Abstract
An increase has been observed not only in the absolute number of CT examinations but also in the length of coverage and number of scanning phases, with the result that exposure to ionising radiation from CT is becoming an increasingly serious problem. The extent of the problem is not entirely known and cannot be adequately addressed without proper knowledge of all the phases that leads to the effective dose calculation. In light of the growing awareness of the issue of ionising radiation dose and the possible risk for the individual and the population, there is a need for radiologists, medical physicists and radiographers to play an active role in dose management. In this review, the authors try to delineate the problem in a consequential and multifaceted way: radiation–patient interaction, possible mechanisms of damage, main CT dose units, risk and its quantification in the population, with the aim of optimising the acquisition dose without diagnostic drawbacks. For an “up-to-date” use of CT, radiologists must know the dose concerns for the single patient and population, and use the CT apparatus with the best dose care; substitute CT with other diagnostic techniques when possible, especially in children; reduce the number/extension of scans and phases, and the dose in single scans and single examinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exposure to ionising radiation in computed tomography (CT) is a problem that is becoming progressively more important as CT has acquired the role of a rapid, total-body exploratory examination; it is very popular with both patients and clinicians and is considered a “defensive” tool in the diagnostic setting. An increase not only in the absolute number of CT examinations, but also in terms of both length of coverage and number of phases obtained while scanning (baseline, arterial, sometimes two, venous, late) has been observed [1, 2]. In some places, this is the product of an expedient exchange of the ease and speed of acquisition against appropriateness; however, there is an abuse of the technique. This is often associated with the adoption of suboptimal protocols, as is the case when suitable technique modifications would allow inappropriate exposures to be reduced without loss of quality. The problem is compounded by scanners that seem “friendly”, but are in fact becoming increasingly complex, favouring the adoption of new practices without giving full regard to the optimal use of dose reduction algorithms. It is thus possible that a given instrument, in a single department, as used by various operators for examination of a single body district may yield doses that no longer depend predominantly on the limits of the machine but are operator dependent, with the important implication that some choices of the technicians and radiologists may not be justifiable from diagnostic or dose limitation perspectives. The extent of this problem is not entirely known and cannot be adequately addressed without proper knowledge of all the phases that lead to the effective dose calculation [3, 4]. However, there is no doubt that CT investigations are increasing from every point of view: numbers of requests, extension, and scan phases [1, 2].
In light of the growing awareness of the issue of radiation dose and the possible risk for the individual and population there is a need for radiologists, medical physicists and radiographers to take on an active role in dose management. The purpose of this review is to delineate the problem in a consequential and multifaceted manner: radiation–patient interaction, possible mechanisms of damage, main CT dose units, risk and its quantification in the population, with the aim of optimising the acquisition dose without diagnostic drawbacks. This is extremely important because of the increased awareness of patients, as well as some recent decisions by the American College of Radiology and legislative measures by individual American states (California State “Dose Bill” [5]), which change the professional and legal perspective on the practice of CT, establishing a model that might be quickly and uncritically emulated elsewhere.
Radiation damage
Ionising radiation interacts with the medium in which it propagates yielding its energy. The energy delivery modalities are described by the LET (linear energy transfer). In the case of photons (X-rays), low LET radiation, it is more probable that the interaction takes place with the water molecules that are present in billions of copies in the cell and represent 80 % of the weight. In this case, water undergoes radiolysis with breaking up of the fundamental bond and creation of two highly unstable and reactive species such as free H. and OH. radicals. This primary transfer, in living organisms, starts a complex series of chemical reactions that are a prerequisite for cell changes and the possible beginning of pathological alterations. There is solid experimental evidence that DNA is the main target of ionising radiation and that the damage is not uniformly distributed along the molecule. Even repair is influenced by the structure; in fact the transcription zones are repaired faster than the silent zones, using enzymes and co-factors that recognise the damage. Their lack does not allow a timely repair influencing the survival and then the probability of inducing mutations and carcinogenesis processes [6].
