Abstract
Since the turn of the last millennium, the pediatric radiology community has blazed a patient-quality and safety trail in helping to effectively address the public and the news media’s concerns about the implications of ionizing radiation from CT scanners in children. As such, this article (1) reviews the potential deleterious effects of ionizing radiation, (2) discusses why limiting radiation exposure in children is so important, (3) tells the history of pediatric CT radiation exposure concerns, (4) explains the interventions that took place to address these concerns and (5) touches on the current school of thought on pediatric CT dose reduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the turn of the last millennium, the pediatric radiology community has blazed a patient-quality and safety trail in helping to effectively address the public and the news media’s concerns over the implications of ionizing radiation from CT scanners in children. Our coordinated, effective and highly convincing actions alerted patients, practitioners and the public at large as to how these issues should be addressed. It also demonstrated what we as a professional body could do to ensure that the unrecognized dangers of ionizing radiation were being addressed and that information concerning this topic was available, understandable and rectifiable. This mechanism of addressing concern over medical imaging radiation exposure quickly spread to the adult community and led to a national debate on the necessity of keeping ionizing radiation from medical imaging as low as reasonably achievable (ALARA).
As such, this article (1) reviews the potential deleterious effects of ionizing radiation, (2) discusses why limiting radiation exposure in children is so important, (3) tells the history of pediatric CT radiation exposure concerns, (4) explains the interventions that took place to address these concerns and (5) touches on the current school of thought on pediatric CT dose reduction.
Biological effects of ionizing radiation
Radiation is all around us [1]. It emanates from the earth and from the sky and is in the air. Its presence is unavoidable and its effects, while often harmless, can also be extremely severe. Radiation is a mechanism whereby energy travels through space and takes the form of an electromagnetic wave. Different types of radiation exist on an electromagnetic spectrum, with indolent low-frequency radio waves at one end and damaging high-frequency ionizing radiation on the other [2]. These high-frequency electromagnetic waves include X-rays and, unlike low-frequency waves, contain enough energy to displace electrons from atoms, hence their term “ionizing.”
The reason for the deleterious effects of radiation stems from this ionization potential. Although an ionized atom can adopt a different structure, this does not cause any problems unless the atom in question is part of a critical molecule such as that in a chromosome. However the electron displaced by the X-ray can also cause damage as it moves through neighboring structures. The results of this can include cell death or damage to critical cell components, such as chromosomes.
The consequent radiation effects on the human body can therefore be divided into two categories: deterministic and tissue effects (also known as stochastic) [3]. Deterministic effects are those that occur as a result of cell death and include effects such as erythema, epilation, tissue necrosis and cataracts. Deterministic effects only occur over a known threshold and therefore are avoidable during routine medical imaging. Despite this, deterministic effects have been encountered in interventional radiology and in CT studies [4] where excessive doses were given. Stochastic effects occur because of chromosomal damage, and while theoretically these can result in birth defects, they are more commonly recognized as a potential cause of carcinogenesis. Indeed, since 2012 the International Agency for Research on Cancer has classified ionizing radiation as a carcinogen [5]. Unfortunately these stochastic effects do not have a known dose threshold, although the risk of occurrence rises as the dose increases. The cause of stochastic effects is thought to be a result of cell mutations taking place secondary to chromosomal damage. The human body addresses these mutations in three ways: successful repair with cell survival, unsuccessful repair with cell death, and unsuccessful repair resulting in an ongoing mutation. This last outcome has the potential to cause cancer.
Specific importance of radiation exposure in pediatric patients
Data from the atomic bomb survivors have been used by many authors and research groups to demonstrate that children are more vulnerable than adults to the effects of ionizing radiation [6]. Conclusions from the literature suggest that the higher sensitivity of children to radiation could be attributed to some specific considerations. One is children’s longer life expectancy from the time of exposure, which provides more time for a cancer to manifest. Because of their longer latency period, many radiation-induced malignancies, particularly solid cancers, do not manifest for decades. Therefore radiation effects and associated cancer risk in children are evidently higher in children than in adults. Another consideration is that radiation effects are more likely to occur in proliferating cells [6, 7]. Growing children have more dividing cells, and hence greater radiation sensitivity than adults.
