Abstract
The gel polymer electrolytes composed of the blend of polyvinylchloride (PVC) and polyvinylidene fluoride (PVdF) as host polymers, the mixture of ethylene carbonate (EC) and propylene carbonate (PC) as a plasticizer, and LiClO4 as a salt was studied. An attempt was made to investigate the effect of PVdF in the plasticized PVC + LiClO4 system in three blend ratios. The differential scanning calorimetry study confirms the formation of polymer–salt complex and miscibility of the PVC and PVdF. The X-ray diffraction results of plasticized PVC (S1, S2, S3) and PVdF-blended films (S4, S5, S6) were compared, in that an increase in PVC concentration decreases the degree of crystallinity for S1 and S3, respectively, but drastically increases for PVC (S2). The increase in PVC content has not accounted in the conductivity studies also noted. However, the blending effect of PVdF showed decreases in crystallinity homogeneously for (S6 > S5 > S4), which were reflected in ionic conductivity measurements. The surface morphology of the films were also studied by scanning electron microscope, and it corroborates the same.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several researchers have studied the gel-type polymer electrolytes (GPE), also called as plasticized polymer electrolytes, as a suitable polymer electrolyte for lithium rechargeable batteries. The reported gel-type polymers were polyacrylonitrile [1–3], polyvinylchloride (PVC) [4, 5], and polyvinylidene fluoride (PVdF) [6–9] and poly methyl methacrylate [10–13]. These GPE showed good mechanical strength but often encounter a leakage problem while the battery design is being made because of the phase separation between the polymer matrix and encapsulated electrolyte solution.
The current and potential importance of the polymer blends developed in the last decades is remarkable, and consequently, a lot of research papers have been published that described the conductive properties of polymer blends [14–15, 17–41]. PVdF [–CH2–CF2–] n has been chosen as a support polymer for PVC because it is anodically stable [16] because of its strong electron-withdrawing functional group (C–F). Watanabe et al. [15] found that PVdF can form homogeneous hybrid films with the lithium salt, ethylene carbonate (EC), and propylene carbonate (PC) in proper proportions.
In the recent years, PVC-based blend electrolytes have gotten their popularity because of their easy processibility, inexpensiveness, and good compatibility. In this connection, Muniyandi et al. [42] optimized PVdF polymer electrolytes by blending PVC with 1:1 ratio of plasiticizer (EC/PC) in different inorganic salts and reported that the PVdF–PVC blend polymer electrolyte exhibits improved cyclic behavior. Based on the literature [17–41], the PVC-based polymer electrolyte system plasticized by EC/PC has been reported to be applicable to lithium and lithium ion batteries, in that an attempt has been made to optimize the plasticized PVC + LiClO4 with PVdF, in the 6:4 plasticizer ratio [43].
In the present study, Solid polymer electrolyte films consisting of PVC, PVdF, LiClO4, and EC and PC are prepared for the first time and characterized by differential scanning calorimetry (DSC), X-ray diffraction (XRD), a.c. impedance and scanning electron microscopy (SEM).
Materials and methods
PVC and PVdF (Aldrich, USA) with an average molecular weight of 1.56 × 105 and 5.34 × 105 were used as received in this study. The salt LiClO4 (99.999%, Aldrich, USA) was dried at 110 °C and kept under vacuum for 48 h before used. The plasticizers EC and PC with high purity (>99%) were purchased from E. Merck, Germany, and used without further purification. Polymer electrolytes were prepared by dissolving them in appropriate amounts of the anhydrous tetrahydrofuran (THF; E. Merck, high-performance liquid chromatography Grade, India) and casting them as films, using a doctor blade method. The cast films were allowed to stand in air at room temperature to allow slow evaporation of THF. Any remaining THF was removed by further drying of the films in an air oven at 50 ± 1 °C for 8 h and stored in sealed containers. All the operations were carried out under argon atmosphere in a glove box. The thicknesses of the films obtained were within 40–70 μm. The compositions of the films are given in Table 1.
Conductivity measurements were performed by sandwiching the cast films between two stainless-steel electrodes (ASTM 304) of diameter 2 cm and using an electrochemical analyzer EIS Model 6310A (EG and G Princeton, Applied Research, USA) over a frequency range of 10 Hz–100 kHz at 30, 40, 50, and 60 °C. The thermal analysis of the films was examined with PerkinElmer DSC 2010 (USA) instrument. The crystalline structure of the resultant electrolyte films was investigated by means of XRD (JDX 8030 X-ray diffractometer). The morphology of the films was examined with a Stereoscan 440 scanning electron microscope (Leuica, Cambridge, UK).