The calculation of dose in CT
The rapid evolution of CT technology and the consequent spread of new clinical applications have determined a deep understanding of all information regarding CT dose calculation and awareness of primary definitions of the parameters for this estimate, which should be revised following the evolution of technology. Dose in CT was first described using the computed tomography dose index (CTDI). Now the original definition has been changed to follow the technological improvements of CT. The CTDI is a basic concept to understand dose measurement in CT and is defined by:
where: D (z) is the profile of the absorbed dose along the z axis, n is the number of slices acquired in a single axial rotation; the value of n may be less than or equal to the maximum number of channels available on the system (for example 64 for a multislice CT detector with 64 rows). T is the nominal thickness of the tomographic section or the amplitude of the group of detectors used in the case of multislice CT (for example 5 mm acquisition for a 4 × 5 mm) (Table 1).
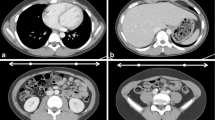
The CTDI can be measured using a 100-mm-long pencil ionisation chamber, either in air (CTDIair) or in a dedicated cylindrical polymethyl methacrylate (PMMA) phantom simulating the head (head-H, 16 cm diameter) and the body (body-B, 32 cm diameter) (Fig. 1). The CTDIair is characteristic for each scanner and depends on tube current intensity and voltage, beam collimation, filtration and the geometric characteristics. Since dose distribution in the phantom is generally not uniform, the measurements are acquired at five different positions (in the centre and at the four cardinal points), thus introducing the weighted CTDI (CTDIw).
where CTDI100,c and CTDI100,p are measured at the centre and at the periphery of the phantom, respectively, and the index 100 indicates that the CTDI was measured with a 100-mm-long ionisation chamber. The CTDIw, however, does not take into account the pitch used during a spiral acquisition.
It was, therefore, necessary to define the volumetric CTDI, the CTDIvol, i.e. CTDIw corrected for pitch. \( {\text{CTDI}}_{\text{vol}} = \frac{{{\text{CTDI}}_{\text{w}} }}{\text{pitch}} \)
It should be noted that the term “mAs” indicated on some manufacturers’ consoles does not refer to true mAs, but to mAs per rotation divided by the pitch. They are commonly defined as “effective” mAs. In this case, the CTDIvol will not change with pitch. Finally, as we do not acquire a single-slice but a whole volume, we should also consider the length of the scan and introduce a second dose descriptor: the dose length product (DLP). The DLP provides information on the total exposure in the case of a complete CT examination and is defined as the product of the CTDIvol multiplied by the irradiated scan length “L”. The DLP expressed in mGy cm is:
The DLP is a comprehensive dose descriptor and allows the assessment of risk by an estimation of the effective dose using the appropriate conversion factors defined by anatomical region. These conversion factors have been defined in a document of the European Commission [7] and updated after the release of ICRP 103 in 2007 to consider the weighting factors for the different tissues shown in Table 2 [8].
Obviously, this approach does not take into account the individual patient size or the specific examination, but allows a rough estimate of the effective dose for protocol optimisation. However, there is a debate in the scientific community about the fact that CTDIvol and consequently, DLP are no longer adequate CT dose descriptors for a number of reasons. The 100-mm-long ionisation chamber is not suitable for multislice CT, because it is not long enough to measure the wider beam width or to include the contribution of the tails of the dose profile, resulting in an underestimation of the measured CTDIvol. Also the commercial phantoms used for CTDI measurements are shorter than the chest of an adult and do not produce enough scattered radiation, as would happen in a standard adult (i.e. average dose received at the thorax underestimated up to 40 %). Finally, the CTDI is not suitable for CT exposures where the patient remains immobile (or almost immobile) during acquisition (cone-beam CT or perfusion studies), in which case the value reported on the scanner console is an overestimation of the mean absorbed dose in the scan volume.
