Abstract
Purpose
Crossed cerebellar (CC) diaschisis refers to a decrease in cerebellar perfusion in the presence of contralateral supratentorial lesions. Most of the previous studies have examined stroke patients. In contrast to strokes, seizure-related cerebral cortical lesions (SCCLs) usually show hyperperfusion, and therefore, cerebellar perfusion patterns are expected to be different from those of strokes. With arterial spin labelling (ASL), we evaluated the cerebellar perfusion status in patients with SCCLs.
Materials and methods
Using a search of the recent database over the last 31 months, 26 patients were enrolled in this study. The inclusion criteria were as follows: (1) a history of seizures, (2) MR examination taken within 24 h from the last seizure, (3) the presence of SCCLs on T2/FLAIR or DWI, (4) hyperperfusion in the corresponding areas of SCCLs on ASL, and (5) no structural abnormality in the cerebellum. The perfusion status in the contralateral cerebellum was evaluated and categorized as hyper-, iso- and hypoperfusion. The asymmetric index (AI) of cerebellar perfusion was calculated by ROI measurement of the signal intensity on ASL.
Results
The mean time between the last seizure and MR examinations was 5 h 30 min. CC hyperperfusion was observed in 17 patients (65.4%), hypoperfusion in 7 (26.9%) and isoperfusion in 2 (7.7%). Regarding the location of SCCLs, CC hyperperfusion was more frequent (71.4 vs. 58.3%), and the mean AI was higher (42.0 vs. 11.5) when the lesion involved the frontal lobe.
Conclusions
In patients with SCCLs, CC hyperperfusion occurred more often than hypo- and isoperfusion, especially when the lesions involved the frontal lobe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crossed cerebellar (CC) diaschisis (CCD) refers to a decrease in cerebellar perfusion in the presence of contralateral supratentorial lesions. Because each cerebellum is connected with the contralateral cerebral hemisphere through the cortico-ponto-cerebellar (CPC) projecting fibres, a functional disruption of the CPC pathways is the underlying pathophysiologic mechanism of CCD [1, 2]. Although CCD is typically observed following ischaemic stroke or haemorrhage, it has also been reported in seizure patients [3,4,5]. Prolonged seizure activity may cause cerebral cortical damage. Seizure-related cerebral cortical lesions (SCCLs) are characterized by hyperintensity on the T2/FLAIR image and restricted diffusion on DWI and can mimic acute ischaemic stroke in clinical and radiologic findings. In contrast to stroke, SCCLs usually show hyperperfusion [6]. Therefore, cerebellar perfusion patterns are expected to be different from those of strokes.
The arterial spin labelling (ASL) technique is a noninvasive tool used to measure the cerebral blood flow (CBF) without administering a radiopharmaceutical or contrast agent and can be used repeatedly. Until now, PET and SPECT have been widely used in the evaluation of seizure patients. Compared to PET and SPECT, ASL perfusion MR imaging has several advantages, including high availability, low cost, short scan time and lack of radiation hazard [7,8,9,10].
There have been several reports that SCCLs show hyperperfusion on ASL in patients with acute seizures [11,12,13]. However, to the best of our knowledge, cerebellar perfusion abnormalities using ASL have not been investigated in patients with SCCLs. The purpose of this study was to evaluate the cerebellar perfusion status in patients with acute seizures and SCCLs using ASL perfusion MR imaging.
Materials and methods
Patients
This study was approved by our institutional review board. Informed consent was waived due to the retrospective nature of the study design.
From a database search of our institution during the last 31 months (between August 2014 and March 2017), twenty-six patients were enrolled in this study. The patients visited the emergency department because of seizure or seizure-like symptoms. They were evaluated by neurologists and then underwent MR examinations. Among them, twenty-one patients also received electroencephalography (EEG) studies after MR examinations. The inclusion criteria were as follows: (1) a history of seizures in the patient’s medical record, (2) an MR examination within 24 h of the last seizure, (3) the presence of SCCLs on T2/FLAIR or DWI in the unilateral cerebral hemisphere or bilateral hemispheres with unilateral predominance, (4) hyperperfusion in the corresponding areas of SCCLs on ASL and (5) no structural abnormalities in the cerebellum.
An experienced neurologist reviewed all of the patient EEG data and clinical information, including any abnormalities in seizure-related data.