Earlier studies on leukemia among atomic bomb survivors reported “significantly higher risk” of acute and chronic granulocytic leukemia among those at a younger age at the time of radiation exposure [7,8,9]. Figure 1 shows the effect of age at time of exposure on the incidence of all forms of leukemia (except chronic lymphocytic leukemia) among atomic bomb survivors [7]. This figure shows that the younger the person was at the time of exposure, the shorter the cancer latency period and the period of expression. Bushberg [10] suggested that “this evidence, together with subsequent studies of medically and occupationally exposed cohorts, indicate that excess leukemia cases can be detected within a year or two after exposure and would peak at about 10–12 years after. Those findings were subsequently supported by reports on children’s medical and occupational exposure to radiation. Indeed, the BEIR VII [11] summary on studies of children treated with X-rays or gamma radiation for tinea capitis, large-appearing thymus, and benign head and neck tumors reported high excess cancer risk among the exposed population.
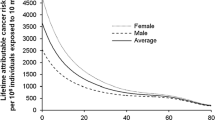
In a follow-up study of patients with ankylosing spondylitis treated using therapeutic X-rays [12], the authors also noted a correlation between excess risk increase for developing leukemia and age at treatment. Similarly, age–time patterns in radiation-associated cancer risk for solid cancer incidence and leukemia mortality has also been reported [11, 13]. Leukemia mortality expressed as function of age at exposure obtained from gender-averaged estimates of the risk from an effective whole-body dose of 1 Sv also shows increased risks at younger ages [11] (Fig. 2). Figure 3 shows the lifetime attributable risk of solid cancer mortality by age of exposure and demonstrates the increased risk effect of age on radiation sensitivity. Table 1 data, obtained from Fig. 3, compare solid cancer mortality risk and cancer incidence at ages 0, 5 and 15 years old to that at 30 years old for females and males. The table shows an increase in children’s excess cancer risk and about a factor of two difference in sensitivity between girls and boys. Those results are consistent with updated atomic bomb survivor data by Brenner [14], which showed a noticeable increase in lifetime cancer risk mortality in children compared to adults and in females compared to males.
Lifetime attributable risk of solid cancer mortality representing the number of deaths per 10,000 persons exposed to a whole-body dose of 10 mSv. The graph is based on data adapted from Table 11-7 published by Bushberg [10] and from Table 12D-2 in BEIR (Biologic Effects of Ionizing Radiation) VII [11]. (Reprinted with permission from Wolters Kluwer)
It is worth mentioning that although there seems to be no disagreement in the scientific community on children’s increased sensitivity to radiation [15], there is considerable disagreement on the carcinogenicity of low-dose radiation to all age groups, including children. BEIR VII defines low effective doses as those in the range of near 0 mSv to about 100 mSv. The disagreement centers around attempts to extrapolate excess cancer risk from high-dose exposures, like those from atomic bomb and radiation incidents, to obtain low-dose risk estimates [16] as a conservative measure of radiation safety. Many reports emphasize that credible evidence of carcinogenic risk from imaging-related low dose (below 100 mGy) are nonexistent, or if they exist, would be too small to demonstrate [16,17,18].
History of pediatric CT radiation exposure
Computed tomographic imaging was instituted in radiology departments in the 1970s, but early scanners were initially too slow to be used routinely in pediatric practice. The original single-slice models required anesthesia or sedation for young children and, while revolutionary in terms of cross-sectional imaging, had limited pediatric indications. However with the advent of slip-ring technology and spiral capabilities in the late 1990s, CT became faster and much more attractive to pediatric radiologists. The technique became popular and children in the United States were increasingly being exposed to CT [19] because it quickly became an available and effective imaging modality.
