP blended polymer, those could show semi-crystalline nature [13, 14]. Upon addition of Ag nanofiller to the lithium-doped polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) polymer system, we could observe the broadening of the XRD peaks relating to PEO and PVP resulting in the polymer matrix augmented by the amorphous domains. As the Ag nanofiller concentration increases, the intensity of the polymer diffraction peaks weakens, suggesting that the interaction between the polymer chains and nanofiller leads to decrease of the intermolecular interaction of polymer chains, thereby resulting in an increase of the amorphousity of the blended polymer films. This facilitates significant motion of the polymer chains in the amorphous phase. Higher concentration of Ag nanofiller-incorporated PEO + PVP blended polymer films could exhibit the semi-crystalline nature with the existing conformational Ag nanofiller peaks at (1 1 1) at 38.13° (111), 44.24° (200), 64.64° (220) and 77.22° (311), which are the fcc crystalline planes of Ag nanostructures, respectively [15]. At lower concentrations of the Ag nanofiller-dispersed polymer films, the crystalline peaks pertaining to Ag nanofiller are found to disappear, indicating the involvement of the nanofiller in disrupting the structure. Persistence of crystalline peaks corresponding to nanofiller in the higher concentration of Ag nanofiller-incorporated composite polymer films suggests the presence of agglomerated nanoparticles in the polymer matrix. These peaks are attributed to the Ag nanofiller as a separate phase. Similar behaviour has been noticed by other researchers [14].
In order to understand the possible interactions between the salt and the PEO + PVP blended polymer films along with Ag nanofiller, the FTIR spectral measurement has been performed in the wavenumber range of 3000–400 cm−1 as shown in Fig. 2. It explores the comparative FTIR spectra of pure polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) and lithium-doped polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) with and without Ag nanofiller at different concentrations. A broad band located between 2975 and 2813 cm−1 relates to the inherent bands of asymmetric (CH) stretching mode of CH2 of PEO [16]. The band region in the range of 1480–1413 cm−1 is for CH2 scissoring mode of PEO. The CH2 bending mode is at 1466 cm−1, the CH2 wagging doublet at 1368 cm−1 and 1343 cm−1 and the CH2 twisting modes at 1282 cm−1 and 1243 cm−1. The band at 1488 cm−1 represents the C-H bending of CH2, respectively, which is a characteristic band of PEO. The relative weak band at 1256 cm−1 is assigned to CH2 symmetric twisting of PEO, and the vibrational bands at 1100 cm−1 is because of C-O-C stretching of PEO [17, 18]. These significant bands are more sensitive to macromolecular interaction conformation and hence provide an evidence of semi-crystalline nature due to the presence of PEO. The factors supporting this assignment of these two bands near 946 and 845 cm−1 are assigned to CH2 rocking vibrations of methylene groups, and these bands are related to PEO [19]. The band region between 1346–1245 cm−1 and 1280 cm−1 indicates the –CH- wagging motion of the PVP. The bands at 1296–1242 cm−1 correspond to CH2 twisting or waging of both PEO and PVP. The characteristic bands of PEO at 1145, 1275, 1352 and 1466 cm−1 are observed in the FTIR spectral profile. In addition, a vibrational band at 2906 cm−1 is due to aliphatic C-H stretching of PVP [20]. Another band at 1700–1610 cm−1 is due to the symmetric and asymmetric of C=O stretching modes of PVP. The CH2 wagging mode of PVP is observed at 1445 cm−1 [21].
Due to the addition of salt to the host polymer matrix, the width of the vibrational band at 1100 cm−1 becomes slightly broadened and its wavenumber is slightly shifted. This may be due to the formation of coordination bonds between lithium ions and etheric oxygen units (−O-) of PEO. This leads to the interruption of crystallization so that the fraction of amorphous phase of the substance increases. Also, a hump-like peak at 1130 cm−1 indicates the presence of anion. The region 1160–1086 cm−1 is the location of C-O-C stretching, due to the interaction of lithium cations with ether oxygen atoms in PEO. C-H bending of CH2 in both PEO and PVP at 1455 cm−1 is shifted gradually towards 1429 cm−1 with the presence of the dopant ions in the host blended polymer matrix.