MR imaging protocol
MR examinations were performed using a 3.0-Tesla scanner (Ingenia; Philips Healthcare, Best, Netherlands) with a 32-channel head coil. Imaging sequences included FLAIR, DWI (b value = 0, 1000 s/mm2), ASL perfusion and conventional MR images. ASL perfusion imaging was performed using a 2D pseudo-continuous ASL (pCASL) pulse sequence with the following scan parameters: TR/TE 4000 ms/12 ms, slice thickness/gap 5/2 mm, postlabel delay 1600 ms, label distance 98 mm, FOV 220 mm, voxel size 3.0 × 3.0 × 5.0 mm, flip angle 90°, number of dynamic scans 25 and total scan 3 min and 28 s. From the ASL source data, the rCBF map images were generated automatically by the scanner software. T2-weighted and pre- and postcontrast T1-weighted images were also acquired to assess the presence of structural alterations.
Image analysis
All MR images were reviewed by two neuroradiologists with 3 and 25 years of experience on a picture archiving and communication system. First, we evaluated the location and extent of SCCLs on T2/FLAIR and DWI and evaluated perfusion abnormalities on ASL without knowledge of the clinical information except for the presence of a current clinically and/or electrophysiologically confirmed seizure.
Second, a qualitative analysis (visual assessment) was performed to evaluate the cerebellar perfusion status on the ASL rCBF colour map. Each reviewer who was blinded to the presence and location of SCCLs independently assessed the perfusion status of each cerebellar hemisphere from the bottom to the top and classified these as symmetric (isoperfusion) or asymmetric perfusion. In the cases of asymmetric perfusion, the contralateral cerebellar perfusion (CCP) was divided into hyper- or hypoperfusion based on the location of the supratentorial SCCLs. Finally, the CCP was classified as hyper-, iso- or hypoperfusion compared to the ipsilateral cerebellum. In cases in which there was a discrepancy, two reviewers later reached a consensus.
Third, a quantitative analysis was also performed. The asymmetric index (AI) of cerebellar perfusion was calculated by measuring the rCBF in each cerebellum. On a slice of an axial scan representing the greatest perfusion asymmetry, ROI circles measuring 15–30 mm in diameter were manually drawn on the affected and mirrored cerebellar hemispheres (Fig. 1) [14, 15]. ROIs were placed to avoid the major vessels and cerebellar vermis. The AI of cerebellar perfusion was calculated by using the following formula [15, 16]:
Positive and negative AI values indicate the relative hyperperfusion and hypoperfusion of the contralateral cerebellum, respectively. To assess the reproducibility of the measurements, the AI was measured twice by the same reader at 3-week intervals. The mean value of the repeated measurements was used to determine the AI [17].
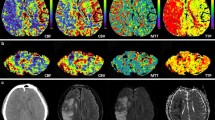
A 47-year-old woman with a generalized tonic–clonic seizure (patient 16). a The axial FLAIR and b DWI show a focal hyperintense lesion in the left frontal lobe, which is presumed to be cortical dysplasia. c The signal intensity of the lesion is isointense on the ADC map image. d The ASL perfusion image reveals increased perfusion in the corresponding area. e The colour and f greyscale ASL images show increased perfusion in the right cerebellum compared to the left. ROI circles are located in both cerebella (f). The calculated asymmetry index was a positive value
Statistical analysis
To assess the interobserver agreement for CCP (hypoperfusion, isoperfusion, hyperperfusion) in the qualitative analysis, the kappa statistic was used [15]. The κ values of < 0.20, 0.20–0.40, 0.41–0.60, 0.61–0.80 and > 0.81 indicated poor, fair, moderate, good and excellent agreement, respectively. Intraobserver reproducibility for the AIs was assessed by calculating intraclass correlation coefficients (ICCs) [18]. The ICCs of < 0.40, 0.40–0.59, 0.60–0.74 and > 0.75 indicated poor, fair, good or excellent reproducibility, respectively [19]. The differences in the AI values according to the CCP status were analysed by using the analysis of variance (ANOVA) test.
Regarding the location of the SCCLs, the patients were divided into frontal-positive and frontal-negative groups according to the presence or absence of frontal lobe involvement. Statistical analysis was performed to determine the difference in the frequency of CC hyperperfusion and AI values between the groups by using the Fisher’s exact test and the Mann–Whitney U test. The significance level for the test was p < 0.05.