At about the same time, studies were appearing in the literature citing the first estimates for medical imaging radiation-induced fatal cancer risks in adults [20]. These were based on data collated from the Radiation Effects Research Foundation, with risks from imaging being extrapolated from dose and outcomes encountered in the ensuing 40 years following the Hiroshima and Nagasaki atomic bomb drops. In view of these findings in adults, it soon became apparent that the lifetime risks for cancer from medical imaging ionizing radiation exposures were more marked in children [21]. Studies estimated that a CT scan on a child delivered a dose sufficient to increase the lifetime radiation risk of cancer at an order of magnitude higher than that of adults. They also demonstrated that the collective ionizing radiation dose (high-dose CT studies being delivered with increasing frequency to pediatric patients) was affecting the quantitative lifetime radiation risk for children. In one study at this time, a university center demonstrated that while their pediatric CT activity accounted for only 11% of cases, these patients received 70% of the total radiation dose being delivered from medical imaging exposures [22]. The situation was compounded by additional research showing that the doses being given to children from CT were unnecessarily high because of the suboptimal imaging parameters being used [23]. This was illustrated in a sobering study performed in a pediatric radiology department where researchers analyzed the scan dose parameters on all CT cases transferred from non-pediatric centers [23]. The results from this analysis showed that children would often receive doses six times higher than necessary because, in part, of the scan parameters (tube current or mA) not being adjusted for the specific needs of pediatric patients. Whether this neglect in catering to the specific imaging needs of a powerful tool was a result of lassitude, ignorance or a combination was not known, but the news media had their story. Multiple articles appeared in the news media during this time spelling out the dangers of CT scanning and the risks of children developing cancer as a result. Pediatric radiologists at the time recall the difficult conversations they were having with concerned parents and the increasing reluctance by providers to refer children for CT imaging. Pediatric radiologists were concerned about the potential liability of performing CT scans in children, and the concept of requiring consent surfaced [24]. It became clear that something needed to be done if CT scanning in pediatric practice were ever going to be deemed an acceptable, safe and trusted tool. This rehabilitation was going to require concerted efforts from multiple stakeholders to restore the profession, the referrers and the public’s faith in CT. Interestingly, industry was also going to need to be part of the solution. CT equipment at the time was designed almost exclusively for speed and for image quality, with little emphasis (or perceived need) being placed on dose-reduction features. It was clear that the profession was reaching a watershed moment; how would it respond?
Interventions to reduce CT dose in pediatric radiology
In 2001, Dr. Tom Slovis of the Society for Pediatric Radiology (SPR) organized a multidisciplinary conference on the concept of ALARA (as low as reasonably achievable) as applied to pediatric CT radiation dose [25]. The concept was an old one, commonly invoked in the context of nuclear radiation safety; the SPR’s application of it to medical dosing was revolutionary. The following year, the National Council on Radiation Protection held a 2-day symposium on the topic of CT radiation dose [26]. The symposium was attended by radiologists, medical physicists, scanner manufacturers and radiologic technologists. Attendees recognized a need to record doses for children (and for that matter, adults) undergoing CT scans, or at least to record reasonable estimates thereof, allowing for prospective and retrospective studies to be done. Actual patient dose recording at the time was much less straightforward than today [27, 28]. In 2002, with dose metrics in its infancy, there was a veritable alphabet soup of terms in the lexicon, with CTDI (CT dose index), CTDI 100, CTDIw (weighted CT dose index), CTDIvol (volumetric CT dose index), MSAD (multiple scan average dose) and DLP (dose–length product) being examples, and with none of these parameters reporting actual patient dose. The need for a standardized language was pressing. A respected professor of medical physics and biomedical engineering from the Mayo Clinic suggested “a moratorium on any new dose concepts or terms until we can sort out what we have now” [26].
Attendees at the National Council on Radiation Protection and Measurement (NCRP) Symposium were also determined to facilitate child-sizing the dose. Many existing CT scanners in 2002 had the capabilities to expose the child to lower levels of radiation, but this required technical adjustments that could be impractical for time-pressed CT technologists. In addition, technologists were often learning how to scan on the job rather than as part of their education, making the onus of added attention to patient dose burdensome. Improved training for technologist students was established as a goal. There was also a plea for manufacturers to create scanners with more automation [26]. As a stopgap measure, technique charts were created; these were intended to be machine-specific and to assist the technologist in making appropriate adjustments. In the years following, technological innovations, including more dose automation, became widespread practice, with dose modulation (automatic exposure control) being one of the most powerful mechanisms. Dose modulation is automatic adjustment of tube current as the gantry rotates around the patient [29]. Many current scanners can perform these adjustments in three dimensions (x, y and z planes). Other key innovations included the ability to scan at lower voltage (kV) and the reintroduction of iterative reconstruction. Iterative reconstruction had actually been used on the earliest CT scanners [30]; however it was limited by processing speed and consequently was replaced by filtered back-projection by about 2000. Much faster computer processors were developed subsequently, allowing for the reintroduction of iterative reconstruction. State-of-the-art scanners used either partial- or model-based iterative reconstruction methods to produce images with better image quality at a lower doses [29, 30].