Upon addition of the nanofiller to the blended polymer film, the intensity of the peaks in the range of 2500–1500 cm−1 decreased. This indicates that the nanofiller is homogeneously dispersed in the polymer [22, 23]. In the FTIR spectra of Ag nanofiller-dispersed lithium-doped PEO + PVP polymer, the polymer is probably through weak physical forces rather than strong chemical bonding forces. The transmittance decreases when increasing the Ag nanofiller to the lithium-doped polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) polymer films; this might be due to the presence of Ag nanofiller within the polymer matrix [24]. From the FTIR spectral comparison, it is observed that no distinct changes in the peak positions of the polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) blended complexes have been noticed after the dispersion of the Ag nanofiller. The intensity of the FTIR bands decreases when increasing Ag nanofiller, and a very slight shift has been noticed towards the higher wavenumber side; this indicates that the transparency of the film has been decreased when increasing the Ag nanofiller, and it also explores the Ag nanofiller which is well distributed in the blended polymer films. These results have been further confirmed from the XRD analysis.
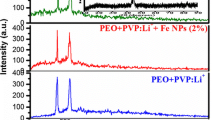
Figure 3a shows the Raman spectra of pure and lithium ion-doped PEO + PVP polymer films along with Ag nanofiller in different concentrations as aforementioned FTIR elucidation studies. The bands at 1236, 1427 and 1666 cm−1 are attributed to C-N stretching, C-H bending and C=O vibrations of PVP, respectively [25]. The bands pertaining to PEO in the measured region are 1068, 1042, 932, 860, 828, 845 and 1396 cm−1 [26]. Three bands at 860, 829 and 845 cm−1 are active modes of CH2 rocking and CO stretching modes. The spectral band at 932 cm−1 is assigned to CO stretching mixed with CH2 rocking vibrations of PEO [19]. The bands observed at 1340 and 1367 cm−1 are in good agreement with the earlier reports. In addition to these bands, new bands are also observed at 1344, 1445 and 1465 cm−1 due to the presence of PEO.
Upon adding lithium ions to the PEO + PVP blended polymer, the intensity of the band at 1064 cm−1 is decreased. This might be due to C-O stretching and/or rocking modes of CH2 vibrations of polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) polymer matrix [27]. This indicates the complex formation between the lithium salt and the polymer matrix. It has been noticed that the intensity of the Raman band corresponding to C-C stretching vibration at 845 and 860 cm−1 in the pure blended polymer decreases and becomes asymmetric with the addition of lithium salt and also Ag nanofiller; this is shown in Fig. 3b. This indicates the formation of ion pairs. Moreover, due to the addition of Ag nanofiller, weak physical forces have been working between the lithium-doped polymer and Ag nanofiller rather than chemical forces. The significant changes in the C-C vibrational band can be associated with the formation of ionic bonds with less polarization. The intense band at 1666 cm−1 attributed to PVP is also found to be diminished with the addition of dopant. This variation in intensity might be due to strong interaction taking place between the dissociated salt and the blended polymer. It is well recognized that the incorporation of anion from the salt to the polymer matrix helps to improve the decreasing nature of the degree of crystallinity of the host polymer because of its role as plasticizer. The semi-crystalline nature found with the addition of the salt has been confirmed by broadening of the Raman modes at 1445 and 1365 cm−1 that could be attributed to a mixture of C-H bending and O-H bending vibrations. This has been in good agreement with the XRD results. The most intense feature band at around 932 cm−1 which is assigned with the symmetric stretching mode of the anion in the blended polymer film as shown in Fig. 3c. This mode is very important and suitable for investigating ionic association. The intensity of the Raman band 932 cm−1 decreases with the addition of lithium salt along with Ag nanofiller. At higher concentration of the Ag nanofiller (8 wt%) in the lithium-doped PEO + PVP blended polymer film, the main band of 932 cm−1 shifts to a higher wavenumber and becomes asymmetric; this is shown in Fig. 3c. This is thought to be associated with the formation of ion pair aggregation. The broadening in Raman spectra is usually an indication of amorphous or semi-crystalline nature of the blended polymer [28]. The semi-crystalline nature of the polymer blend has also been confirmed by XRD analysis.
