Abstract
This paper describes preparation and characterization of polyvinyl chloride and polyethylene glycol 2000 blend polymer electrolytes with LiX (X=ClO −4 , BF −4 , and CF3SO −3 ) salt by solution casting technique. Ethylene carbonate and propylene carbonate mixture was used as the plasticizers. LiClO4-based electrolytes exhibited better ionic conduction behavior than other salts. The thermal profiles ascertain the stability of the membranes up to 120°C by differential scanning calorimetry. Complexation and crystallinity were studied through X-ray diffraction measurements. Phase morphological study reveals the porous nature of the polymer electrolyte membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the recent years, there has been an increasing interest to develop the polymer electrolytes for various applications, such as high energy density batteries, fuel cells, electrochemical capacitors, solar cells, sensors, etc. due to their versatility and dimensional stability [1–3]. Polyethyleneoxide-based electrolyte is the first system and extensively studied by many researchers. But its applicability in ambient temperature is excluded due to the crystalline nature of the host at ambient temperature, and coordination of Li+ ions with ether oxygen atoms in the amorphous phase exhibits the conductivity in the order of 10−8 to 10−5 S/cm [1, 4].
Gel polymer electrolytes, in which liquid counterparts, are immobilized in a polymer matrix, and it exhibits a conductivity >10−5 S/cm at ambient temperature conditions. However, the mechanical properties are not sufficient for the practical applications. The problem of poor mechanical strength can be circumvented by blending the polymer-like polyvinyl chloride (PVC), which also exhibits excellent phase separated morphology due to the poor miscibility with plasticizers [5, 6]. Since electrolytes based on polymer blend are free from any additional supporting layer and any chemical cross-linking reaction, they are highly promising for large-scale production in terms of simplicity and cost. The proof of the PVC blend with poly methylmethacrylate (PMMA) concept was demonstrated by Rhoo et al. [7] with phase separated morphology, and the characteristics of lithium ion polymer battery employed with PVC/PMMA electrolytes were reported by Kim et al. [8].
In this context, we have made an attempt to study the blend polymer electrolytes based on PVC and polyethylene glycol 2000 (PEG) with LiClO4, LiBF4, and LiCF3SO3 salts. The ethylene carbonate (EC) and propylene carbonate (PC) binary mixture has been used as the plasticizer.
Materials and methods
The high purity PEG 2000, PVC, LiClO4, LiBF4, and LiCF3SO3 were procured from Aldrich, USA. Plasticizers EC, PC, and the solvent tetrahydrofuran were obtained from E. Merck, India and used without any further purification. The film was cast by using doctor blade method. The cast films were allowed to stand in air at 60°C for 10 h and stored in sealed containers. The thickness of the films obtained were within 40–70 μm. The compositions of the films were given in Table 1.
Conductivity was measured by sandwiching the cast films between the stainless steel electrodes (ASTM 304) of 1 cm2 surface area by using EG&G, Princeton Applied research, USA over a frequency range of 10–100 kHz at different temperatures. The thermal analyses of the films were examined with Perkin Elmer differential scanning calorimetry instrument (DSC) 2010, USA. The XRD analysis was performed by means of JDX 8030, X-ray diffractometer. The morphological features of the films were investigated by scanning electron microscope Stereoscan 440, Leuica, Cambridge, USA.
Results and discussion
A.C. impedance studies
In the present work, the ionic conductivity of the polymer electrolytes are measured as a function of the PVC/PEG 2000 blend ratios (Table 1). We are unable to get the free standing films for higher PEG 2000 content (say the PVC/PEG 2000 3/7 blend ratio (S1)). The conductivity (σ) of the polymer electrolytes are calculated from the following equation using the electrochemical impedance spectroscopy,
where l is thickness of the polymer electrolyte, A is area of the SS electrode, and R b bulk resistance of polymer electrolyte.
