Abstract
Lithium ion conducting poly(ethylene oxide) (PEO):poly(vinyl pyrrolidone) (PVP) polymer blend electrolytes have been prepared by solution casting technique. The XRD and FTIR analysis confirm the complexation between salt and polymers. The conductivity of the samples is analyzed by impedance spectroscopy technique within the frequency range of 42 Hz to 1 MHz in the temperature range of 303 to 363 K. The conductivity of polymer electrolytes increases up to 20 wt % of lithium acetate concentration and the higher conductivity is found to be 2.56 × 10–6 S/cm at room temperature. The transference number measurements are also carried out using Wagner polarization technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
In the recent years, polymer electrolytes are broadly considered because of their potential electro-chemical applications in batteries, energy units, super capacitors, electro-chromic sensors [1]. Polymer electrolytes have numerous points of interest such as adaptability, simplicity of processing into thin films, electrochemical strength and sealing nature [2]. There are few procedures used to prepare polymer electrolytes, for example, blending of polymers [3], co-polymerization, addition of plasticizers and ceramic nanofillers [4] to enhance the ionic conductivity of Polymer electrolytes. PEO has both crystalline and amorphous nature at room temperature [5]. In 1972, Wright detailed about the Ionic conductivity of PEO with soluble alkali metal salt complexes [6]. The main short-comings of PEO based solid polymer electrolytes are poor ionic conductivity (10−8–10−7 S/cm) at surrounding temperature [7] and low cation transference number (<0.3) [7–10]. As of late, PEO has been easily formed as films and has good lithium ion stability. So that, it has been widely contemplated as an electrolyte and separator materials. The coordination bonds have been formed by wrapping the lithium ion with the polymeric chain of PEO [11, 12].
To improve the amorphous nature and conductivity, the blending of polymers is one of the viable ways. Blending of polymers frequently display improved properties that are better than the single polymer [13]. Among the conjugated polymers, PVP is unique organic one because of its high amorphous nature that allows quicker ionic mobility. The development of various complexes with diverse inorganic salts is possible by using PVP because of the existence of the carbonyl group (C=O) in the side chain. PVP is exceedingly solvable in polar solvents, for example, alcohol, water, etc. And also having high glass transition temperature Tg with great eco-friendly, thermal and mechanical stability [14]. The another preferred point of PVP is that the formation of thermally cross connected polymer chain and accordingly, it has extraordinary thermal stability and high mechanical strength of blend materials [15, 16]. On account of its exceptional attributes, Poly(vinyl pyrrolidone) (PVP) was chosen as another polymer to prepare polymer blends with PEO.
In last couple of years, ion based strong polymer electrolytes have been prepared. Lithium batteries have high energy density because of high polarizing energy of lithium ions and little cationic size. The interesting points like no-spillage of electrolyte, higher energy density, adaptable geometry and enhanced well-being perils attract the researchers to work on lithium polymer batteries [17–19]. In the current work, the solid polymer blend electrolytes based on the PEO/PVP in addition of different ratios of lithium acetate have been prepared by solution casting technique and characterized by various techniques.
EXPERIMENTAL
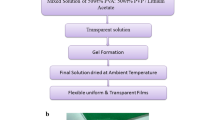
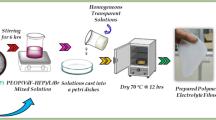
PEO (Molecular weight 4 × 106 g/mol) is purchased from Aldrich chemicals USA, PVP (molecular weight, Mw = 9 × 105) is purchased from (S-d fine chem-Ltd), India and lithium acetate of molecular weight Mw = 102.02 is purchased from Merck, India. The solution casting technique is simple and flexible method to prepare polymer electrolyte. Double distilled water is used as solvent. PEO, PVP and lithium acetate are taken in the ratios of (67.5 : 27.5 : 5) (65 : 25 : 10) (62.5 : 22.5 : 15) and (60 : 20 : 20). Initially the polymers PEO, PVP and lithium acetate are dissolved in distilled water separately. Then it is mixed and stirred well. The resultant homogeneous solution is poured into Petri dish and allowed for solvent evaporation.