The size of the nanoparticles is confirmed from the TEM analysis, and it is found to be ∼70 nm as in Fig. 4. The results from the TEM analysis indicate that deagglomeration and dispersion of the Ag nanofiller at 4 wt% concentration had occurred while loading the nanofiller into the blended polymer films. This is an important parameter for a good dispersion of the nanoparticles within the blended polymer composite materials and consequently for an advance of the enriched properties of the polymer nanocomposites [29].
Figure 5 shows the optical absorption spectra of pure and lithium-doped PEO + PVP blended polymer film embedded with different concentrations of Ag nanofiller. For all the samples, there is no specific absorption bands observed in the pure and lithium-doped polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) blended polymer films. Significantly, the surface plasmon resonance absorption band of Ag at 420 nm has been noticed in the Ag nanofiller-embedded PEO + PVP blended polymer films. The absorption intensity of the plasmon band gradually increases with the increasing concentration of nanoparticles. This confirms that the Ag nanofillers are homogeneously distributed in the lithium-doped PEO + PVP blended polymer films [30, 31].
Impedance spectroscopy is a powerful method to investigate the electrical and dielectric properties of the solid polymer electrolyte films. The impedance, dielectric constant and dielectric loss parameters were clearly reported in previous work [32]. In the present study, the samples were vacuum dried at 300 K for 1 h and the measurements were done by sandwiching the polymer film between two aluminium electrodes. Figure 6 shows the complex impedance plots (Z′ Vs Z″) of PEO + PVP blended film doped with LiClO4 salts along with different concentration of Ag nanofiller at room temperature. Among all the concentrations of Ag nanofiller in the blended polymer film, 4 wt% concentration exhibited the prominent ionic conductivity at room temperature. From the figure, two well-defined regions have been observed such as a high-frequency semicircle related to the parallel combination of a resistor and capacitor and a low-frequency spike representing the formation of double-layer capacitance at the electrode–electrolyte interface due to migration of ions at low frequency. The low-frequency response appearing as an inclined spike at an angle less than 90° to the real axis indicates the inhomogeneous nature of the electrode–electrolyte interface. By the intersection of a semicircle with the real axis, we can find the bulk resistance (R b) of the polymer electrolytes. The magnitude of the bulk resistance decreases when increasing the Ag nanofiller concentration. The ionic conductivity of the solid polymer electrolyte was calculated by the following formula
where l is the thickness of the polymer electrolyte (cm), A is the area of the blocking electrode (cm2) and R b is the bulk resistance of the solid polymer electrolyte film [33]. At room temperature, the Li+-doped PEO + PVP blended polymer electrolyte film exhibits higher ionic conductivity in the order of 1.53 × 10−6 S cm−1. It could be due to the coordination interaction of the etheric oxygen atoms of PEO or/and carbonyl oxygen atoms of the PVP, with Li+ cations resulting in an increase in the number of dissociated mobile charge carriers and a reduction in the crystallinity of the PEO + PVP polymer matrix. This is responsible for the ionic conductivity. Upon addition of the Ag nanofiller to the Li+-doped PEO + PVP blended polymer electrolyte, the ionic conductivity has been enhanced and it is in the order of 1.14 × 10−5 S cm−1 at 4 wt% of the Ag nanofiller concentration.