Another important recommendation to come out of the 2002 meeting was that of a collaborative effort on dose reduction across many professions. Effective action to reduce CT dose would require a commitment from not only radiologists and radiologic technologists but also referring clinicians [31, 32], medical physicists, manufacturers and regulatory bodies [26]. The seeds were planted for the second red-letter moment on this journey.
In 2006, a committee was formed within the SPR to address the topic of radiation exposure from medical imaging in children; this was the origin of the Image Gently Alliance [33, 34]. In 2007, the SPR leadership partnered with the American College of Radiology, the American Society of Radiologic Technologists and the American Association of Physicists in Medicine (AAPM) to form a true alliance: the formal Image Gently campaign was launched that summer. The primary objective of the Image Gently Alliance was “to raise awareness in the imaging community of the need to adjust radiation dose when imaging children. The ultimate goal is to change practice.” With respect to CT, the Alliance made several simple recommendations, such as to scan only when necessary, scan only the indicated region, and scan only once. In addition to these straightforward recommendations, the Alliance initially (in 2008) published CT protocols for children on its website; these protocols were “independent of equipment manufacturer, age of machine, or number of detectors” [33]. Despite good intentions, automatic exposure control and an inability to select lower tube voltages or the use of iterative reconstruction made adoption difficult. Other innovations after 2000 such as bow-tie filters, focal-spot to detector distance, and detector efficiency contributed further to the complexity of the issue. Ironically, the better the scanners got at reducing dose, the harder it became to create a universal child-size protocol. By 2014, it was impossible to do so. The Image Gently Alliance accordingly revised its website. In place of the earlier “universal” protocols for children, there is now an 8-page document titled “Pediatric CT Protocols and Instructions 2014” [34]. However the creation of this useful document would not have been possible had early efforts by the Image Gently Alliance not taken place.
Although the Image Gently Alliance struggled with simplifying pediatric protocols, it was phenomenally successful when it came to education, outreach and collaborative efforts. The Alliance is now an international organization with more than 100 member groups with a presence on all six populated continents. Since its inception, a vast amount of scholarly work has gone into the topic of radiation safety in medical imaging and dose optimization for children [35,36,37,38,39,40].
In the post-Image Gently campaign world, radiologists and referring providers were also encouraged to consider alternative modalities such as ultrasound or MRI for pediatric evaluation. A 7-year effort to reduce CT dose at St. Louis Children’s Hospital included not just lower CT dose protocols but also streamlined access to US and MRI for pediatric patients. A subsequent decline in CT volume at the St. Louis hospital coincided with an increase in its US and MRI volumes. Meanwhile, the CT volume at the adjacent adult hospital increased, suggesting that the St. Louis efforts to provide alternative methods of diagnosis were successful [36]. At our hospital, CT is rarely used on pediatric patients for indications other than cancer staging (primarily lung and lymphoma assessment), or emergent indications. In the emergency setting, US is now the initial imaging modality for appendicitis. These practices are now commonplace across the United States and the rest of the world.
Pediatric radiology’s effect on CT dose reduction
CT dose monitoring for adult and pediatric patients during the last 10 years has shown a favorable trend in patient dose reduction and better dose management concurrent with optimal image quality. Collective and collaborative implementation efforts made by the radiology community, professional organizations in imaging, the clinical medical community and the radiation imaging industry were instrumental in lowering risk from the relatively higher-dose CT procedures. This was mainly a result of different levels of technical, scientific, clinical and regulatory interventions proposed and implemented by these groups. Over the last decade, improvements in CT acquisition chain technology using pediatric-size-based CT protocols and innovations in image reconstruction algorithms have made it possible to achieve lower doses at the same, if not better, image quality. From study justification for the exam to dose optimization at the exam time, to dose management, reporting and benchmarking after the exam, a team of providers works to achieve the scan optimization objective of balanced risk versus benefit.