The ionic conductivity of the polymer electrolyte increased when decreasing the PVC content from 7/3 to 5/5 (PVC/PEG 2000) and also with temperature for all the three salt systems studied (Fig. 1). The LiClO4 salt system with 5/5 blends ratio (PVC/PEG 2000) containing electrolytes exhibited enhanced conductivity [9]. Polymer electrolyte comprising LiBF4 salt showed slightly lower conductivity than LiClO4-based system, which may be due to the higher mobility of soft BF −4 anion, however, its dissociation constant is significantly smaller than other salts, and this leads to the moderate ionic conductivity [9, 10]. LiCF3SO3 (is based on the conjugate bases of the organic super acids, where acid strength is increased because of the stabilization of the anions by strong electron withdrawing groups usually perfluorinated alkyls) containing electrolytes exhibited poor ionic conductivity. This may be reason that the dissociation constant of LiCF3SO3 is very high even in the low dielectric media leads to the formation of free triflate anions, monodendate ion-paired triflates (LiX, \( {\text{LiX}}_2^{-} \), and \( {\text{LiX}}_3^{2 - } \)) and highly aggregated triflates (Li2X+ and Li3X2+) causing poor ionic conductivity [11]. The conductivity profiles are excellent agreement with the earlier report by Xu [9] in liquid electrolytes based on these salts. On the other hand, the poor conductivity profile observed while increasing the concentration of the PVC is evident of its poor miscibility with the plasticizers [12]. The temperature dependence on ionic conductivity is also observed and presented in Fig. 1. The conductivity variation of polymer electrolytes obeys the Arrhenius behavior, which describes the transport properties in a viscous polymer matrix.
Thermal studies (DSC)
High ionic conductivity, high lithium transport number, and wide electrochemical stability, although desirable properties, are not sufficient to make an electrolyte in useful practical applications. Thermal stability of the polymer electrolyte is also an important parameter to guarantee acceptable performance when it is operated at elevated temperature wherein the safety is concerned. In order to ascertain the thermal stability of the polymer electrolyte, differential scanning calorimetric studies were performed, and the results are presented in Fig. 2.
All the polymer electrolytes exhibited melting endotherm (Tm) ~135°C is due to the crystalline phase of PEG 2000 [13]. The DSC thermograms indicate a lot of melting events occurred above 250°C to 300°C indicative of melting of PVC and boiling of the plasticizers used in this study for the LiBF4 and LiCF3SO3 based systems. In contrary, LiClO4-based electrolytes do not show such kind of melting events, but they exhibited a sharp double exothermic events (one with kink-like appearance) around ~228°C to 300°C. As far as LiClO4 systems are concerned, the exothermic events are highly prominent compared to the minuscule variations of endothermic events that these distinct appearance ascribed to the different level of intake of plasticizers of atatic and syndiotactic sequences of PVC, suggesting the strong influence of PVC-content with other constituents[12]. The strong influence of polymers with electrolyte counterparts leads to the improved conductivity than other systems based on LiBF4 and LiCF3SO3, which is clearly observed from the conductivity studies [13, 14]. Some very small melting events around 60°C to 90°C are attributed to the removal of moisture from the sample, which may absorb when loading of electrolyte film. The DSC thermograms reveal that these electrolytes may be used in the lithium batteries at elevated temperatures (up to 120°C).