The Powder X-ray Diffraction pattern of the polymer blend electrolytes is detailed using Bruker make X-Ray diffractrometer using Cu Kα radiation (λ = 1.540 Å) with scanning rate 5° per minute in the range of 10°–80°. The FTIR transmittance spectra of the films are detailed using “SHIMADZU IR Tracer 100” Spectrometer with a resolution of 4 cm–1 in the wave number range of 400–4000 cm–1. The impedance measurements are done by the computer controlled HIOKI 3532-50 LCR Hi-tester within the frequency range of 42 Hz–1 MHz in the temperature range of 303–363 K. Wagner’s polarization technique is used to determine the ionic and electronic transference numbers.
RESULTS AND DISCUSSION
X-Ray Diffraction Studies
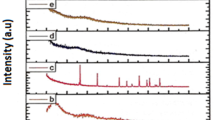
X-ray diffraction is a main technique to define the amorphous nature of the blend polymers. Figure 1 displays the X-ray diffraction pattern of various composition of lithium acetate complexed with PEO/PVP polymer electrolytes. The sharp diffraction peaks appeared at 18° and 23° corresponding to PEO indicates the crystalline nature [20]. After blending PVP with PEO, the peak intensity decreases. As increasing the concentration of salt with the polymer blend, the sharp diffraction peaks become, reducing that indicates the increase in amorphous nature in whole polymer system. There is no peak corresponding to lithium acetate were observed that specifies the nonexistence of extra salt in the blend polymer films and the broad dispersion of salt with the polymer system. The characteristic peaks of PEO/PVP/20 wt % lithium acetate have decreased to a maximum amount than the other prepared blend polymers.
FTIR Analysis
FTIR investigation is performed to determine the interaction between the salt and polymers in the electrolytes. The distinctions in the spectral features have been seen on looking at the spectra of PEO/PVP with various concentration of lithium acetate are exhibited. FTIR spectra of different compositions of (PEO/PVP/lithium acetate) are given in Figs. 2a and 2b. It is conceivable to ascribe the transmittance bands of lithium acetate mixed blend polymer complex. The diverse vibrational modes of interaction strengths and the occurrence of specific groups may possibly affirm the presence of different element of the electrolyte films.
The bands appearing at 3500–3000 cm–1 demonstrates the OH vibration, the band increases by increasing the concentration of lithium acetate. A peak at 2885 is seen owing to the asymmetric (CH) bending of CH2 of PEO [21]. The band at 1343 cm–1 is because of CH2 bending of PEO. The robust interaction in PEO is CH2 twisting at 1282 cm–1, which is moved to 1278 cm–1, may be because of the bonding of CH3COO-Li with PEO/PVP. The slight band at about 1238 cm–1 is allotted to CH2 symmetric stretching of PEO [21]. The vibration band at 1099 cm–1 is fixed to C–O–C (symmetric and asymmetric) stretching of PEO [23] that is moved somewhat in all the complexes. The mode in charge of the band at 845 cm–1 is essentially owing to the CH2-rocking through a slight commitment from C–O stretching movement of PEO while band at 947 cm–1 starts fundamentally in the C‒O stretching with some CH2-rocking [22].
The vibrational band at 1020 cm–1 might be because of the stretching of C–O in PEO. The intensity of the peak increases by increasing the salt concentration. These outcomes form the coordination bonds between Li+ particle and ether units (–O–) of PEO. So that the spectral changes are probable in the C–O–C stretching and deformation ranges. The band around 1463 cm–1 are ascribed to C=O stretching and CH2 wagging of PVP of the all complexes respectively. Conversely, for pure PVP, a vibration band observed at 2885 cm–1 may possibly be ascribed to aliphatic C–H stretching of PVP [23]. The bands around 1463 cm–1 are ascribed to C=O stretching and CH2 wagging of PVP of all complexes respectively. Consequently, the spectral analysis approves the complexation of polymer blend and lithium salt. Table 1 shows the vibrational frequencies of PEO/PVP/lithium acetate polymer electrolytes.