These criticisms are in fact based on the false assumption that the CTDIvol should estimate the dose to the patient. In reality, the variety of patient types, scan protocols and clinical applications is such that no single existing phantom is able to accurately estimate the dose in all patients. The estimations of patient dose for a “standard man” will underestimate the dose received by a paediatric or thin patient and overestimate the dose truly absorbed by an obese subject. Furthermore, as the CTDIvol is displayed on the console before starting a scan and recorded in the dose report at the end of the examination, many users assume that this is the individual patient dose. The CTDIvol is no doubt still useful, but it should be clear that it is a standardised phantom measurement of the CT system output, which enables users to optimise and compare radiation output between different protocols or different scanners and not the real dose delivered to the patient.
Patient dose
Population dose from CT was a radiation protection concern before the advent of ultrafast multidetector scanners; in fact, diagnostic reference levels (DRLs) for CT examinations have been defined in European guidelines [7] and in the Italian law “D.Lgs. 87/00” since 2000 [9].
Even though DRLs should not be applied to individual exposures but are reference doses for common examinations, they can help to optimise radiation protection to avoid unnecessarily high doses to the patient. They are provided for four major anatomical regions: head, chest, abdomen, and pelvis. For CT, the CTDIW and the DLP were suitable quantities to be used as DRLs.
The Italian DRLs for CT are derived from a European document based on a British study performed in the early 1990s. Since then, CT has undergone major evolution and while CTDIW was a good metric at the time of single-slice CT scanners, now it has been replaced by the CTDIvol commonly displayed by the CT scanner console.
In 2006, Shrimpton et al. [10] published a national survey, a review of patient doses from CT examinations in the UK in 2003, conducted on the basis of data received from over a quarter of all UK scanners, of which 37 % had multislice capability. The study collected data for protocols established at each scanner for 12 common types of CT examination on adults and children. The mean UK doses for adult patients were in general lower by up to 50 % than previous ones, although doses were slightly higher for multislice relative to single-slice scanners. The relative increase in reference dose was larger for scans of the head and the chest (high resolution). These examinations both involve axial scanning with narrow beam collimations, where beam penumbral effects and differences in z axis geometrical efficiency between single and multislice scanners were most pronounced [10].
A similar investigation, prior to the widespread adoption of multislice CT was conducted in Italy in 2004 and published in 2006 [11]. The survey was carried out for seven adult clinical CT protocols, and showed that CTDIw and DLP were always below the DRLs set by the European guidelines [7]. Now the first Italian nationwide survey about adult exposures from MDCT and including multiphase studies is at last available [12].
The subsequent advances in CT technology made it possible to collect multiple images per rotation and to acquire a given volume of data in a much shorter time interval. This caused a general increase in the DLP, due to the wide use of multiphase examinations and a larger application in paediatric radiology.
In paediatric patients, CT radiation protection became greater concern due to inherently higher radiosensitivity of growing tissues (such as cartilage, red marrow), and children’s longer life expectancy that involves a longer interval in which one can develop a possible neoplasm.
Analysis of the risks associated with paediatric CT mainly refers to the study by Brenner [13], which highlighted the increase in the probability of occurrence of tumours when patient age decreased. It should be noted that abdominal CT in a child increases the probability of tumour occurrence to more than 20 %.
However, the main dosimetric aspects connected with the use of CT in paediatrics are still poorly standardised:
-
1.
The weighting factors for effective dose are not age specific, and therefore, some authors suggest estimating them on an organ-by-organ basis [14].
-
2.
The classic CT dose descriptors are based on phantoms with diameter simulating the geometry of an adult and introducing significant uncertainties in the evaluation of organ doses in children.