As mentioned in the previous section, one of the challenges radiology providers faced was identifying a dose descriptor that could be used to make a patient dose estimate and determine associated risk. A weighted CT dose index (CTDIw) and dose–length product (DLP) were the most common CT dose descriptors. With the introduction of helical CT scanning of a volume of tissue in one rotation, CTDIvol was introduced and was estimated from the CTDIw adjusted for the scan pitch. It is important to remember that those dose descriptors were measured in tissue-equivalent phantoms of specific size and diameter (head, body) when scanned under similar patient scanning parameters and system radiation output. They are, to a large extent, dependent on and correlate well with changes in CT acquisition parameters and post-processing methods. Those parameters include kilovoltage (kV), milliamperes (mAs), dose modulation, detector configuration, scan field of view, beam filtration, scan mode (helical/axial), patient positioning, dose reduction algorithms, reconstruction algorithms and post-processing kernels.
The effective dose, which was intended for developing occupational dose limits and radiation safety measures as stated in the International Commission on Radiological Protection (ICRP) report 103 [41], has been widely used by the imaging community to predict cancer incidence in large populations exposed to medical radiation. Effective dose is defined as the dose that, if delivered uniformly to the whole body, would produce the same effects as those produced by a dose delivered to one or more specific organs [41]. As a CT dose descriptor, the effective dose has been linked to the CTDIvol and the DLP [14, 42,43,44]. It became the most reported dose descriptor relating CT doses delivered to specific organs to cancer risk predicted in BEIR VII report from the Japanese survivors model [11, 45, 46]. Effective dose is calculated by multiplying the dose delivered to organs in the body by an organ-specific biological factor and a radiation-type weighting factor (1 for X-rays), and summing the products for all exposed organs [41, 47].
Using these measurements, the introduction of patient-size and age-specific acquisition parameters had a significant effect on lowering both adult and child CT doses. Table 2 compares the evolving CT acquisition parameters used by several authors over the years and their consequent effect on CT effective doses [14, 21, 23, 37, 48,49,50,51]. Some authors reported using actual scan parameters on physical phantoms to determine organ doses, while others used synthesized data from computer-based models. Data from our institution are based on our departmental patient age- and weight-based pediatric CT protocols. We obtained CTDIvol and DLP from individual patient scan dose reports through the picture archiving and communication system (PACS) and by using a commercial radiation dose reporting and management system (Radimetrics Enterprise Platform; Bayer HealthCare, Whippany, NJ). Review of the referenced authors’ notes and results in Table 2 demonstrates success in lowering pediatric CT doses through better utilization of available technology, lowering scanner radiation output to a reasonable minimum, and reducing scanned volume of irradiated tissue. Using age- or weight-based mAs values and using pitch values above 1 helped reduce reported effective doses. An example from Table 2 shows 5-year-old effective doses from head CT in 2001 and 2007 at about 70 mSv and 52 mSv, respectively, when adult protocols were used for pediatric head CT exams [21, 51]. The subsequent use of more appropriate pediatric head protocols [48, 51] shows reduced effective doses to about 4 mSv and 1.4 mSv. Our results show mean effective doses to the same age group at values about 1 mSv. The same can be seen with abdominal CT studies. Effective doses reported by the same authors from body CT showed lower mAs values and consequently reduced effective doses over the years. For a 5-year-old child, effective doses from body CT obtained over time were 33 mSv in 2001 [21] and 9 mSv in 2012. With advanced dose modulation technology, our results show a 5-year-old effective dose from chest/abdomen CT to now be about 0.4 mSv. These figures illustrate the remarkable achievements in pediatric CT dose reduction that have taken place over the years.
Current thinking
Have we gone too far on pediatric CT dose reduction? At our institution, as described, weight- and age-based protocols are readily available. One of our scanners has the capability to scan at a tube voltage as low as 80 kVp with no compromise in diagnostic ability. However, major children’s hospitals are still moving forward, using size-specific dose estimates as a patient dose estimate. This makes sense because the photon path depends more on patient thickness than on patient weight or age. Cincinnati Children’s Hospital Medical Center, which has measured every fluoroscopy patient to ensure appropriate machine settings, now does the same for its CT patients. The ultimate goal is to gather enough data to develop target dose guidelines based on patient thickness (K. Strauss, personal communication). In the meantime, the AAPM has individualized pediatric protocols (brand-specific) for select scanners freely available on its website; these protocols can serve as a starting point for hospitals unaccustomed to scanning pediatric patients. These take patient thickness into account, and more than 30 scanners are included in the chest section [52]. This suggests there is still room for improvement.