AC Impedance Analysis
The characteristic impedance plots (Z ' vs. Z ") for PEO/PVP/X wt % lithium acetate polymer electrolytes at ambient temperature are shown in Fig. 3. There exists a depressed semicircular portion in all the polymer systems. In the complex impedance, representation, by increasing the salt concentration, there exists a spike. The appearance of the spike is mainly because of the typical jamming double-layer capacitance. The bulk response of the films in high frequency region is indicated by the semicircle. In the equivalent circuit, the semicircle is noted by the parallel combination of resistance and capacitance and this combination produces the impedance effect due to the passage of ions and the polarization of immobile polymer chains [24].
The intercept of the spike on X-axis of the complex impedance plot gives the bulk resistance. The ionic conductivity is calculated by the formula:
where l—thickness of the film, A—area of the film and Rb—bulk resistance of the material. By increasing the salt concentration, the conductivity increases due to the raise in number of movable charge carriers and a drop in the crystallinity nature of polymer electrolytes. The polymer electrolyte PEO/PVP/20 wt % lithium acetate shows the higher conductivity of 2.56 × 10–6 S/cm. The ionic conductivity demonstrates the effective collaboration among oxygen atoms and Li+ cations in the electrolytes [25, 26]. From Fig. 4, it is detected that the conductivity is increasing by increasing the temperature, indicating the raise in amorphous nature of the polymer electrolytes. The polymer electrolytes have more flexible chain that promotes the segmental motion of polymer that encourages higher mobility [27].
Temperature Dependent Conductivity
Figure 5 exhibits the linear dependence of log σT with respect to inverse temperature (1000/T) for blend and lithium acetate complexed films. The direct deviation in log σT versus 1000/T plot prescribes an Arrhenius-type of thermally started process denoted by
where σ0 is the pre-exponential factor, Ea—activation energy, K—Boltzmann constant and T—absolute temperature. In all the compositions of the polymer blend electrolytes, ionic conductivity is found to increase with increase in temperature [28–30].
At the point, when the temperature increases, there is a creation of little measure of space encompassing its specific volume because of the vibrational energy of a fragment of polymer chain. The vibrating energy winds up adequate to thrust against the hydrostatic pressure forced by its nearby atoms in this manner making free volume [31]. In this manner, the free volume all over the polymer chain creates the movement of the ions and the segmental movement of polymer chain and along these builds the conductivity. The expansion in amorphous nature of the polymer blend electrolyte gives a more free volume in the polymer electrolyte framework on expanding temperature [32]. By using the linear fitting of Arrhenius plots, the activation energy values are calculated for the prepared polymer electrolytes and are listed in Table 2. By increasing the concentration of lithium acetate, the value of activation energy is found to be decreased steadily. Figure 6 shows the composition dependent conductivity of polymer electrolytes.
Dielectric Studies
The dielectric constant of the polymer blends with different concentrations of lithium acetate is denoted in Figs. 7a and 7b.
The complex dielectric constant of a system is defined by,
where ε' and ε'' denotes the real (dielectric permittivity) and imaginary (dielectric loss) part of dielectric constant.
Figure 7a illustrates the deviation of the dielectric permittivity ε through frequency for the PEO/PVP/20 wt % lithium acetate polymer electrolyte at different temperatures. The plot obtained for dielectric permittivity denotes the monotonic diminish in the permittivity with low frequencies and achieves a steady rate at higher frequencies [39–45]. However by raising the electric field, the dipoles in the polymer electrolytes are not having the capacity to take after the field variations at high frequencies and there is an impact due to the electrode polarization. The dielectric permittivity begins to go down [47]. The occasional inversion of the field happens so quick at higher frequencies and there is an absence of over abundance ion dissemination towards the field.