Various tools have been used by different research groups to perform size-dependent dose evaluations. Axelsons et al. [15] or Giacco et al. [16] used a physical anthropomorphic phantom, while other investigators used Monte Carlo voxelized phantoms [17]. In both methodologies, unlike what happens in the adult, the phantoms are size and age specific [18]. Khursheed et al. [19] used the Monte Carlo N-particle (MCNP) radiation transport code to calculate normalised effective dose values for three different scanners and mathematical anthropomorphic phantoms with ages ranging from newborn to adult. They demonstrated the high dependence on patient age and size: the effective dose in a newborn was 1.5 times greater than that of an adult for all types of examinations. Other dosimetric aspects associated with paediatric CT regard optimisation procedures [20].
Dose reduction systems
Dose reduction is one of the main problems in CT, and many techniques have been developed to face this problem, such as tube current modulation and voltage reduction. Automatic dose modulation is based on the principle that the operator decides on the desired image quality in terms of “noise index”, “reference mAs”, or “reference image”, depending on the CT system manufacturer, and the scanner automatically selects the right tube current–time product (mAs). The mAs are changed during the acquisition on the basis of the different density and deepness of the anatomical region being investigated. The system automatically regulates tube current referring to scout images in the z (longitudinal modulation) and x–y (transverse modulation) axes.
The limits of these modalities in obtaining additional dose reduction are determined by the inverse correlation between mAs value and image noise. Using the conventional reconstruction with filtered back-projection (FBP), this limit seems impossible to overcome. Hence, a new algorithm called iterative reconstruction (IR) has been proposed by manufacturers—used in the past and abandoned because of the long reconstruction time imposed by older computers—which is currently capable of additional dose reduction [21]. Beister et al. [22] published an extensive review of the different modalities of dose reduction systems (Table 3).
Iterative modalities are based on mathematical algorithms, which are not fully accessible because various vendors produce them as “black boxes” mainly for the commercial impact of these tools. However, it is possible to divide these modalities into two types: those working on the control of the images, using a statistical method for noise reduction, and those directly managing the raw data domain. The latter allow for a further dose reduction, but imply a complex analysis with longer processing time [22].
Radiation dose and associated cancer risk
Although CT accounts for less than 19 % of radiological examinations [3], its contribution in radiation dose for the general population is becoming significantly higher. For this reason, low-dose CT has become the subject of numerous research publications. Epidemiological studies failed to answer the various questions regarding cancer risk related to low radiation levels; nonetheless the data were robust, and the topic is challenging. For doses lower than 100 mSv (100 mGy with w R equal to 1), the risk calculation is based on the linear no-threshold model (LNT) that is mainly accepted and adopted by the large majority of international organisations in the field of radiation protection as ICRP, BEIR, NRPB, UNSCEAR, etc. [23–26].
In March 2007, the new ICRP no. 103 guideline modified the estimation of cancer risk from low radiation levels. In fact, they increase the risk for the induction of somatic damage in the population up to 6 % per Sv received, remaining unchanged at 0.2 % for genetic risk. In the new IRCP guidelines (no. 103), also a significant increase of tissue weighting factors is established for breast tissue (0.12 vs. 0.05) and a decrease for gonads (0.08 vs. 0.20) [23].
The relation between age and risk is fully considered in the BEIR VII publication of 2005 [24], which reports different risks of cancer induction related to life time (lifetime attributable risk, LAR). LAR is updated using data from atomic bomb survivors and more recent occupational and epidemiological studies. LAR coefficients are calculated according to age, sex and different organs for exposure to 100 mSv. For risk reduction, at low dose levels, in addition to the linear model without threshold DDRF (dose and dose rate effectiveness factor), a factor of 1.5 was used; conversely, the ICRP adopted a factor equal to 2 [23].
Estimation of cancer risk represents a complex problem and many factors must be considered in cancer induction, not only low-dose X-rays used in CT. So a more complete approach should balance the “X-ray cancer risk” against the “natural cancer risk”.