However the recent focus on quality and outcomes in patient care further complicates matters. Historically, we have spoken about dose optimization with reference to factors such as contrast-to-noise ratios. While dose reduction is a challenging goal in isolation, successfully managing imaging quality requires simultaneously considering patient-centered care needs, operational variability and efficiency, along with dose. But if measuring dose is challenging, try simultaneously measuring dose (or even just exposure) with quality with a capital Q. This requires accounting for additional factors such as patient-centered care needs, operational variability, and efficiency. All these need to be considered in moving forward.
Manufacturers have also responded with admirable vigor to the challenge of record-keeping for CT radiation exposure and dose tracking. There are 13 software packages and three open-access packages on the market. About two-thirds of these can send data directly to the ACR National Radiation Dose Registry. If users are appropriately trained, dose alerts can now be sent to smartphones, so institutions must assess their needs before choosing from the (numerous) options. This adds another layer of complexity to the issue of CT dose.
The issue of dose metrics appearing in patient reports has also become one of legislative concern. Select states, including Connecticut, California, Texas and New York, have passed legislation regarding some form of mandatory CT dose reporting [53]. An well-known case in California where a young boy was mistakenly subjected to the equivalent of 151 CT scans within 68 min [54] precipitated the first legislative effort to report “dose” (actually CTDIvol, phantom used, and DLP). Some benefits of such legislation include public reassurance, more experience with dose tracking for trainees, technologists and radiologists, and potentially useful data archiving. However, as mentioned, these numbers are not actual patient doses and can be misleading. The method of reporting dose tracking is not yet standardized, and there is concern that such documentation could encourage malpractice litigation [55].
Finally, to come full circle, it has also recently been pointed out that the linear-no-threshold model of radiation risk is a hypothesis [56]. While this has generally been acknowledged in articles on CT dosimetry, it is usually mentioned in passing before the ensuing detailed discussion of dose optimization or reduction. Is there a problem with using an unproven hypothesis as the basis for a worldwide patient safety campaign? It sounds like a rhetorical question, and indeed certain authors have aggressively attacked the idea, claiming that the ALARA concept is actively harmful to patients and even that the Image Gently Alliance should be dissolved [57, 58]. The argument is that no increased cancer risk has been demonstrated at dose levels below 100 mSv, and to emphasize continuing dose reduction serves only to fuel public fear and unnecessarily compromise image quality. However, these authors concurrently endorse the controversial principle of hormesis, itself unprovable in the absence of massive population cohort studies [17]. While there is no proven harm at doses of 10 mGy, neither is there proven benefit. Hypothetical statements to the contrary notwithstanding, the lowest effective CT dose is a laudable goal, as is the lowest effective medication dose. Large cohort studies are underway to more directly assess radiation risk, at least at the population level. However, even with an expected cohort of 1.2 million patients, the studies will only be adequately powered to assess leukemia and possibly brain tumor risk. A cohort of 5 million patients would be needed before any accurate quantification of cancer risk could be definitively performed for doses of 10 mSv. Even size-specific dose estimate (SSDE), likely the next step forward, is not individualized; individual patients are more or less radiation sensitive depending on their unique genome, epigenetic factors, and confounding radiation sensitization at any given time. All of this invites the question, just how accurate are we going to need to be?
Summary
Pediatric CT dose optimization is a work in progress and will continue to be for several years. Measuring dose to actual patients is not easy. Optimizing an entity that is hard to measure is harder still. However, since the beginning of the millennium, the medical imaging community has shifted its focus from best possible image quality to a balance of image quality with patient safety. On the way, we have partnered with technologists, medical physicists, manufacturers, referring clinicians and regulatory bodies. This partnership, rising in large part out of concerns for our children, has and will continue to benefit our patients, child-size and otherwise.