DSC studies
The DSC thermograms of plasticized PVC salt complexes with increasing PVC concentration (S1, S2, S3) and PVdF-blended PVC–salt complexes (S4, S5, S6) in the temperature range of 0–350 °C with a heating rate of 5 °C/min are shown in Fig. 1.
The two glass transition thermal events take place, one at 61.14 °C (S2) and another at 112.68 °C (S3), in the temperature region of 0 to 100 and 105 to 150 °C. The existence of two glass transition temperatures indicate the existence of two amorphous phases (atatic and syndiotactic) in the plasticized PVC–salt complex, which is consistent with the report of earlier investigators [44–46] that atatic and syndiotactic sequences would accept the plasticizer in different degrees. The decrease in T g with an increase in PVC concentration (S1 and S3) may be taken to indicate the increase in polymer flexibility/fluidity implying that the amorphous phase is relatively high at the maximum content of PVC (S3), also reflected in the XRD studies.
There are two crystalline exothermic events in the temperature range of 225 to 350 °C, which were observed in the S1, S2, and S3 systems, that have the same height, which may be due to the interaction of PVC and PVdF through the plasticizer-rich phase in S4. When the PVdF content is decreased (S5 and S6), the lower temperature crystalline peak decreases in height and shifts toward the lower temperature side, whereas the higher temperature crystalline peak increases in height and shifts to the lower temperature side. This may be taken to mean that the crystallinity decreases as the PVdF content increases, and this result is consistent with the XRD results.
In sample S4 (Fig. 1b), a small hump-shaped exothermic events at 170.52 °C is observed, which is absent in S1. This temperature corresponds to the Curie transition of PVdF, which is nearly coincident with its melting temperature [47]. A small melting event appears at 139.52 °C in the temperature region of 125 to 175 °C, which corresponds to the melting of the crystalline region of PVdF [48, 49]. The melting of one crystalline form of PVdF, which occurred as a small event in S5, is well prominent in S6 in which the temperature region of 100 to 150 °C is also noted [49].
XRD studies
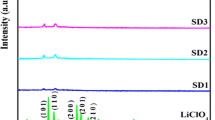
For the system containing LiClO4 at PVC concentration S1, there are three crystalline peaks that correspond to the 2θ values at 10.96, 22.12, and 24.06 ° with 313 counts, and for sample S2, several crystalline peaks are formed that correspond to the 2θ values at 10.64, 11.72, 19.40, 23.4, 33.34, 39.12, 40.52, 48.88, 49.82, and 58.75 ° with a high total intensity of 533 counts, and there are only two peaks that appeared in S3 that correspond to the 2θ values at 11.5 and 23.50 ° with I = 166 counts, respectively. These indicate that the crystallinity of the complex is high for S2 and low for samples S1 and S3 (Fig. 2).
The PVdF-blended films S4, S5, and S6 (Fig. 2) are recorded, and in the higher content of the PVdF-blended sample S4 electrolyte, there are only two crystalline peaks with the I = 199, where in S5, an equal proportion of PVC and PVdF caused a decrease in crystallinity with the absence of some of peaks of S2 with I = 368 counts, and in S6, a meager content of PVdF enhanced the degree of crystallinity with a new appearance of several peaks with I = 600 counts. The XRD results reveal that PVdF blending to plasticized PVC–LiClO4 in three blend ratios describes the degree of crystallinity of polymer electrolytes.
a.c. impedance studies
The XRD and DSC studies have shown that the decrease in crystallinity resulted in the increase of conductivity in S1 and S3 for the LiClO4 system (Fig. 3; this is contrary to our earlier studies [50]). Blending PVdF to samples S1 (5.82 × 10−7 S/cm), S2 (5.27 × 10−7 S/cm), and S3 (5.1 × 10−7 S/cm) increases the conductivity to S4 (1.74 × 10−3 S/cm) and S5 (5.84 × 10−5 S/cm) and decreases to S6 (2.31 × 10−5 S/cm) at ambient temperature (Fig. 4). Thus in the case of LiClO4, where blending leads to high conductivity in three blend ratios as it is evidently seen from the XRD studies in terms of their decrease in crystallinity. Therefore, the blending of PVdF to PVC does seem to be suitable as far as the LiClO4 salt is concerned.