Individual cancer risk is a multifactorial entity that is difficult to estimate; however, age, sex and delivered examination dose (in mSv) are important contributing factors, as reported in the BEIR VII, IRCP and UNSCEAR publications [23–26]. For instance, a 40-year-old male subjected to an effective dose of 100 mSv (at least 4–5 total-body CT examinations) has, according to BEIR VII, a risk of fatal cancer of 0.04 %. The risk is calculated by multiplying the “natural” cancer risk (not considering X-ray dose) by 0.4.
Nowadays, the risk of falling ill with cancer throughout one’s lifetime is calculated at around 25 %, while the radiation-related “estimated excess rate ratio” after an exposure of 100 mSv is calculated as 0.01 (i.e. 0.04 × 0.25 plus an additional 1 %). Due to the greater sensitivity of young subjects to radiation damage, the risk factor is 3–4 times more than in adults [27, 28].
In CT examinations, only body segments and not the entire body (such as brain, chest and abdomen) are usually exposed, so the risk calculation should take into account tissue-specific weighting factors (such as the breast in chest exposure, which increases the risk).
Many publications consider it incorrect to calculate a single individual risk, and underline the lack of sound evidence of cancer risk for doses less than 50 mSv. Finally, the risk estimation based on the Japanese nuclear bomb survivors compared to the dose commonly used in CT is considered questionable [29].
As the ICRP guidelines well outline, it is incorrect to extrapolate an individual’s cancer risk [30], as the guidelines are for the cumulative cancer risk of a population. Following Brenner’s publication [31, 32] some papers calculated the effective cancer risk with a simple arithmetical calculation (number of CT examinations multiplied by mean exposure in mSv of the population) resulting in impressive and overestimated rates of cancer related to medical exposures in the general population [29]. Valid evidence against this approach was described by Meer in the US, who considered a population of 10 million (Medicare) patients exposed to CT examinations (1998–2005) and found a cancer risk between 0.02 and 0.04 % against the expected 1.5–2.0 %, applying the simple Brenner derived arithmetical calculation [33].
Two relevant epidemiological studies on low-dose cancer risk have recently been published by Pearce in the Lancet and Mathews et al. in the BMJ and regarded British and Australian young subjects affected by leukaemia and other solid cancers [34, 35]. Mathews et al. compared the cancer incidence rates in individuals exposed to a CT scan more than 1 year before any cancer diagnosis with the incidence rates in unexposed individuals. The study determined that “the increased cancer incidence rates after CT exposure in these cohorts are mostly due to irradiation” [35].
Another additional criticism of exponential cancer risk is related to the evidence of the linear relation of the damage in opposition to the nonlinear relation of the cellular reparative mechanism, with consequent overestimation of the damage [36]. Otherwise two different biological theories negate the LNT model [37]. The first [36] considers that there is no biological evidence for low dose. In fact, data on atomic bomb survivors fit more with “a nonlinear biological response” or “with threshold” models, than with LNT. The second theory refers to “hormesis”, which in the cellular environment might promote an adaptive mechanism for low doses determining higher resistance of cells to radiation damage. Chen et al. [38] underlined that the accidental chronic exposure to low gamma radiation from Cobalt 60 determined protective effects against tumours; in fact the expected tumour rate in this population is lower than expected.
Although the scientific community is still debating the effects of low dose levels, some misinterpretations of scientific papers and some questionable CT practices have resulted in internet forums and newspaper articles on “CT linked to cancer” in single groups of patients. Media pressure has probably induced the FDA and the California State government to create protocols on CT exposure. The FDA has established an initiative to reduce unnecessary radiation exposure from medical imaging; the California State government has promoted a law imposing a dose report in every CT scan (Dose Bill) [5], including cases of overdose and a strict annual control of the dose used for every protocol by the medical physicist. In Italy as well, several Regions are planning to introduce a dose report in the radiological report. Moreover, a European Community (EC) directive aiming to impose the dose report in the radiological report (Council Directive 242, 2012 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation) is under evaluation. Therefore, “risk denial” and “risk strengthening” appear equally wrong, and the major problem is represented by the information that the radiologist should give to the patient. Are radiologists and clinicians sufficiently prepared on the subject of cancer risk?