References
National Research Council (1998) Health risks from exposure to low levels of ionizing radiation: BEIR VII, phase I, letter report. The National Academies Press, Washington, DC. https://doi.org/10.17226/9526
United States Environmental Protection Agency, Office of Radiation and Indoor Air (2012) Radiation facts, risks and realities https://www.epa.gov/sites/production/files/2015-05/documents/402-k-10-008.pdf. Accessed 11 Sept 2018
Dendy PP, Heaton B (2012) Physics for diagnostic radiology Series in medical physics and biomedical engineering, vol 17, 3rd edn. CRC Press, Boca Raton
Williams R (2010) Stroke scans and radiation risk. Lancet Neurol 9:1150–1151
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2008) IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr Eval Carcinog Risks Hum 97:3–471
Pierce DA, Shimizu Y, Preston DL et al (1996) Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990. Radiat Res 146:1–27
Ichimaru M, Ishimaru T, Belsky JL (1978) Incidence of leukemia in atomic bomb survivors belonging to a fixed cohort in Hiroshima and Nagasaki, 1950-71. Radiation dose, years after exposure, age at exposure, and type of leukemia. J Radiat Res 19:262–282
Tomonaga M (1966) Statistical investigation of leukemia in Japan. N Z Med J 65S:863–869
Bizzozero OJ Jr, Johnson KG, Ciocco A et al (1967) Radiation-related leukemia in Hiroshima and Nagasaki 1946-1964. II. Ann Intern Med 66:522–530
Bushberg JT (2012) The essential physics of medical imaging, 3rd edn. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia
National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation (2006) Health risks from exposure to low levels of ionizing radiation: BEIR VII, Phase 2. National Academies Press, Washington, DC
Court-Brown WM, Doll R (1957) Leukaemia and aplastic anaemia in patients irradiated for ankylosing spondylitis. Medical Research Council
Linet MS, Slovis TL, Miller DL et al (2012) Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin 62:75–100
Brenner DJ (2002) Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol 32:228–221
United Nations Scientific Committee on the Effects of Atomic Radiation (2012) Sources, effects and risks of ionizing radiation: 2012 report to the general assembly, with annexes. United Nations, New York
Land CE (1980) Estimating cancer risks from low doses of ionizing radiation. Science 209:1197–1203
Siegel JA, McCollough CH, Orton CG (2017) Advocating for use of the ALARA principle in the context of medical imaging fails to recognize that the risk is hypothetical and so serves to reinforce patients' fears of radiation. Med Phys 44:3–6
Doss M (2015) Counterpoint: should radiation dose from CT scans be a factor in patient care? No Chest 147:874–877
Brenner DJ, Hall EJ (2007) Computed tomography — an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Pierce DA, Preston DL (2000) Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res 154:178–186
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296
Mettler FA Jr, Wiest PW, Locken JA, Kelsey CA (2000) CT scanning: patterns of use and dose. J Radiol Prot 20:353–359
Paterson A, Frush DP, Donnelly LF (2001) Helical CT of the body: are settings adjusted for pediatric patients? AJR Am J Roentgenol 176:297–301
Frush DP (2003) Responsible use of CT. Radiology 229:289–291
(2002) The ALARA (as low as reasonably achievable) concept in pediatric CT intelligent dose reduction. Multidisciplinary conference organized by the Society of [sic] Pediatric Radiology. August 18-19, 2001. Pediatr Radiol 32:217–313
Linton OW, Mettler FA Jr, National Council on Radiation Protection Measurements (2003) National conference on dose reduction in CT, with an emphasis on pediatric patients. AJR Am J Roentgenol 181:321–329
Strauss KJ (2014) Developing patient-specific dose protocols for a CT scanner and exam using diagnostic reference levels. Pediatr Radiol 44:479–488
Siegel MJ, Schmidt B, Bradley D et al (2004) Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape. Radiology 233:515–522
McCollough CH, Bruesewitz MR, Kofler JM Jr (2006) CT dose reduction and dose management tools: overview of available options. Radiographics 26:503–512
Costello JE, Cecava ND, Tucker JE, Bau JL (2013) CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol 201:1283–1290
Jacob K, Vivian G, Steel JR (2004) X-ray dose training: are we exposed to enough? Clin Radiol 59:928–934