Argand Plot Analysis
The Argand plot analysis gives the nature of relaxation processes present in the polymer electrolytes at different temperatures. Figure 8 shows the Argand plot of 20 wt % lithium acetate doped PEO/PVP polymer matrix at different temperature. The figure shows incomplete semicircle which explains the non Debye nature of the polymer electrolytes. So that two relaxation processes occur in polymer electrolytes. One is due to the dipolar relaxation and the other is occurred because of the existence of permanent dipoles on the side chains of the polymer backbone. The existence of relaxation time is because of the hopping of ions, space charge polarization and inhomogenetic of the material [48]. The semicircle with diameter below the axis indicates that the motion of ions and the polymer segmental motion are strongly coupled. The radius of the arc in the Argand plot is highly related to the electrical conductivity. The larger arc relates to the lower conductivity [49].
Tangent Spectra Analysis
Dissipation of energy takes place during the ionic transport and polarization of a dipole and it can be explained by the tangent loss. The morphology of the polymer, crystallinity level and molecular weight are the influencing factors of loss tangent or dissipation factor. In Fig. 9a, the peak in the loss tangent plot demonstrates the event of phase transition when the temperature changes. From the XRD, it is clear that the intensity of PEO peak declines by increasing lithium acetate concentration and further-more the expansion in amorphous nature.
In Fig. 9b, the peaks observed tends to move in the direction of the higher frequency region with the rise in temperature that move ahead to decrease in relaxation time of polymer systems. The swiftness of the polymer chain segmental motion motivates the amorphous nature of the polymer blend electrolytes while rising the temperature [48]. In this figure, it is observed that all the scaled tangent loss spectrum disintegrate into a single curve. Thus, dissipation of energy is temperature independent. From this, the scaling property of the tangent spectra is determined from the potency of the Coulomb interaction between movable particles. The relaxation frequency and the relaxation time of the prepared blend polymer electrolytes are presented in the Table 3.
Transference Number Studies
For the characterization of polymer electrolyte materials, transference number is considered as the utmost significant parameter. The transference number is used to relate the dispersion phenomena to the conductivity behavior of prepared polymer electrolytes. Wagner’s polarizing technique is used to determine the Transference number [49]. In this method, the prepared polymer electrolyte is sandwiched between the two silver electrodes. By applying the fixed dc voltage (1.5 V) and observing the value of current by increasing the time, the transference number can be calculated.
The current consequently begins decreasing with time. The cell gets polarized by the passage of ions due to the electric field and thereby introduces a concentration gradient. Electrons or holes then only convey the polarization current. The transference numbers tele and tion are computed by the equations.
Figure 10 denotes the graph between the polarization current versus time for different polymer electrolytes. From Table 4, transference number of polymer electrolytes are in the range of 0.86–0.96. From this, it is confirmed that the charge transport of the investigated polymer electrolytes is predominately due to ions. The electron contribution is highly negligible in all the samples as suggested by the other workers [50, 51]. The transference number (tion) of the prepared polymer electrolyte films is closed by unity and denotes the conductivity is mainly due to lithium ions. So that, these kinds of polymer electrolytes are appropriate for solid-state electrochemical cells applications.
M.V. Leena Chandra, reported that charge carrier of the prepared polymer electrolytes are calculated using the following equation [54]:
where NA—Avogadro’s number, ρ—density of the salt.
From the charge carrier and transference number, diffusion coefficient and mobility of both anion and cations are calculated by the following formula by Arof et al. [30]:
and
Charge carrier and carrier concentration and mobility of both anion and cations are tabulated in Table 4.
CONCLUSIONS
PEO/PVP/lithium acetate polymer membranes are prepared in different compositions. In the X-ray spectrum, there is a decrease in the peaks corresponding to PEO by increasing the lithium acetate concentration. The increase in the amorphous nature point out the absolute dissolvation of the salt with the blend polymer matrix. The complexation between the polymer and the salt is confirmed by the FTIR spectroscopic analysis. There exists an ever-changing bands by changing the concentration of salt. The conductivity of the polymer blend electrolytes improved by increasing the concentration of lithium acetate as well as temperature. The blend polymer electrolyte PEO/PVP/20 wt % lithium acetate is having the higher conductivity as 2.56 × 10–6 S/cm. From the dielectric analysis, obviously the dielectric consistent and loss diminishes monotonically with expanding frequency and accomplishes a steady value at higher frequencies. The field variation is coupled with the creation of electric dipoles. From the tangent plot, the variation of temperature shifts the peaks towards the higher frequencies and thereby diminish the relaxation time. The acquired transference number for the prepared polymer blend electrolytes are in the range of 0.86–0.96 which means that the conductivity is fundamentally because of ions. Thus the prepared polymer electrolytes can be employed for electrochemical applications.