Results
The baseline characteristics of the patients and their results are provided in Table 1. Of the 26 patients with SCCLs, 13 were males and 13 were females, with an age range of 15–89 years (mean, 61.7 years). The mean time between the last seizure and the MR examinations was 5 h 30 min. The potential aetiology of the seizures varied. These aetiologies included brain parenchymal tissue loss due to previous infarction or haemorrhage (n = 8, 30.8%), subdural haemorrhage (n = 4, 15.4%), viral encephalitis (n = 3, 11.5%), metabolic (hyponatremia) (n = 2, 7.7%), cortical dysplasia (n = 2), hippocampal sclerosis (n = 1, 3.8%), alcohol withdrawal (n = 1), and idiopathy (n = 5, 19.2%).
In the qualitative assessment, CC hyperperfusion was observed in 17 (65.4%), hypoperfusion in 7 (26.9%) and isoperfusion in 2 patients (7.7%) after a consensus was reached (Table 2). There was one case of discrepancy. The case was classified as isoperfusion by observer 1 and was classified as hyperperfusion by observer 2, respectively. However, the interobserver agreement was excellent (κ value = 0.93).
For the quantitative analysis, intraobserver reproducibility was excellent for the AI (ICC = 0.92). The AI demonstrated general agreement with the CCP status of the visual assessment. All cases with contralateral cerebellar hypoperfusion showed negative AI values (median, − 38.4; range, − 63.0 ~ − 19.8), and CC hyperperfusion showed positive AI values (median, 49.3; range, 10.5–176.4). Cerebellar isoperfusion showed narrow AI values (median, 0.59; range, − 7.5–8.7) (Table 2, Fig. 2). There were significant differences in the AI values between the CC hyperperfusion and hypoperfusion groups (p = < 0.001, ANOVA). However, no significant differences were found between the CC hyperperfusion and isoperfusion groups (p = 0.147) as well as between the isoperfusion and hypoperfusion groups (p = 0.641).
Regarding the location of SCCLs, the SCCLs involved the frontal lobe in 14 patients (53.8%). CC hyperperfusion was more frequently observed with higher AI values in the frontal-positive group [10/14 patients (71.4%), mean AI ± SD, 42.0 ± 63.1] than in the frontal-negative group [7/12 patients (58.3%), mean AI ± SD, 11.5 ± 42.8] (Table 2). However, there was no statistically significant difference (p = 0.401, p = 0.230, respectively).
Discussion
In the present study, CC hyperperfusion was observed in 65.4% of acute seizure patients with SCCLs on ASL perfusion MR imaging (Fig. 1, 3), and it was more common than CC hypoperfusion (26.9%). CCD is a well-known phenomenon in which blood flow and metabolism in the contralateral cerebellum decrease when there is a supratentorial lesion. The interruption of CPC pathways causes a remote functional deactivation with reduced excitatory input and decreased cerebellar blood flow [1, 2]. This phenomenon is commonly observed on perfusion MR imaging, including ASL as well as PET and SPECT, in stroke patients [14, 16, 17, 20, 21]. In contrast to stroke, during seizure activity, CCD is believed to arise from excessive excitatory input along the CPC pathways, which produces a phenomenon similar to the deafferentation syndrome observed after stroke [3]. Therefore, our results of the CCP appear to reflect both the excessive excitatory input due to seizure activity and the reduced excitatory input due to SCCLs. Among them, the excessive excitatory input from the seizure activity appears to have a greater influence on the cerebellar perfusion status in our patients. Until now, the frequency and clinical significance of CC hyperperfusion in patients with SCCLs have not been yet fully established. According to the previous reports of ictal SPECT, CC hyperperfusion was observed in 33–75% of patients with ictal hyperperfusion foci [22,23,24]. Although there are differences in the patient population, imaging modality and timing (ictal vs. postictal), the results of our study are consistent with theirs.
A 73-year-old man with a complex partial seizure (patient 3). a The DWI shows subtle hyperintense lesions in the right frontoparietal lobes (arrows). There is also parenchymal tissue loss due to a previous infarction in the right parietal lobe (arrow head). b On the ADC map image, the lesions of the frontoparietal lobes reveal restricted diffusion (arrows) and the parietal lobe lesion reveals increased water diffusion (arrow head), respectively. c The ASL perfusion image shows markedly increased perfusion in the corresponding areas of DWI hyperintensity. d The left cerebellum reveals hyperperfusion on the ASL image regardless of the tissue loss in the right parietal lobe
Regarding the location of SCCLs, CC hyperperfusion was more frequently observed (71.4 vs. 58.3%), and AI was higher in the frontal-positive group than in the frontal-negative group (mean ± SD, 42.0 ± 63.1 vs. 11.5 ± 42.8). Previous studies using ictal SPECT have also reported that CC hyperperfusion was frequently observed when the ictal focus involved the frontal lobe [23, 24]. This finding seems to be caused by the densest CPC projection fibres arising from the precentral and prefrontal cortical areas [1, 25]. The presence of CC hyperperfusion in seizure patients may be helpful for the lateralization of epileptic foci in clinical situations [22].