Dose risk knowledge
“Are all the subjects involved in CT prescription and execution prepared on dose risk?” According to some experiences published in the BMJ [39, 40], doctors working in the UK National Health Service have insufficient knowledge about radiation protection, despite serious continuous education programmes for health professionals and a widely available publication for referring physicians entitled “How to make the best use of the diagnostic radiology department”, in which the practice of dose reduction is extensively described [41].
Introduction of radiation protection in medical school programmes is also recommended in European guidelines to all state members (European Commission Medical Exposure Directive) [10, 42, 43].
In Italy, possible knowledge gaps among referring physicians, radiographers and radiologists have not been investigated despite the fact that since 1995 Italy has had detailed legislation regulating the patient’s radiation protection and operator education and training [43]. An increased attention to the problem should be adopted in young radiologists, who are more involved in emerging radiation technologies and new applications. In June 2009, the American College of Radiology and the Radiological Society of North America set up a task force to increase radiation protection knowledge and reduce unnecessary imaging in particular in paediatric patients.
Conclusions
Dose reduction in CT relies on the correct implementation/optimisation of protocols and everyday practice. We can significantly reduce unnecessary radiation exposure by being aware of patient risk and using all the available resources in the CT apparatus to the best advantage. To synthesise what radiologists are required to do to ensure an “up-to-date” use of CT, we can state that they should:
-
Know the dose concerns for the single patient and population, involving referring physicians in the knowledge of possible risks and using the CT apparatus with the best dose utilisation;
-
Substitute CT with other diagnostic techniques when possible especially in children;
-
Reduce the number/extension of scans, phases, the dose in single scans and examinations, also optimising contrast and reducing costs.
Glossary and definitions
To facilitate reading of this review, this section recalls the unit measures used in radiation protection and dosimetry in alphabetical order.
Absorbed dose D is the measure of all types of ionising radiation and is defined a \( D = \frac{{{\text{d}}e}}{{{\text{d}}m}} \) where “de” represents the mean energy deposited to the mass “dm” by the ionising radiation. The adsorbed dose in SI system is measured in Joule per kilogram (J kg−1) and named Gray (Gy).
The absorbed dose is calculated from the energy deposited (e), does not reflect the single event of interaction and its value is obtained as a mean on a generic element of mass (dm).
The radiation damage for low dose levels is supposed to be correlated with the value of adsorbed dose related to a specific organ or tissue. To account for the different modalities of radiation interaction and different radiation sensitivities of tissue two other values need to be introduced: the equivalent dose (H T) and the effective dose (E). The unit measure is the same as the adsorbed dose J kg−1, but it is called Sievert (Sv).
Diagnostic references levels (DRL) are defined dose levels used in diagnostic and nuclear medicine for typical groups of patients of standard body mass or a standard phantom defined for different systems. The defined levels should not be exceeded for standard procedures in normal conditions (DLR for paediatric CT practice are not yet available in Italy).
Equivalent dose H T is the result of the sum Σ of adsorbed dose multiplied by the weight of radiation w R. The type of radiation that interacts may contribute with a factor of 1, 5, 10 in the case of photon, neutron, and alpha particles, respectively.
Effective dose E is the sum of the equivalent dose (considering radiation weighting factor) weighted for different organs or tissues (w T), according to the expression
The sum is considered with the weight of the global detriment of all organs or tissue according to the weighted value w T described in Table 4 [20].
These values are the result of epidemiological studies on cancer induction in exposed populations and on the evaluation of the risk of hereditary effects according to ICRP guideline 103 [23].