The ionic conductivity of the polymer electrolyte depends on the concentration of the conducting species and their mobility. The variation of ionic conductivity at room temperature as a function of Ag nanofiller concentration is shown in the insert in Fig. 6. Interestingly, the ionic conductivity increases and reaches to maximum (1.14 × 10−5 S cm−1) when increasing the Ag nanofiller concentration until 4 wt% of the Ag nanofiller. It is believed that the nanosized Ag nanofiller prevents the local PEO + PVP chain reorganization due to its large surface area with the results of locking in at ambient temperature a high degree of disorder characteristic of the amorphous phase, which in turn favours high ionic transport. However, the enhancement of the conductivity in the nanocomposite blended polymer electrolytes cannot be solely attributed to the retention of the amorphous phase caused by the steric hindrance effect of the Ag nanofiller. Thus, the model must be extended to consider other effects involving the Ag nanofiller, such as Lewis acid–base interactions, in order to explain the overall conductivity enhancement observed in these nanocomposite polymer electrolytes. The dispersed nanofiller could be promoting the specific structural modifications via Lewis acid–base reactions between their surface states and the PEO + PVP polymer segments. It might be taken for granted that the Lewis acid groups of the filler could probably compete with the Lewis acid Li+ cations for the formation of the complexes with the polymer chains, as well as with the anions of the added LiClO4 salt. These results might be the local structural modifications occurring at the nanofiller surface, due to the particular cations of the polar surface groups of the nanofiller which may act as the following: first one is cross-linking centres for the polymer segments, thus lowering the tendency to polymer reorganization and promoting structural modification of the polymer chains. The pre-expected outcome effect is the promotion of the conducting pathways of Li+ at the nanofiller surface, which results in enhancement of the ionic conductivity. Second one is Lewis acid–base interaction centres for the electrolyte ionic species lowers ionic coupling, which is expected to take place the salt dissociation via a sort of “ionic-nanoparticle complex” formation, and it results in an enhancement of the Li+ ion transport.
The ionic conductivity is found to be decreased above an optimized concentration (8 wt%) of the Ag nanofiller at 4 wt%. This is imputable to the agglomerated nanofiller portion (a continuous non-conducting phase). The small nanofiller tends to form large agglomerates due to the ultrafine nature of the nanofillers. This agglomeration nanofiller portion of the polymer matrix acts as an electrically inert component, which would block the lithium ion transport, resulting an increase in the total resistance of the polymer composite [34].
The dielectric relaxation parameter of the polymer can be obtained from the study of Tan δ as a function of frequency. The dielectric loss tangent, Tan δ, can be defined by the equation Tan δ = ε″/ε′. The variation of Tan δ with frequency for all the prepared Ag nanofiller-embedded Li+-doped PEO + PVP blended polymer complexes at room temperature is presented in Fig. 7a. Higher values of Tan when decreasing the frequency at room temperature may be attributed to the face charge build-up at the interface between the sample and the electrode (space charge polarization). The appearance of the peaks in the loss spectrum (tangent loss) suggests the presence of relaxing dipoles in the polymer film. The strength of the relaxation depends on characteristic property of dipolar relaxation. The tangent loss peaks shift towards the higher frequency side on increase in the Ag nanofiller. On addition of nanofiller, it is believed that there is an increase in the segmental motion of the lithium ions. The segmental motion of the Li+ ion could be increased by the available free volume due to addition of Ag nanofiller. It is evidenced by the peak shifting towards the higher frequency side, thereby reducing the relaxation time. The relatively fast segmental motion coupled with mobile ions enhances the transport properties of Ag nanofiller-embedded polymer nanocomposites [35]. The electrode polarization (EP) relaxation frequency f EP, which is used to evaluate the EP relaxation time f EP = (2πf EP)−1 [36].