Lee CI, Haims AH, Monico EP et al (2004) Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology 231:393–398
Goske MJ, Applegate KE, Boylan J et al (2008) The Image Gently campaign: working together to change practice. AJR Am J Roentgenol 190:273–274
Image Gently Alliance (2014) Image Gently development of pediatric CT protocols 2014. https://radiologiadetrinchera.files.wordpress.com/2018/01/ig-ct-protocols-111714.pdf. Accessed 01 June 2018
Brody AS, Frush DP, Huda W et al (2007) Radiation risk to children from computed tomography. Pediatrics 120:677–682
Greenwood TJ, Lopez-Costa RI, Rhoades PD et al (2015) CT dose optimization in pediatric radiology: a multiyear effort to preserve the benefits of imaging while reducing the risks. Radiographics 35:1539–1554
Huda W, Vance A (2007) Patient radiation doses from adult and pediatric CT. AJR Am J Roentgenol 188:540–546
Larson DB, Rader SB, Forman HP, Fenton LZ (2007) Informing parents about CT radiation exposure in children: it's OK to tell them. AJR Am J Roentgenol 189:271–275
Shah NB, Platt SL (2008) ALARA: is there a cause for alarm? Reducing radiation risks from computed tomography scanning in children. Curr Opin Pediatr 20:243–247
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
International Commission on Radiological Protection (2007) The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 37:1–332
Huda W, Atherton JV, Ware DE, Cumming WA (1997) An approach for the estimation of effective radiation dose at CT in pediatric patients. Radiology 203:417–422
Huda W, Ogden KM, Khorasani MR (2008) Converting dose-length product to effective dose at CT. Radiology 248:995–1003
Huda W, Ogden KM (2008) Computing effective doses to pediatric patients undergoing body CT examinations. Pediatr Radiol 38:415–423
Hendee WR, O'Connor MK (2012) Radiation risks of medical imaging: separating fact from fantasy. Radiology 264:312–321
Parakh A, Kortesniemi M, Schindera ST (2016) CT radiation dose management: a comprehensive optimization process for improving patient safety. Radiology 280:663–673
Hendee WR (1992) Estimation of radiation risks. BEIR V and its significance for medicine. JAMA 268:620–624
Huda W, Chamberlain CC, Rosenbaum AE, Garrisi W (2001) Radiation doses to infants and adults undergoing head CT examinations. Med Phys 28:393–399
Hollingsworth C, Frush DP, Cross M, Lucaya J (2003) Helical CT of the body: a survey of techniques used for pediatric patients. AJR Am J Roentgenol 180:401–406
Arch ME, Frush DP (2008) Pediatric body MDCT: a 5-year follow-up survey of scanning parameters used by pediatric radiologists. AJR Am J Roentgenol 191:611–617
Bernier MO, Rehel JL, Brisse HJ et al (2012) Radiation exposure from CT in early childhood: a French large-scale multicentre study. Br J Radiol 85:53–60
American Association of Physicists in Medicine (2017) Routine pediatric chest CT. https://aapm.org/pubs/CTProtocols/documents/PediatricRoutineChestCT.pdf. Accessed 01 June 2018
Lauing B (2013) Radiation dose legislation: is your state next? Advisory Board. https://www.advisory.com/research/imaging-performance-partnership/the-reading-room/2013/06/radiation-dose-legislation-is-your-state-next. Accessed 11 Aug 2018
Domino D (2010) Court transcripts don't resolve questions in Mad River CT case. https://www.auntminnie.com/index.aspx?sec=sup&sub=imc&pag=dis&ItemID=91193. Accessed 01 June 2018
Mezrich JL, Siegel EL (2013) Dose reporting legislation in California: are we placing the idea of patient safety ahead of reality? J Am Coll Radiol 10:814–816
Shore R, Beck H, Boice JD Jr et al (2018) Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 38:1217–1233
Siegel JA, Sacks B, Pennington CW, Welsh JS (2017) Dose optimization to minimize radiation risk for children undergoing CT and nuclear medicine imaging is misguided and detrimental. J Nucl Med 58:865–868
Siegel JA, Pennington CW, Sacks B (2017) Subjecting radiologic imaging to the linear no-threshold hypothesis: a non sequitur of non-trivial proportion. J Nucl Med 58:1–6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Goodman, T.R., Mustafa, A. & Rowe, E. Pediatric CT radiation exposure: where we were, and where we are now. Pediatr Radiol 49, 469–478 (2019). https://doi.org/10.1007/s00247-018-4281-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4281-y