The principal uses of effective dose are prospective dose evaluation way to plan and optimise radiation protection and to comply with dose limits in accordance with guidelines and dose reference levels. It may also be interesting to compare doses from different diagnostic procedures and/or technologies or the use of similar technologies or procedures across hospitals or countries to compare technologies for the same medical investigations. The effective dose, however, is not recommended for epidemiological evaluation either for detailed retrospective analysis on exposure and risk of single individuals. The interpretation of effective dose, in patients exposed for medical purposes, remains complex especially when organs or tissue is subjected to partial or heterogeneous exposure.
Pitch in multislice spiral CT is defined as the ratio of the table increment over the detector collimation. One can assume that the detector collimation for each of N detector arrays is the same, excluding cases of either different collimations or combined ‘‘measurement row.’’
References
SIRM SAGO (2010) Censimento nazionale delle risorse umane e tecnologiche dell’area radiologica. Il Radiologo Suppl 2:3–39
IMV (2012) CT benchmark report. GreenBelt, USA
Frush DP (2004) Review of radiation issue for CT. Sem Ultras CT MRI 25:17–24
Balonov MI, Shrimpton PC (2012) Effective dose and risks from medical x-ray procedures. Ann ICRP 41:129–141
California Senate Bill 1237. http://www.leginfo.ca.gov/pub/09-10/bill/sen/sb_1201-1250/sb_1237_bill_20100929_chaptered.html. Accessed Jan 2013
Mancuso MT, Pasquali E, Leopardi S et al (2008) Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. PNAS 105:12445–12450
Jessen KA, Panzer W, Shrimpton PC et al (2000) EUR 16262: European guidelines on quality criteria for computed tomography. Office for Official Publications of the European Communities, Luxembourg
Huda W, Magill D (2011) CT effective dose per dose length product using ICRP 103 weighting factors. Med Phys 38:1261–1265
D.Lgs 187 (2000) Attuazione della direttiva 97/43/Euratom in materia di protezione sanitaria delle persone contro i pericoli delle radiazioni ionizzanti connesse ad esposizioni mediche
Shrimpton PC, Hillier MC, Lewis MA, Dunn M (2006) National survey of doses from CT in the UK: 2003. Br J Radiol 79:968–980
Origgi D, Vigorito S, Villa G et al (2006) Survey of computed tomography techniques and absorbed dose in Italian hospitals: a comparison between two methods to estimate the dose-length product and the effective dose and to verify fulfillment of the diagnostic reference levels. Eur Radiol 16:227–237
Palorini F, Origgi D, Granata C, Matranga D, Salerno S (2014) Adult exposures from MDCT including multiphase studies: first Italian nationwide survey. Eur Radiol 24:469–483
Brenner DJ, Elliston CD, Hall EJ, Berdon WE (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol 176:289–296
Huda W, Vance A (2002) Patient radiation doses from adult and pediatric CT. Pediatr Radiol 32:540–546
Axelsson B, Persliden J, Schuwert P (1996) Dosimetry for computed tomography examinations of children. Radiat Prot Dosim 64:221–226
Giacco G, Cannata V, Furetta C et al (2001) On the use of paediatric phantoms in the dose evaluation during computed tomography (CT) thorax examinations. Med Phys 28:199–204
Lee C, Lee C, Staton RJ et al (2007) Organ and effective doses in pediatric patients undergoing helical multislice computed tomography examination. Med Phys 34:1858–1873
Varchena V (2002) Pediatric phantoms. Pediatr Radiol 32:280–284
Khursheed A, Hillier MC, Shrimpton PC, Wall BF (2002) Influence of patient age on normalized effective doses calculated for CT examinations. Br J Radiol 75:819–830
Kalra MK, Maher MM, Toth TL et al (2004) Strategies for CT radiation dose optimization. Radiology 230:619–628