The variation of ε′ (the real part of the complex dielectric permittivity: ε* = ε′ − j ε″) as a function of frequencies at room temperature for the polymer samples which are incorporated with Li+ ions along with Ag nanofiller is shown in Fig. 7b. As expected, the variation tendency of dielectric constant with frequency is the reverse of the electrical conductivity. ε′ attains high value at low frequency and decreases exponentially with an increase in frequency. In all cases, it has been found that there is a sharp decrease in the ε′ value in the lower frequency region as shown in Fig. 7b. It is seen that with addition of Ag nanofiller, the ε′ value increases in lower frequency region and nearly same in the higher frequency region. The high permittivity in the polymer system can be attributed to the localization of charge carriers [37]. In the lower frequency region, the larger value of the dielectric constant (ε′) may be the contribution of moving ions causing the higher ionic conductivity, resulting in electrochemical double layers at the electrodes. This type of conduction often makes it difficult or impossible to detect dipole relaxation due to permanent dipoles.
The reduction of dielectric constant is primarily assigned to the mismatch of interfacial polarization of composites to external electric fields at elevated frequencies. The permittivity enhances near the percolation threshold. It is usually behind this percolation threshold, which is an important point at which electrical property varies a great deal. Therefore, study of conducting composites in the vicinity of percolation threshold is an efficacious way to know the electrical transport behaviour of composites. The dielectric response is explained by complex permittivity ε* = ε′ − j ε″, where ε′ and ε″ are the dielectric components for energy storage and energy loss of applied electric field. Dielectric properties of ionic conducting polymers are due to the contribution of electronic, ionic, dipole orientations and space charge polarizations. The complex permittivity of the polymer is obtained from the impedance data.
where Z* is the complex impedance and C o is the capacitance of free medium. The real part of the permittivity (dielectric constant) ε′ represents the polarizability, while the imaginary part (dielectric loss) ε″ represents the energy loss due to polarization and ionic conduction. The dielectric constant ε′ is calculated from
where C is the capacitance of the sample, ε 0 is the permittivity of the free space (8.85 × 10−12 F/m) and A is the cross-sectional area of the electrode [38].
Polymer electrolyte battery is a solid-state battery in which the electrolyte is the salt complexed polymer electrolyte film. The negative electrode is a metal foil. In the present investigation, cathode material contains three components: particles of interaction material, normally called the active cathode; particles of electronic conductors such as carbon and a surrounding materials consisting of polymer electrolyte, which acts an ionic conductor. These three components together form the catholytic compartment. The choice of anode in a solid-state battery depends on the mobile species in the electrolyte [39]. In the present case, lithium is used as an anode material.
The schematic diagram of the battery preparation for discharge characteristics is shown in Fig. 8. Discharge characteristics (variation of cell parameters as a function of time) of electrochemical cells with the configuration Li | polymer electrolyte | (I2 + C + polymer electrolyte) for PEO + PVP:Li+ and also along with the Ag nanofiller (4 wt%)-loaded polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):Li+ polymer electrolytes at ambient temperature for constant load 100 kΩ are shown in Fig. 9. It has been identified that during discharge, the cell voltage decreases initially and then remains constant for a particular duration (time of stable performance of the cell which is showing almost constant voltage) after which voltage declines. The initial sharp decrease in voltage could be attributed to the activation polarization and/or the formation of a flimsy layer of lithium at the electrode–electrolyte interface [40] as shown in Fig. 9. The cell parameters such as open-circuit voltage (OCV), short-circuit current (SCC), the plateau region of the cell which shows the voltage remains constant in particular duration, energy density, etc, for PEO + PVP:Li+ and also along with the Ag nanofiller (4 wt%)-loaded PEO + PVP:Li+ polymer electrolyte cells are listed in Table. 1. From the table, it is found that the Ag nanofiller (4 wt%)-loaded PEO + PVP:Li+ polymer electrolyte cell showed better performance in terms of all the parameters. This may be ascribed to the higher ionic conductivity and greater amorphousity of the electrolyte system; the cell parameters of the present electrolyte systems are comparable with those of the earlier work reported on different polymer electrolyte systems [41]. Thus, based on these results, 4 wt% Ag nanofiller-dispersed PEO + PVP:Li+ blended polymer system could be found to be a promising electrolyte material in practical electrochemical cell applications.
