Abstract
Background
Crossed cerebellar diaschisis is a rare finding of hemispheric cerebellar depression following contralateral cerebral injury, hypothesized to result from excessive neuronal excitatory synaptic activity along cortico-pontine-cerebellar pathways. The phenomenon is typically observed following ischemic stroke, but has also been characterized during seizure activity—in particular, status epilepticus (SE). Neurological outcome has varied widely in published reports, with some patients achieving full neurologic recovery, while others experience persistent disability.
Methods
Case report and literature review.
Results
We present a 54-year-old man found unresponsive with a right hemispheric syndrome several days after discharge following amygdalohippocampectomy for refractory right temporal lobe epilepsy. Prolonged electroencephalogram demonstrated one subclinical right frontal seizure, along with right frontal periodic lateralized epileptiform discharges, presumed to be associated with SE preceding his admission. Initial MRI demonstrated restricted diffusion on diffusion weighted imaging in the right cerebral hemisphere, ipsilateral thalamus, and contralateral cerebellum. A head CT one week later showed diffuse sulcal effacement with loss of gray–white differentiation in the right frontal and insular regions with low attenuation changes of right thalamus. An MRI showed worsened diffusion restriction, despite a corresponding increase in perfusion. The patient remained paretic at discharge and follow-up. Follow-up MRI at 2 months demonstrated pronounced right cerebral and left cerebellar atrophy, loss of gray matter in much of the right cerebrum, and scattered areas of T2 hyperintensity, consistent with permanent right fronto-temporal neuronal loss.
Conclusions
Collectively, these observations indicate that imaging findings of persistent cerebral restricted diffusion and cytotoxic edema in the subacute post-ictal period may predict irreversible neuronal injury and poor long-term outcome—even when accompanied by evidence of cortical hyperperfusion and recovery of second- and third-order neurons along the involved circuit.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Crossed cerebellar diaschisis (CCD) is a rare phenomenon that was first described clinically in 1914 as contralateral symptoms in the cerebral and cerebellar hemispheres [11]. The first radiographic description was published in 1980, using positron emission tomography (PET) in acute infarct patients [2]. Subsequent reports have described CCD using magnetic resonance imaging (MRI) and characterized the finding in neoplasms, arteriovenous malformations, hemorrhages, and—in conjunction with continuous electroencephalography (cEEG)—have correlated CCD with seizures [9, 10, 12, 13].
Physiologically, CCD results from a decline in cerebellar blood flow and metabolism that occurs contralateral to a supratentorial injury [13]. In the setting of seizures, CCD is thought to arise from excessive excitatory activity along the cortico-pontine-cerebellar pathways, which produces a similar phenomenon to the deafferentation syndrome observed after acute infarct [9, 10, 12]. Cases documenting seizure-associated CCD vary considerably in terms of outcome, ranging from full neurologic recovery to permanent disability. We present a patient with severe injury and poor outcome whose subacute post-ictal imaging demonstrated ischemic-pattern neuronal injury in spite of robust perfusion, and hypothesize that this constellation of findings predicts a poor prognosis.
Case Report
The patient is a 54-year-old right-handed man with a history of intrauterine thalidomide exposure and prior traumatic brain injuries in 1988 and 1995, who presented with medically refractory focal epilepsy with secondary generalization. Prior to evaluation at our facility, he failed multiple anti-epileptic drugs (AEDs), including carbamazepine, valproate, and gabapentin, and was maintained preoperatively on levetiracetam and lacosamide with moderate efficacy.
MRI of the brain demonstrated right mesial temporal sclerosis (Fig. 1). Preoperative assessment included evaluation by the adult epilepsy monitoring unit at our facility for seizure localization. cEEG demonstrated right temporal discharges precipitating focal seizures with secondary generalization.