Tricarico F, Hlavacek AM, Schoepf UJ et al (2013) Cardiovascular CT angiography in neonates and children: image quality and potential for radiation dose reduction with iterative image reconstruction techniques. Eur Radiol 23:1306–1315
Beister M, Kolditz D, Kalender WA (2012) Iterative reconstruction methods in X-ray CT. Phys Med 28:94–108
ICRP (2007) The 2007 Recommendations of the International Commission on radiological protection. ICRP Publication 103. Ann ICRP 37:2–4
BEIR VII (2005) Health risks from exposure to low levels of ionizing radiation. National Academies Press, Washington, DC
NCRP report 160. Ionizing radiation exposure of the population of the United States. http://www.ncrppublications.org/reports/160. Accessed Jan 2013
UNSCEAR (2006) Effects of ionizing radiation. UNSCEAR 2006 report, vol. I, Annex A. United Nations, New York
Verdun FR, Bochud F, Gundinchet F et al (2008) Quality initiatives radiation risk: what you should know to tell your patient. Radiographics 28:1807–1816
Krille L, Zeeb H, Jahnen A et al (2012) Computed tomographies and cancer risk in children: a literature overview of CT practices, risk estimations and an epidemiologic cohort study proposal. Radiat Environ Biophys 51:103–111
Pauwels EK, Bourguingon M (2011) Cancer induction caused by radiation due to computed tomography: a critical note. Acta Radiol 52:767–773
ICRP (2005) Low-dose extrapolation of radiation-related cancer risk. ICRP Publication 99. Ann ICRP, vol 35(4), Elsevier
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Brenner DJ, Doll R, Goodhead DT et al (2003) Cancer risks attributable to low dose of ionizing radiation: assessing what we really know. PNAS 100:13761–13766
Meer AB, Basu PA, Baker LC, Atlas SW (2012) Exposure to ionizing radiation and estimate of secondary cancers in the era of high-speed CT scanning: projections from the Medicare population. J Am Coll Radiol 9:245–250
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Matthews JD, Forsythe A, Brady Z et al (2013) Cancer risk in 680000 people exposed to computed tomography scans in childhood or adolescent: data linkage study of 11 million Australians. BMJ 346:1–18
Neumaier T, Swenson J, Pham C et al (2012) Evidence for formation of DNA repair centers and dose–response nonlinearity in human cells. Proc Natl Acad Sci USA 109:443–448
Hoel DG, Li P (1998) Threshold models in radiation carcinogenesis. Health Phys 75:241–250
Chen WL, Luan YC, Shieh MC et al (2007) Effects of cobalt-60 exposure on health of Taiwan residents suggest new approach needed in radiation protection. Dose Response 5:63–75
Gower-Thomes K, Lewis MH, Shiralkar S et al (2002) Doctors’ knowledge of radiation exposures is deficient. BMJ 324:919
Shiralkar S, Rennie M, Snow M et al (2003) Doctor’s knowledge of radiation exposure: questionnaire study. BMJ 327:371–372
Royal College of Radiologists (2007) Making the best use of clinical radiology services (MBUR) referral guidelines, 6th edn. Royal College of Radiologists, London
Commissione Europea. Linee guida per l’esposizione a radiazioni a scopo medico (2000) http://ec.europa.eu/energy/nuclear/radioprotection/publication/doc/099_it.pdf. Accessed Jan 2013
D.Lgs 230 (1995). Attuazione delle direttive 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 2006/117/Euratom in materia di radiazioni ionizzanti e 2009/71/Euratom, in materia di sicurezza nucleare degli impianti nucleari
Conflict of interest
Stefano Colagrande, Daniela Origgi, Giovanna Zatelli, Andrea Giovagnoni, Sergio Salerno declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colagrande, S., Origgi, D., Zatelli, G. et al. CT exposure in adult and paediatric patients: a review of the mechanisms of damage, relative dose and consequent possible risks. Radiol med 119, 803–810 (2014). https://doi.org/10.1007/s11547-014-0393-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0393-0