In our patients, all the 7 cases of CC hypoperfusion were in the left cerebellar hemisphere with a consequent SCCL in the right cerebral hemisphere. In the group of CC hyperperfusion, 12 out of 17 patients (70.6%) were lateralized in the right cerebellar hemisphere. We reviewed the literature on previous studies. However, we could not find any differences in the cerebellar perfusion patterns according to whether the location of SCCLs was the right cerebral hemisphere or the left cerebral hemisphere. We are not convinced at present whether these results are due to coincidence or whether there is actually a difference in laterality according to the location of the SCCLs. Further studies with a large number of patients are needed.
A seizure can mimic an acute stroke, and a stroke can also present with a seizure [26]. In clinical practice, the differentiation of SCCLs from acute stroke is important to accurately diagnose and provide appropriate treatment. An acute stoke usually shows hypoperfusion, and SCCLs are characterized by hyperperfusion [13]. Nonetheless, an acute infarction showing focal hyperperfusion (luxury perfusion) or reperfusion with spontaneous recanalization of a clot on MR is challenging to differentiate from SCCLs [27]. The CC hyperperfusion associated with SCCLs is an ancillary finding and supplementary useful for the differential diagnosis between an infarct and a SCCL. This finding is different from an acute stroke, which shows hypoperfusion or isoperfusion of the contralateral cerebellum rather than CC hyperperfusion.
Another advantage of this study is the use of the ASL method, which does not require the administration of an exogenous tracer or contrast agent. The ASL technique has been shown to be beneficial in many clinical trials, especially for paediatric patients, patients with impaired renal function or patients who need periodic follow-up perfusion studies. To the best of our knowledge, this report is the first that evaluates cerebellar perfusion using ASL perfusion MR in patients with SCCLs. In addition, the CCP status on ASL was determined with excellent interobserver agreement and was quantified using AI with excellent reproducibility.
In our study, comparing the results of qualitative analysis (visual assessment) and quantitative analysis, the AI demonstrated general agreement with the CCP status of the visual assessment. All cases with CC hypoperfusion showed negative AI values, and CC hyperperfusion showed positive AI values. Cerebellar isoperfusion showed narrow AI values. The mean AI values of the hyperperfusion group and the hypoperfusion group showed statistically significant difference. However, in the isoperfusion group, there were only two cases and there was no statistically significant difference with other groups. Considering the results of visual assessment and quantitative analysis, it seems that the cerebellar perfusion asymmetry can be visually distinguished when the AI value differs by 10 or more.
The present study has several limitations. First, this study was a retrospective study, and only patients with SCCLs and corresponding hyperperfusion were enrolled. This feature may lead to a selection bias because potential patients with only hyperperfusion without T2/FLAIR or DWI abnormality were eliminated. Eight patients had old infarctions or haemorrhage adjacent to the SCCLs, a finding that could affect the results of the CCP. Nonetheless, CC hyperperfusion was also observed in four patients (50%) (Fig. 3), but the frequency was slightly lower than the average.
Second, because the number of subjects was relatively small, we did not find a statistically significant difference between the two groups according to the presence or absence of frontal lobe involvement. Further studies with a larger number of patients will be needed.
Third, we could not determine the evolution of the cerebellar perfusion status because follow-up examinations were not performed in most cases. Because seizure activity is a dynamic process, serial follow-up studies are required for the complete understanding of its pathophysiology. Further studies with serial follow-up are needed.
In conclusion, with ASL perfusion MR imaging, we evaluated the cerebellar perfusion status in acute seizure patients with SCCLs. CC hyperperfusion was observed in 63.0% of the patients, and it occurred more frequently than hypo- and isoperfusion, especially when the lesions involved the frontal lobe.