Conclusions
In summary, it could be concluded that we have successfully synthesized the polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP) blended polymer films with and without Li+ ions along with Ag nanofiller. Their semi-crystalline nature has been confirmed based on XRD features. FTIR and Raman spectral studies suggested the variation in the environment of the functional groups upon addition of the salt along with Ag nanofiller. It confirms the coordination of ions (cations and anions) with the polar groups of blended polymer host during the formation of complexes. The ionic conductivity of the Li+ (0.1 wt%)-doped PEO + PVP blended polymer has been found to be 1.53 × 10−6 S cm−1 at room temperature. The enhancement of the ionic conductivity of the polyethylene oxide (PEO) + polyvinyl pyrrolidone (PVP):Li+ polymer electrolyte has been found with the addition of Ag nanofiller to the polymer electrolyte. Ag nanofiller-added PEO + PVP:Li+ polymer electrolyte has exhibited high ionic conductivity, which is in the order of 1.14 × 10−5 S cm−1 at 4 wt% concentration of the Ag nanofiller. PEO + PVP:Li+ + Ag nanofiller (4 wt%) cell exhibited better performance in terms of cell parameters like OCV, SCC and discharge time for the plateau region, etc. This is attributed to the presence of flexible matrix and high ionic conductivity. The applicability of the present 4 wt% Ag nanofiller-dispersed PEO + PVP:Li+ polymer electrolyte system could be suggested as a possible prospective candidate for solid-state electrochemical cells.
References
Li J-X, Du Z-X, Wang J-G, Wang T, Lv J-N (2012) Zinc and manganese coordination polymers constructed by a new coordination mode of 4,5-dicyanoimidazolate ligand: syntheses, crystal structures, fluorescent and magnetic properties. Inorg Chem Commun 15:243–247. doi:10.1016/j.inoche.2011.10.036
Gray FM (1991) Solid polymer electrolytes. VCH, New York
Kiran Kumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, Narasimha Rao VVR (2012) Electrical conduction mechanism in NaCl complexed PEO/PVP polymer blend electrolytes. J Non-Cryst Solids 358(23):3205–3211
Rajeswari N, Selvasekarapandian S, Prabu M, Karthikeyan S, Sanjeeviraja C (2013) Lithium ion conducting solid polymer blend electrolyte based on bio-degradable polymers. Bull Mater Sci 36:333–339. doi:10.1007/s12034-013-0463-2
Sivaiah K, Buddhudu S (2012) Photoluminescence spectra of Sm3+ and Dy3+: PVP polymer films. Indian J Phys 86(12):1079–1085. doi:10.1007/s12648-012-0176-1
Vignarooban K, Dissanayake MAKL, Albinsson I, Mellander B-E (2014) Effect of TiO2 nano-filler and EC plasticizer on electrical and thermal properties of poly(ethylene oxide) (PEO) based solid polymer electrolytes. Solid State Ionics 266:25–28. doi:10.1016/j.ssi.2014.08.002
Tripathi SK, Gupta A, Jain A, Kumari M (2013) Electrochemical studies on nanocomposite polymer electrolytes. Indian J Pure Appl Phys 51:358–361
Abraham R, Thomas SP, Kuryan S, Isac J, Varughese KT, Thomas S (2009) Mechanical properties of ceramic-polymer nanocomposites. Express Polym Lett 3(3):177–189
Majles Ara MH, Naderi H, Mobasheri A, Rajabi MH, Malekfar R, Koushki E (2013) Characterization and nonlinear optical properties of PVP/TiO2 nano-fibers doping with Ag colloid nano-particles. Phys E 48:124–127. doi:10.1016/j.physe.2012.11.027