Thus, the study indicates that the polymer electrolytes developed using PVdF-blended plasticized PVC are capable of giving modest conductivities at an ambient temperature of 30 °C, notable among them being PVC/PVdF/EC/PC/LiClO4 with S4 and S5 samples giving a conductivities of 1.74 × 10−3 and 5.84 × 10–5 S/cm, respectively, at ambient temperature. The contribution to ionic conductivity by decoupled viscous modes also cannot be ruled out, and the PVC-based system exhibits the Arrhenius behavior (Fig. 4).
Morphological studies
The morphology of the films of plasticized PVC + LiClO4 in three concentrations (S1, S2, and S3) and the PVdF-blended plasticized PVC in three blend ratios (S4, S5, and S6; Fig. 5) with a fixed plasticizer and salt content were investigated using a scanning electron microscope. The SEM images of Plasticized PVC in all the three concentrations show (Fig. 5—S1, S2, S3) the phase separation of PVC and plasticizer, and homogeneously dispersed pores were seen in the maximum content of PVC [Fig. 5, S3]. This may be due to the degree of miscibility of the plasticizer with PVC that affects the morphology of the resultant polymer electrolyte prepared by the solution-casting technique with the sudden/steady evaporation of THF [43]. Depending on the rate of evaporation, the driving force for the phase separation increases, and consequently, the PVC may attain its coagulation state. Thus, the pores appearance indicates the phase separation of PVC and the plasticizer. The LiClO4 being dissoluted is seen (Fig. 5, S3) in the plasticizer-rich phase.
When minimum/maximum (S6/S4) content of PVdF is added to the maximum/minimum (S3/S1) content of PVC, the appearance of pores is seen (Fig. 5—S4, S5, S6). The large numbers of pores prominently seen in S4 than in S3 indicates the rate of coagulation of PVC. The pores that are increased in sizes in the S5 blend ratio (Fig. 5—S5) are due to the increase in driving force for phase separation. The pores widened in porosity or increased in porosity in S4 (Fig. 5—S4) creating a large number of voids for lithium ion migration that resulted in an increase in amorphousity of the polymer electrolyte that caused it to have increased ionic conductivity. The driving force for phase separation is reduced with an increase in PVdF content with plasticized PVC + LiClO4 indicating the PVdF compatibility with PVC and the plasticizer [43].
Conclusion
The conclusions of this study are as follows:
-
The blending of PVdF to PVC, which seems to be suitable for the LiClO4 salt, is concerned in a 3/7 blend ratio.
-
The XRD and DSC studies confirm the degree of crystallinity and miscibility of PVC and PVC blend complexes as a function of PVC content and PVC /PVdF blend ratio.
-
SEM images of the samples showed phase-separated morphology and an increase in porosity accounting for an increase in conductivity.
References
Abraham KM (1993) Electrochim Acta 38:1223
Croce F, Gerace F, Dautzemberg G, Passerini S, Appetecchi GB, Scrosati B (1993) Electrochim Acta 39(14):2187
Kim HS, Wicho B, Kim JT, Yun KS, Chun HS (1996) J Power Sources 62:21
Alangir M, Abraham KM (1993) J Electrochem Soc 140(6):L96
Pistoia G, Anotonini A, Wang G (1996) J Power Sources 52(2):1319
Tsuninimi K, Ohono H, Tsuchida E (1983) Electrochim Acta 28(6):833
Tsuchida E, Suninimi KT, Ohono H (1983) Electrochim Acta 28(5):591
Jiang Z, Karrol B, Abraham KM (1997) Electrochim Acta 42:2667
Gozdz AS, Tarascon JM, Gebizlioglu OS, Schmutz CN, Warren PC, Shokoohi FK (1994) Electrochem Soc Proc 94–98:400
Feuillade G, Perche Ph (1975) J Appl Electrochem 5:63
Lijima T, Tyoguchi Y, Eda N (1985) Denki Kagaku 53:619