REFERENCES
S. Ramesh, G. B. The, R.-F. Louh, Y. K. Hou, P. Y. Sin, and L. J. Yi, Ind. Acad. Sci. 35, 87 (2010).
P. Santhosh, T. Vasudevan, A. Gopalan, and K. P. Lee, J. Power Sources 160, 609 (2006).
S. Rajendran, R. Kannan, and O. Mahendran, Mater Lett. 49, 172 (2001).
M. Sundar and S. Selladurai, Ionics 12, 281 (2006).
F. M. Gray, Solid Polymer Electrolytes (VCH, New York, 1991).
D. E. Fenton, J. M. Parker, and P. V. Wright, Polymer 14, 589 (1973).
K. Kesavan, C. M. Mathew, S. Rajendran, C. Subbu, and M. Ulaganathan, Braz. J. Phys. 45, 19 (2015).
M. Kovac, M. Gaberscek, and J. Grdadolnik, Electrochim. Acta 44, 863 (1998).
A. Ghosh and P. Kofinas, ECS Trans. 11, 131 (2008).
Z. Ahmad, N. A. Al-Awadi, and F. Al-Sagheer, Polym. Degrad. Stab. 92, 1025 (2007).
P. G. Bruce, Solid State Electrochemistry (Cambridge University Press, Cambridge, 1995), p. 344.
Y. G. Andreev and P. G. Bruce, Electrochim. Acta 45, 1417 (2000).
D. A. J. Rand, J. Power Sources 4, 1 (1979).
K. KiranKumar, M. Ravi, Y. Pavani, S. Bhavani, A. K. Sharma, and V. V. R. Narasimha Rao, Phys. B (Amsterdam, Neth.) 406, 1706 (2011).
J. J. Zieba, Y. Zhang, and P. N. Prasad, Sol-Gel Opt. II 1758, 287 (1992).
H.-D. Wu, I.-D. Wu, and F.-C. Chang, Polymer 42, 555 (2001).
B. K. Money, PhD Thesis (IIT Madras, Madras, 2019).
P. Knauth, Solid State Ionics 180, 911 (2009).
Lithium Batteries-Science and Technology, Ed. by G. Nazri and G. Pistoia (Kluwer Acad. Publ., London, 2004).
A. M. Abdelghany, M. S. Meikhail, and H. Y. Salah, Res. J. Pharm., Biol. Chem. Sci. 6 (1), 1352 (2015).
B. L. Papke, M. A. Ratner, and D. F. Shriver, J. Electrochem. Soc. 129, 1434 (1982).
I. Sireerat, PhD Thesis (School Chem. Inst. Sci., Suranaree Univ. Technol., Suranari, 2005), pp. 54–175.
C. V. Subba Reddy, A.-P. Ji, Q.-Y. Zhu, L.-Q. Mai, and W. Chen, Eur. Phys. J. E: Soft Matter Biol. Phys. 19, 471 (2006).
S. Ramesh and A. K. Arof, Mater. Sci. Eng., B 85, 11 (2001).
N. Ammakutti Sridevi, K. Karuppasamy, S. Balakumar, and X. Sahaya Shajan, Chem. Sci. Trans. 1, 311 (2012).
O. Borodin, G. D. Smith, R. Bandyopadhyaya, P. Redfern, and L. A. Curtiss, Model. Simul. Mater. Sci. Eng. 12, S73 (2004).