The right temporal lobe was approached via a frontotemporal craniotomy. Intraoperative EEG using both surface and depth electrodes demonstrated slowing with spontaneous sharp waves within the amygdala, anterior hippocampus, and anterior aspect of the inferior and superior temporal gyri. An extensive resection of the right temporal lobe to the level of the trigone with amygdalohippocampectomy was completed without complication. The patient awoke without focal neurologic deficits, and was transferred to the postanesthesia care unit followed by the neurological intensive care unit (NICU) in stable condition. His postoperative course was unremarkable, and he was dismissed from the hospital on postoperative day two, on his preoperative AEDs. No postoperative seizures were observed.
Two days following dismissal, the patient was found down in his hotel room. He was awake, alert, and oriented to self and location, but amnesic to recent events. Neurologic examination demonstrated a right hemispheric syndrome including dense left-sided hemiplegia, hemianesthesia and hemineglect. The patient was readmitted to the NICU. Computed tomography (CT) imaging showed expected postoperative changes, without evidence of acute vascular, hemorrhagic, or compressive injury.
Levetiracetam level was subtherapeutic at 2.1 mcg/mL (reference range 12.0–46.0 mcg/mL), suggesting AED non-compliance following dismissal. cEEG demonstrated right temporal seizure activity along with clustering of periodic lateralized epileptiform discharges (PLEDs). Complete seizure control was achieved on hospital day one with levetiracetam, valproic acid, and lacosamide. cEEG monitoring confirmed seizure resolution for 48 h prior to discontinuation of EEG monitoring. Laboratory evaluation revealed severe rhabdomyolysis with a peak creatine kinase beyond the upper limit of detection 195,000 U/L (reference range 0–336 U/L) and acute kidney injury (creatinine 5.8 mg/dL, reference range 0.8–1.3 mg/dL) necessitating continuous renal replacement therapy. The combination of electrographic and clinical findings suggested a prolonged period of unwitnessed SE prior to admission.
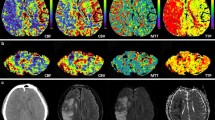
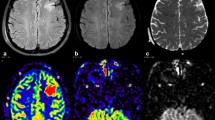
MRI of the brain was obtained 1 day after admission (Fig. 2) and demonstrated restricted diffusion and FLAIR hyperintensities of the right frontal cortex, thalamus, and left cerebellum. MRA of the head was normal. Given the clinical history, pattern of injury on neuroimaging and normal vasculature, the MRI findings were attributed to excitotoxic-mediated neuronal injury and resultant cytotoxic edema from prolonged seizure activity. Seven days later a head CT was obtained for reevaluation (Fig. 3), which demonstrated diffuse loss of gray–white matter differentiation and diffuse sulcal effacement of the right frontal lobe. This raised concern for permanent injury in the area previously shown to have restricted diffusion from his seizures. A second MRI was acquired the next day (Fig. 4), which demonstrated continued restricted diffusion in the right frontal/insular cortex and thalamus with new hazy restricted diffusion involving the white matter. To further evaluate the findings, MR perfusion via arterial spin-labeling technique (Fig. 5) was performed, which demonstrated increased perfusion in the regions of diffusion restriction.
Noncontrast head CT demonstrates diffuse loss of gray–white matter differentiation in the right frontal lobe, with relative preservation of the right basal ganglia. The affected area has mild mass effect with effacement of the sulci, mild effacement of the right lateral ventricle and 2 mm of subfalcine herniation
At the time of dismissal, the patient had poor cognitive function and was noncommunicative. He inconsistently followed commands on the right and had a persistent, dense left hemiplegia. He was unable to follow commands with major axial or bulbar muscles including muscles of facial expression and mastication, and his ability to fixate and track examiners was inconsistent. He was transferred to a long-term rehabilitation facility for further cares.