References
Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522
Gold L, Lauritzen M (2002) Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A 99:7699–7704
Graffeo CS, Snyder KA, Nasr DM et al (2016) Prognostic and mechanistic factors characterizing seizure-associated crossed cerebellar diaschisis. Neurocrit Care 24:258–263
Bailly P, Bazire A, Prat G et al (2017) Crossed cerebellar diaschisis in status epilepticus. Presse Med 46:117–118
Pantano P, Baron J, Samson Y et al (1986) Crossed cerebellar diaschisis: further studies. Brain 109:677–794
Cianfoni A, Caulo M, Cerase A et al (2013) Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol 82:1964–1972
Wolf RL, Detre JA (2007) Clinical neuroimaging using arterial spin-labeled perfusion magnetic resonance imaging. Neurotherapeutics 4:346–359
Detre JA, Rao H, Wang DJ et al (2012) Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging 35:1026–1037
Chen Y, Wang DJ, Detre JA (2011) Test–retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging 33:940–949
Deibler AR, Pollock JM, Kraft RA et al (2008) Arterial spin-labeling in routine clinical practice, part 2: hypoperfusion patterns. AJNR Am J Neuroradiol 29:1235–1241
Matsuura K, Maeda M, Okamoto K et al (2015) Usefulness of arterial spin-labeling images in periictal state diagnosis of epilepsy. J Neurol Sci 359:424–429
Pizzini FB, Farace P, Manganotti P et al (2013) Cerebral perfusion alterations in epileptic patients during peri-ictal and post-ictal phase: PASL vs DSC-MRI. Magn Reson Imaging 31:1001–1005
Miyaji Y, Yokoyama M, Kawabata Y et al (2014) Arterial spin-labeling magnetic resonance imaging for diagnosis of late seizure after stroke. J Neurol Sci 339:87–90
Chen S, Guan M, Lian H et al (2014) Crossed cerebellar diaschisis detected by arterial spin-labeled perfusion magnetic resonance imaging in subacute ischemic stroke. J Stroke Cerebrovasc Dis 23:2378–2383
Kang KM, Sohn CH, Kim BS et al (2015) Correlation of asymmetry indices measured by arterial spin-labeling MR imaging and SPECT in patients with crossed cerebellar diaschisis. AJNR Am J Neuroradiol 36:1662–1668
Lin DD, Kleinman JT, Wityk RJ et al (2009) Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol 30:710–715
Kang KM, Sohn C, Choi SH et al (2017) Detection of crossed cerebellar diaschisis in hyperacute ischemic stroke using arterial spin-labeled MR imaging. PLoS ONE 12:e0173971
Varoquaux A, Rager O, Lovblad K et al (2013) Functional imaging of head and neck squamous cell carcinoma with diffusion-weighted MRI and FDG PET/CT: quantitative analysis of ADC and SUV. Eur J Nucl Med Mol Imaging 40:842–852
Oppo K, Leen E, Angerson WJ et al (1998) Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology 208:453–457
Takasawa M, Watanabe M, Yamamoto S et al (2002) Prognostic value of subacute crossed cerebellar diaschisis: single-photon emission CT study in patients with middle cerebral artery territory infarct. AJNR Am J Neuroradiol 23:189–193
Shinohara Y, Kato A, Kuya K et al (2017) Perfusion MR imaging using a 3D pulsed continuous arterial spin-labeling method for acute cerebral infarction classified as branch atheromatous disease involving the lenticulostriate artery territory. AJNR Am J Neuroradiol 38:1550–1554
Won JH, Lee JD, Chung TS et al (1996) Increased contralateral cerebellar uptake of technetium-99 m-HMPAO on ictal brain SPECT. J Nucl Med 37:426–429
Marks DA, Katz A, Hoffer P et al (1992) Localization of extratemporal epileptic foci during ictal single photon emission computed tomography. Ann Neurol 31:250–255
Shin WC, Hong SB, Tae WS et al (2001) Ictal hyperperfusion of cerebellum and basal ganglia in temporal lobe epilepsy: SPECT subtraction with MRI coregistration. J Nucl Med 42:853–858
Poretti A, Boltshauser E (2012) Crossed cerebro-cerebellar diaschisis. Neuropediatrics 43:53–54
Long B, Koyfman A (2017) Clinical mimics: an emergency medicine-focused review of stroke mimics. J Emerg Med 52:176–183
Deibler AR, Pollock JM, Kraft RA et al (2008) Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR Am J Neuroradiol 29:1428–1435
Acknowledgements
The authors would like to thank ‘Wiley Editing Services’ for the English: http://authorservices.wiley.com/bauthor/english_language.asp.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional review board (IRB) after its approval and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Our IRB determined that patient approval and informed consent were not required because of retrospectively reviewing images and records.
Rights and permissions
About this article
Cite this article
Won, J., Choi, D.S., Hong, S.J. et al. Crossed cerebellar hyperperfusion in patients with seizure-related cerebral cortical lesions: an evaluation with arterial spin labelling perfusion MR imaging. Radiol med 123, 843–850 (2018). https://doi.org/10.1007/s11547-018-0921-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0921-4