Bhonhe O, Frand G, Rezrai M, Rousselot C, Truche C (1993) Solid State Ion 66:97
Bohnke O, Frand G, Rezrai M, Rousselt C, Truche C (1993) Solid State Ion 66:105
Sung HY, Wang YY, Chaowan C (1998) J Electrochem Soc 145:1207
Watanabe M, Kanba M, Matsuda H, Mizoguchi K, Shinohara I, Tsuchida E, Tsunemi K (1981) Makromol Chem Rapid Commun 2:741
Song JY, Wan YY, Wan CC (1999) J Power Sources 77:183
Carre C, Hamaide T, Guiot A, Mai C (1988) Br Polym J 20:269
Orihara J, Yonecara H (1990) J Macromol Sci 27A:1217
Przyluski J, Such K, Wycislik H, Wieczook W, Florianczyk Z (1990) Synth Met 35:241
Florjanezyk Z, Krawice W, Gresta D, Wieczorek W, Siekierski M (1992) Bull Electrochem 8:524
Wieczorek W, Such K, Florjanczyk Z, Prezyluski J (1992) Electrochim Acta 37:1565
Florjanczyk Z, Wieczoerk W (1994) Solid State Phenom 29–40:161
Paullmer RDA, Kullkarni AR (1994) Solid State Ion 68:243
Alamgir M, Moulton RD, Abraham KM (1991) In: Abraham KM, Salomon M (eds) Proc Symp, primary and secondary lithium batteries, vol. 91–93. The Electrochem Soc, Pennington, NJ, p 131
Abraham KM, Alangir M (1990) J Electrochem Soc 137:1657
Abraham KM (1993) Electrochim Acta 38:1233
Hong W, Liquan C, Xueje H, Rorgian X (1992) Electrochim Acta 37:1671
Armand MB (1980) In: Yeager EB, Schumm B, Blomgren G, Blankenship DR, Leger V, Akridge J (eds) Proc workshop on lithium non-aqueous battery electorchemistry, vol. 80–87. The Lectochem Soc, Pennigton, NJ, pp 261–275
Ue M, Kaitoh M, Yasukawa E, Mori S (1993) Electochim Acta 38:1301
Bohnke O, Rousselot C, Gillet PA, Truche C (1992) J Electrochem Soc 139:1862
Alamgir M, Abraham KM (1997) J Electrochem Soc 140:L96
Kelly IE, Owen JR, Steele BCH (1985) J Power Sources 14:439
Matsuda Y, Morita M, Tsutsumi H (1993) J Power Sources 43/44:439
Nagasubramanian G, Di ste fano S (1990) In: Subbarao S, Koch VR, Owens BB, Smyh WH (eds) Proc symp rechargeable lithium batteries, vol. 90–95. The Electrochem Soc, Pennigton, NJ, p 262
Guglielmi M, Aldebert P, Pineri M (1989) J Appl Electrochem 19:167
Shigehara K, Kobayashi N, Tsuchda E (1984) Solid State Ion 14:85
Baochen M, Li F, Yangayo X (1993) J Power Sources 43–44:83
Andriew X, Boeune JP, Vicedo T (1993) J Power Sources 43–44:445
Ballard DGH, Chesine P, Mann TS, Prezeworski JE (1990) Macromol 23:1256
Takahashi T, Ashitaka H (1990) J Electrochem Soc 137:3401
Huq R, Tarrington C, Kokshong R, Tonder PE (1991) In: Abraham KM, Salomon M (eds) Proc symp primary and secondary. The Electrochem Soc, Pennigton, NJ, p 142
Muniyandi N, Kalaiselvi N, Periasamy P, Thirunakaran R, Ramesh babu B, Gopukumar S, Prem kumar T, Renganathan NG, Raghavan M (2001) J Power Sources 96:14
Rhoo HJ, Kim HT, Park JK, Hwang JS (1997) Electrochim Acta 42:1571
Bair HE, Warren PC (1981) J Macromol Sci Phys B20:381
Douglass DC (1980) Acs Symp Ser 142:147
Beirnes KJ, Burns CH (1986) J Appl Polymer Sci 31:2561
Teyssedre G, Bernes A, Lacabanne C (1993) J Therm Anal 40:711
Kim KM, Ko JM, Park NG, Ryu KS, Chang SH (2003) Solid State Ion 161:121
Dohany JE, Humphery JS (1989) In: Kroschivtz JI (ed) Encyclopaedia of polymer science and engineering, vol. 17. 2nd edn. Wiley, New York, p 532
Vickraman P, Ramamurthy S (2006) Mater Lett 60:3431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vickraman, P., Aravindan, V. & Shankarasubramanian, N. A study on the blending effect of polyvinyledene fluoride in the ionic transport mechanism of plasticized polyvinyl chloride + lithium perchlorate gel polymer electrolytes. Ionics 13, 355–360 (2007). https://doi.org/10.1007/s11581-007-0125-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0125-8