Ravi M, Kiran Kumar K, Madhu Mohan V, Narasimha Rao VVR (2014) Effect of nano TiO2 filler on the structural and electrical properties of PVP based polymer electrolyte films. Polym Test 33:152–160. doi:10.1016/j.polymertesting.2013.12.002
Aziz SB, Abidin ZHZ, Arof AK (2010) Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosan-silver triflate electrolyte membrane. Express Polym Lett 4(5):300–310. doi:10.3144/expresspolymlett.2010.38
Gaddala B, Nataru S (2015) Synthesis, characterization and evaluation of silver nanoparticles through leaves of Abrus precatorius L.: an important medicinal plant. Appl Nanosci 5:99–104. doi:10.1007/s13204-014-0295-4
Kiran Kumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, Narasimha Rao VVR (2011) Investigations on the effect of complexation of NaF salt with polymer blend (PEO/PVP) electrolytes on ionic conductivity and optical energy band gaps. Physica B 406:1706–1712. doi:10.1016/j.physb.2011.02.010
Dey A, Das K, Karan S, De SK (2011) Vibrational spectroscopy and ionic conductivity of polyethylene oxide–NaClO4–CuO nanocomposite. Spectrochim Acta A 83:384–391. doi:10.1016/j.saa.2011.08.050
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Conductivity and thermal studies of blend polymer electrolytes based on PVA–PMMA. Solid State Ionics 177:2679–2682. doi:10.1016/j.ssi.2006.04.013
Noor SAM, Ahmad A, Talib IA, Rahman MYA (2010) Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics 16:161–170. doi:10.1007/s11581-009-0385-6
Rajendran S, Kannan R, Mahendran O (2001) Ionic conductivity studies in poly(methylmethacrylate)-polyethlene oxide hybrid polymer electrolytes with lithium salts. J Power Sources 96:406–410
Papke BL, Ratner MA, Shriver DF (1982) Vibrational spectroscopic determination of structure and ion pairing in complexes of poly(ethylene oxide) with lithium salt. J Electrochem Soc 129:1434–1438. doi:10.1149/1.2124179J
Matsuura H, Fukuhara K (1986) Vibrational spectroscopic studies of conformation of poly(oxyethylene).II Conformation-spectrum correlations. J Polym Sci B 24:1383–1400. doi:10.1002/polb.1986.090240702
SubbaReddy Ch V, Ji A-P, Zhu Q-Y, Mai L-Q, Chen W (2006) Preparation and characterization of (PVP + NaClO4) electrolytes for battery applications. Eur Phys J E 19:471–476. doi:10.1140/epje/i2005-10076-8
Ramya CS, Selvasekarapandian S, HiranKumar G, Savitha T, Angelo PC (2008) Investigation on dielectric relaxations of PVP-NH4SCN polymer electrolyte. J Non-Cryst Solids 354:1494–1502
Joydeep (2012) Synthesis and characterization of γ-irradiated PVA/PEG/CaCl2 hydrogel for wound dressing. Am J Chem 2:2. doi:10.5923/j.chemistry.20120202.02
Trchova M, Sapurina I, Prokes J, Stejskal J (2003) FTIR spectroscopy of ordered polyaniline films. Syn Met 135:305–306. http://hdl.handle.net/11104/0002617
Kassaee MZ, Mohammadkhani M, Akhavan A, Mohammadi R (2011) In situ formation of silver nanoparticles in PMMA via reduction of silver ions by butylated hydroxytoluene. Struct Chem 22:11–15. doi:10.1007/s11224-010-9671-1
Ramya CS, Selvasekara Pndian S, Savitha T, Harankumar G, Angelo PC (2007) Vibrational and impedance spectroscopic study on PVP–NH4SCN based polymer electrolytes. Phys B Condens Matter 393:11–17. doi:10.1016/j.physb.2006.11.021