The patient was seen in follow-up 10 weeks after hospital discharge at which time the patient and family denied additional seizures. His cognitive status had improved such that he was oriented to self, location, time, and situation. His language and articulation were normal, and he was able to describe several major events during his prior prolonged hospitalization. Examination demonstrated full strength on the right side, but persistent dense left hemiplegia, with involuntary movements isolated to his left foot. The patient reported distal paraesthesias in his left upper and lower extremities. MRI (Fig. 6) demonstrated diffuse atrophy of the right frontal lobe and left cerebellum with loss of gray matter corresponding to the most prominent areas of diffusion restriction on the earlier exams.
a Coronal T1 MPRAGE (TR 1900 TE 5.04) and b axial FLAIR (TR 9500 TE 152) demonstrate diffuse atrophy of the right frontal lobe with multiple areas of T2 hyperintensity involving the right frontal cortical/subcortical areas. Note on the coronal T1 MPRAGE the loss of gray matter in the right frontal region, corresponding to the most prominent areas of diffusion restriction on earlier studies
Discussion
CCD is thought to result from interruption of circuits projecting from the cerebral cortex through the ipsilateral basal ganglia and brainstem to the contralateral cerebellum [5, 13]. In the specific subset of CCD cases associated with seizure activity, the underlying pathophysiology is thought to involve a prolonged ictal event that hyperactivates the first order cerebral neurons, causing excitotoxicity and disabling the circuit in a form of transneuronal depression [12].
As compared to CCD in the setting of an acute infarct, this seizure-associated hyperactivation–deafferentation phenomenon is frequently transient, and most case reports describing CCD document clinical and radiographic resolution soon after seizure control is established [7, 9, 10]. In these patients, the transneuronal depression is akin to stunning, causing an insufficient injury to precipitate long-term dysfunction.
In rare cases, severe transneuronal excitotoxic damage yields permanent disability. One such case occurred in a patient without seizure history, who experienced cocaine-induced SE and associated CCD shown on brain MRI. In spite of optimal treatment and seizure resolution, she remained hemiparetic and moderately aphasic [12]. Similarly, a patient with a chronic, severe seizure history over more than 10 years presented with progressive hemiparesis and CCD on brain MRI [1]. Although surgical intervention ultimately yielded good seizure control, neither his clinical hemiparesis nor his MRI abnormalities improved.
Although the history demonstrates several features that likely predisposed our patient to a poor outcome, it appears that a period of prolonged SE was the catalyst for a severe excitotoxic injury resulting in neuronal death in the seizure circuit. We believe that the clinical findings and correlated sequence of imaging abnormalities offer novel insight into the mechanism of injury in seizure-associated CCD, and may provide prognostically valuable information. Specifically, the progression from diffusion weighted imaging (DWI) restriction involving the cortex, thalamus, and cerebellum, to incremental improvement in the thalamus and cerebellum with persistent cerebral abnormalities, to cerebral and cerebellar atrophy, supports the hypothesis that CCD arises from deafferentation of second- and third-order neurons. When present, permanent disabilities arise from the death of the first order cerebral neurons. On the follow-up MRI, we see T2/FLAIR hyperintensity throughout the regions of cerebral injury, whereas the cerebellum demonstrates atrophy without comparable T2 changes.
This hypothesis is further supported by the persistent hemispheric hypodensity observed on CT, which suggests cytotoxic edema throughout the involved cortex—but not the thalamus or contralateral cerebellum—despite MRI evidence demonstrating regional hyperperfusion. Negative vascular imaging, which demonstrated ischemic injury not respecting a specific arterial territory, is also consistent with an excitotoxicity-mediated mechanism. These findings are particularly interesting in light of the follow-up MRI, where the injury appears similar to chronic post-infarct changes, but without MRA abnormality or evidence of hypoperfusion, providing further evidence that metabolically induced cytotoxic damage underlies neuronal death and poor outcome after seizure-associated CCD.