M. R. Johan and L. M. Ting, Int. J. Electrochem. Sci. 6, 4737 (2011).
J. M. G. Cowie, in Polymer Electrolyte Reviews, Ed. by J. R. Mac Callum and C. A. Vincent (Elsevier Appl. Sci., London, 1987), Vol. 1, p. 92.
B. Tareev, Physics of the Dielectric Materials (MIR Publications, Moscow, 1979) [in Russian].
S. Ramesh, A. H. Yahana, and A. K. Arof, Solid State Ionics 152, 291 (2002).
P. C. K. Pillai, P. Khurana, and A. Trilatera, Mater. Sci. Lett. 5, 629 (1986).
A. A. Mohamad, N. S. Mohamad, M. Z. A Yahya, R. Othman, S. Ramesh, Y. Alias, and A. K. Aroof, Solid State Ionics 156, 171 (2003).
S. Selvasekarapandian, R. Baskaran, and M. Hema, Phys. B (Amsterdam, Neth.) 357, 412 (2005).
S. Rajendran, R. S. Babu, and M. Usha Rani, Bull. Mater. Sci. 34, 1525 (2011).
R. Damle, P. N. Kulkarni, and S. V. Bhat, Ind. Acad. Sci. 72, 555 (2009).
M. Tripathi, S. Trivedi, R. Dhar, M. Singh, N. D. Pandey, and S. L. Agrawal, Phase Transitions 84, 11 (2011).
W. Wang and P. Alexandridis, Polymers 8, 387 (2016).
N. Ahad, E. Saion, and E. Gharibshahi, J. Nanomater. 2012, 857569 (2012).
L. N. Shukla, A. K. Thakur, A. Shukla, and D. T. Marx, Int. J. Electrochem. Sci. 9, 7644 (2014).
H. G. L. Coster, T. C. Chilcott, and A. C. F. Coster, Bioelectrochem. Bioenerg. 40, 79 (1996).
H. S. Tewari and A. Pati, Int. J. Recent Adv. Phys. 2, 4 (2013).
A. Manjunath, T. Deepa, N. K. Supreetha, and M. Irfan, Adv. Mater. Phys. Chem. 5, 295 (2015).
S. Badr, E. Sheha, R. M. Bayomi, and M. G. El-Shaarawy, Ionics 16, 269 (2010).
S. K. Tripathi, A. Gupta, and M. Kumar, Bull. Mater. Sci. 35, 969 (2012).
A. Arya, S. Sharma, A. L. Sharma, D. Kumar, and M. Sadiq, Asian J. Eng. Appl. Technol. 5, 4 (2016).
A. Islam, Z. Imran, T. Yasin, N. Gull, S. M. Khan, M. Shafiq, A. Sabir, M. A.Munawar, M. H. Raza, and T. Jamil, Mater. Res. 18 (6), 1256 (2015).
B. Chatterjee, N. Kulshrestha, and P. N. Gupta, Int. J. Eng. Res. Ind. Appl. 5 (6), 116 (2015).
K. C. Kwan, Dielectric Phenomena in Solids (Elsevier, New York. 2004).
E. S. Matveeva, Synth. Met. 79,127 (1996).
Merhawi Abreha Gebreyesus, Y. Purushotham, and J. Siva Kumar, Heliyon 2 (7), e00134 (2016).
A. K. Joncher, Mater. Sci. 13, 553 (1987).
P. B. Bhargav, V. M. Mohan, A. K. Sharma, and V. V. R. N. Rao, Curr. Appl. Phys. 9, 165 (2009).
K. Funke, B. Roling, and M. Lange, Solid State Ionics 105, 195 (1998).
M. V. Leena Chandra, S. Karthikeyan, S. Selvasekarapandian, D. Vinoth Pandi, S. Monisha, and S. Arulmozhi Packiaseeli, Ionics 22, 2409 (2016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundaramahalingam, K., Muthuvinayagam, M. & Nallamuthu, N. AC Impedance Analysis of Lithium Ion Based PEO:PVP Solid Polymer Blend Electrolytes. Polym. Sci. Ser. A 61, 565–576 (2019). https://doi.org/10.1134/S0965545X19050171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965545X19050171