Rhodes CP, Frech R (1999) Cation–anion and cation–polymer interactions in (PEO)n NaCF3SO3 (n = 1–80). Solid State Ionics 121:91–99, PII: S0167-2738(98)00534-7
Kumar Y, Hashmi SA, Pandey GP (2011) Ionic liquid mediated magnesium ion conduction in poly(ethylene oxide) based polymer electrolyte. Electrochem Acta 56:3864–3873. doi:10.1016/j.electacta.2011.02.035
Hema M, Selvasekarapandian S, Hirankumar G, Sakunthala A, Arunkumar D, Nitya H (2010) Laser Raman and ac impedance spectroscopic studies of PVA: NH4NO3 polymer electrolyte. Spectrochim Acta A 75:474–478. doi:10.1016/j.saa.2009.11.012
Stojanovic DB, Brajovic L, Orlovic A, Dramlic D, Radmilovic V, Uskokovic PS, Aleksic R (2013) Transparent PMMA/silica nanocomposites containing silica nanoparticles coating under supercritical conditions. Prog Org Coat 76:626–631
Faghihi K, Shabanian (2011) Thermal and optical properties of silver-polyimide nanocomposite based on diphenyl sulfone moieties in the main chain. J Chin Chem Soc 56:665–667. doi:10.4067/S0717-97072011000200009
Carotenuto G, Giannini C, Siliqi D, Nicolais L (2011) Nanocomposites based on metal and metal sulfide clusters embedded in polystyrene. Polymers 3:1352–1362. doi:10.3390/polym3031352
Song JY, Wang YY, Wan CC (1999) Review of gel-type polymer electrolytes for lithium-ion batteries. J Power Sources 77:183–197, PII: S0378- 7753 98 00193–1
Mishra R, Baskaran N, Ramakrishnan PA, Rao KJ (1998) Lithium ion conduction in extreme polymer in salt regime. Solid State Ionics 112:261–273, PII: S0167-2738(98)00209-4
Scrosati B, Croce F, Panero S (2001) Progress in lithium polymer battery R&D. J Power Sources 100:93–100
Pradhan DK, Choudhary RNP, Samantaray BK (2008) Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int J Electrochem Sci 3:597–608
Sangwa RJ, Sankhla S (2007) Poly(vinylepyrrolidone)-poly(ethylene glycol) blends colloid. Polym Sci 285:1237
Baskaran R, Selvasekarapandian S, Hirankumar G, Bhuvaneswari M (2004) Dielectric and conductivity relaxations in PVAc based polymer electrolytes. Ionics 10:129
Zhang S, Dou S, Colby RH, Runt J (2005) Glass transition and ionic conduction in plasticized and doped ionomers. J Non-Cryst Solids 351:2825–2830
Sarma RVGK, Radhakrishna S (1990) Silver borotungstate glasses: new electrolyte for solid state electrochemical cell. Solid State Ionics 40–41:483–486
Sasikala U, Naveen Kumar P, Rao VVRN, Sharma AK (2012) Structural, Electrical and parametric studies of a PEO based polymer electrolyte for battery applications. Int J Eng Sci Adv Technol 2:722–730
Madhu Mohan V, Raja V, Sharma AK, Narasimha RaO VV (2005) Ionic conductivity and discharge characteristics of solid-state battery based novel polymer electrolyte (PEO + NaBiF4). Mater Chem Phys 94:177–181. doi:10.1016/j.matchemphys.2005.05.030
Acknowledgments
This work was supported by the Yeungnam University Research Grant (No: 215A345015). The author (KNK) thanks to the Department of Chemistry, Yeungnam University, South Korea, for providing postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, K.N., Kang, M., Sivaiah, K. et al. Enhanced electrical properties of polyethylene oxide (PEO) + polyvinylpyrrolidone (PVP):Li+ blended polymer electrolyte films with addition of Ag nanofiller. Ionics 22, 815–825 (2016). https://doi.org/10.1007/s11581-015-1599-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1599-4