Our case presents a thorough clinical and radiologic description of an exceedingly rare clinical finding—CCD in the setting of SE—including longitudinal follow-up imaging demonstrating permanent cerebral injury with paired atrophy of the cerebrum and cerebellum. This constitutes new evidence describing potential mechanistic differences between patients who do and do not recover from SE-associated injuries. Further, although the majority of CCD cases have described a cortico-pontine-cerebellar pathway, ours is the second documenting a cortico-thalamic-cerebellar circuit [12]. Both of these patients did not recover, suggesting that thalamic involvement may also portend a poor outcome.
A limitation of our report is the absence of MR tractography which could not be obtained during the course of the patient’s evaluation and follow-up. This may provide an important avenue for further characterization of the disease process, particularly as radiographic studies have demonstrated alterations of the cortico-cerebellar circuit on diffusion tensor imaging in CCD patients with chronic infarct [6, 8].
While MRI and CT evidence of hyperperfusion, metabolic hyperactivity, and seizure-related excitotoxic injury have been reported in SE, the interrelatedness of these findings with respect to CCD and their prognostic value regarding permanent injury following SE has not previously been described [3, 4, 12, 14, 15]. The present case describes a novel constellation of radiographic findings that we believe predicts a poor prognosis, and which we anticipate will provide valuable insight in counseling similar patients.
References
Baheti NN, Bansal AR, Rathore C, et al. Teaching NeuroImages: diaschisis: is it always reversible? Neurology. 2009;72:e79.
Baron JC, Bousser MG, Comar D, Castaigne P. “Crossed cerebellar diaschisis” in human supratentorial infarction. Ann Neurol. 1980;8:128.
Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15.
Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45(Suppl 4):72–7.
Feeney DM, Baron JC. Diaschisis. Stroke. 1986;17:817–30.
Johansen-Berg H, Behrens TEJ. Just pretty pictures? What diffusion tractography can add in clinical neuroscience. Curr Opin Neurol. 2006;19:379–85.
Kato T, Okumura A, Hayakawa F, et al. Transient reduced diffusion in the cortex in a child with prolonged febrile seizures. Brain Dev. 2012;34:773–5.
Kim J, Lee SK, Lee JD, et al. Decreased fractional anisotropy of middle cerebellar peduncle in crossed cerebellar diaschisis: diffusion-tensor imaging-positron-emission tomography correlation study. AJNR Am J Neuroradiol. 2005;26:2224–8.
Lansberg MG, O’Brien MW, Norbash AM, et al. MRI abnormalities associated with partial status epilepticus. Neurology. 1999;52:1021–7.
Massaro AM. Teaching neuroimages: crossed cerebellar diaschisis in hemispheric status epilepticus. Neurology. 2012;79:e182.
Monakow CV. Die lokalisation im grosshirn und der abbau der funktion durch kortikale herde. Wiesbaden: J. F. Bergmann; 1914.
Samaniego EA, Stuckert E, Fischbein N, et al. Crossed cerebellar diaschisis in status epilepticus. Neurocrit Care. 2010;12:88–90.
Tien RD, Ashdown BC. Crossed cerebellar diaschisis and crossed cerebellar atrophy: correlation of MR findings, clinical symptoms, and supratentorial diseases in 26 patients. AJR Am J Roentgenol. 1992;158:1155–9.
Warach S, Levin JM, Schomer DL, et al. Hyperperfusion of ictal seizure focus demonstrated by MR perfusion imaging. AJNR Am J Neuroradiol. 1994;15:965–8.
Wasterlain CG, Fujikawa DG, Penix L, et al. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34(Suppl 1):S37–53.
Acknowledgments
The authors would like to thank Dr. Fredric B Meyer, Dr. Alejandro A Rabinstein, and Dr. Eelco F M Wijdicks for their contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graffeo, C.S., Snyder, K.A., Nasr, D.M. et al. Prognostic and Mechanistic Factors Characterizing Seizure-Associated Crossed Cerebellar Diaschisis. Neurocrit Care 24, 258–263 (2016). https://doi.org/10.1007/s12028-015-0155-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0155-4