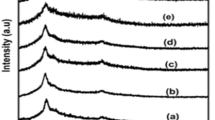
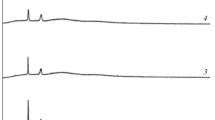
X-ray diffraction analysis
X-ray diffraction studies were carried out to examine the nature of the crystallinity of the polymer electrolytes and to investigate the occurrence of complexation. The PVC shows the characteristic peak at 2θ = 13 and 16 [15–18], and PEG 2000 shows 19 and 23 [16]. Addition of plasticizers and salt in a polymer matrix leads to the complexation of the constituents resulting disappearance and shifting of the bands which are obviously observed (Fig. 3). For example, PEG 2000 peak at 2θ = 19 is observed only in the LiCF3SO3 and LiClO4 salts of 5/5 blend ratio. The 2θ = 23 is only observed in the LiBF4 and LiClO4 salt-based systems of 5/5 blend and is also observed in all the salt systems studied in the 7/3 blend. All the characteristic PVC peaks disappeared due to complex formation of PVC with other constituents of the electrolytes. All the electrolytes exhibited only the broader peak, which is the typical characteristic of amorphous materials. The increase in the amorphous phase supports greater ionic mobility which in turn improves ionic conductivity resultant of fully flexible backbone of the host [17].
Morphological studies
Surface morphological features were analyzed and presented through scanning electron microscope (Fig. 4). All the electrolytes exhibited highly interconnected networks of pores. The pores are indicative of the occurrence of phase separation in the electrolytes, which are indeed necessary for the transportation of Li+ ions between the electrodes [18]. These pores are the sites where the liquid electrolyte (which comprises of lithium salt and plasticizers) solution have been trapped, and the network-like pore walls correspond to the PVC dominance. The difference in pore size (5/5 and 7/3 blends) is related to the driving force for phase separation [7]. The phase separation originates from the immiscibility of plasticizer with PVC. For the 7/3 blend ratio, PVC is coagulated in the earlier stage of phase separation before larger area liquid electrolyte phase is developed. Increasing the PEG 2000 content (say 5/5 ratio) leads to the slower coagulation resulting to smaller pore size [19].
Conclusion
The PVC/PEG 2000 blend polymer electrolytes were investigated with three different lithium salts. Among the systems studied, LiClO4 showed good conductivity than other salt-based systems in 5/5 blend ratio of PVC/PEG 2000. The polymer electrolytes investigated are found thermally stable up to ~120°C, which indicates the possibility of using them at elevated temperatures. The XRD analysis confirmed the phase behavior and complexation of polymer hosts. Surface morphological studies reveal the highly porous nature of the membranes.
References
Gray GM (1993) Solid polymer electrolytes-fundamentals and technological applications, VCH
Tarascon JM, Armand M (2001) Nature 414:359
Armand M, Tarascon JM (2008) Nature 451:652
Abraham KM, Alamgir M (1990) J Electrochem Soc 137:1657
Song JY, Wang YY, Wan CC (1999) J Power Sources 77:183
Stephan AM (2006) Eur Polym J 42:21
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Electrochim Acta 42:1571
Kim HT, Kim KB, Kim SW, Park JK (2000) Electrochim Acta 45:4001
Xu K (2004) Chem Rev 104:4303
Ue M (1995) J Electrochem Soc 142:2577
Frech R (1994) J Solution Chem 23:469
Vickraman P, Aravindan V, Selvambikai M, Shankarasubramanian N (2009) Ionics 15:433
Vickraman P, Aravindan V, Shankarasubramanian N (2007) Ionics 13:355
Panero S, Scrosati B, Greenbaum SG (1992) Electrochim Acta 37:1533
Ramesh S, Arof AK (2001) J Power Sources 99:41
Binesh N, Wan YY, Wan CC (1999) J Power Sources 77:183
Frech R, Chintapalli S (1996) Solid State Ionics 85:61
Aravindan V (2008) Physico-chemical studies of some novel lithium salts based nanocomposite polymer electrolytes for lithium rechargeable batteries, Ph D thesis, Gandhigram Rural University, Gandhigram-624 302, India
Han HS, Kang HR, Kim SW, Kim HT (2002) J Power Sources 112:461
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vickraman, P., Aravindan, V. & Lee, YS. Lithium ion transport in PVC/PEG 2000 blend polymer electrolytes complexed with LiX (X=ClO −4 , BF −4 , and CF3SO −3 ). Ionics 16, 263–267 (2010). https://doi.org/10.1007/s11581-009-0387-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0387-4