Abstract
Radiation protection is a topic of great public concern and of many scientific investigations, because ionizing radiation is an established risk factor for leukaemia and many solid tumours. Exposure of the public to ionizing radiation includes exposure to background radiation, as well as medical and occupational exposures. A large fraction of the exposure from diagnostic procedures comes from medical imaging. Computed tomography (CT) is the major single contributor of diagnostic radiation exposure. An increase in the use of CTs has been reported over the last decades in many countries. Children have smaller bodies and lower shielding capacities, factors that affect the individual organ doses due to medical imaging. Several risk models have been applied to estimate the cancer burden caused by ionizing radiation from CT. All models predict higher risks for cancer among children exposed to CT as compared to adults. However, the cancer risk associated with CT has not been assessed directly in epidemiological studies. Here, plans are described to conduct an historical cohort study to investigate the cancer incidence in paediatric patients exposed to CT before the age of 15 in Germany. Patients will be recruited from radiology departments of several hospitals. Their individual exposure will be recorded, and time-dependent cumulative organ doses will be calculated. Follow-up for cancer incidence via the German Childhood Cancer Registry will allow computation of standardized incidence ratios using population-based incidence rates for childhood cancer. Dose–response modelling and analyses for subgroups of children based on the indication for and the result of the CT will be performed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT) is an important tool for diagnosis and imaging; however, there is a relatively high associated radiation exposure for the patients (Brenner and Hall 2007). Radiation protection is of particular importance for children due to their greater susceptibility to radiation. The risk of cancer associated with CT has so far been assessed by the application of radiation risk models (Berrington de González et al. 2009; Brenner et al. 2001; Chodick et al. 2007; de Jong et al. 2006; Einstein et al. 2007; Huang et al. 2009), but no direct epidemiologic data on cancer risk after CT are available. In the present paper, a literature review is given and results of a feasibility study are presented to investigate whether and how an epidemiological study on this topic could be done in Germany.
Knowledge on computed tomography and ionizing radiation risk
Computed tomography was introduced in 1972 (NCRP 2009). One of the outstanding innovations was the ability of the technique to produce three-dimensional images. All other imaging devices known until then only allowed two-dimensional images and struggled with superimpositions of bones and other structures (NCRP 2009). Furthermore, the sensitivity of a CT scanner image is tenfold higher than that of a conventional X-ray device (ICRP 2007). Nowadays, magnetic resonance imaging (MRI) provides a similar sensitivity as CT imaging. Computed tomography, MRI and ultrasound (US) show individually unique indications, but also some common indications. Concerning the common indications, CT provide considerable benefits. They can be performed within seconds and do not need sedation of very young patients as required for MRI. In contrast to US examinations, CT examinations do not depend on an individual physician’s skills (Prokop 2008).
Given the advantages and fast technological development, annual increases of 8–15% have been observed in the usage frequency of CT in the United States of America (USA) during the last decade (NCRP 2009).
For countries with high healthcare levels, an increase in CT usage frequency from 6.1 to 48 CTs per 1,000 inhabitants from the 1970s to the mid-1990s has been reported (UNSCEAR 2000a; Verdun et al. 2008). This corresponds to a nearly eightfold increase. During the same period, the average effective dose per CT examination rose from 1.3 to 8.8 mSv (UNSCEAR 2000a; Verdun et al. 2008).
In the USA, CT was a part of 15% of all medical examinations and accounted for 49% of the public’s medical radiation dose (1.46 mSv per capita) in 2006 (Mettler et al. 2008a). An even larger increase was observed in Denmark (Hansen and Jurik 2009), where the frequency of CT examinations increased from 14,500 in 1979 to about 300,000 in 2005, which corresponds to a 21-fold increase in 26 years. In Switzerland, the frequency of CT examinations rose by 70% from 1998 to 2003, the effective dose per CT increased by 20%, while the contribution to the average medical effective dose per capita from CT rose by 47% (Verdun et al. 2008). In Germany, the number of CT examinations is lower than in Japan or the USA. However, in terms of average effective dose from medical exposure, Germany clearly belongs to the countries with the highest population doses from medical imaging worldwide (BfS 2010). Here, the frequency of CT examinations increased by roughly 60–80% from 1996 to 2009 (BfS 2008; BfS 2010), and the effective dose per capita increased from 1.5 to 1.8 mSv. During the same period, a general trend of decreasing frequency of X-ray examinations was noted, with the exception of CT (BfS 2010). In 2008, CTs contributed only 8% of all radiological examinations but 60% of the effective dose (BfS 2010).
Countries with different health care systems are difficult to compare. However, the data provided by Brix et al. (2005) showed a lower annual number of CTs per capita in Germany (0.09) than in the USA (0.20) and Japan (0.29). Nevertheless, the increasing usage of CT and the increasing exposure from CT is a common phenomenon in highly developed countries, irrespective of slight ranking differences.
The increased usage of CT and the awareness of associated radiation doses among radiologists are also reflected in the scientific literature. We analysed all publications on adult doses from medical imaging in the British Journal of Radiology for the period 1971–2010 and found that the number of articles dedicated to CT increased from 1971 until 2010. Meanwhile, the absolute number of articles on doses from medical imaging in general has been declining since 2000 (Fig. 1).
Little data are available on paediatric CT practice. Reports from Europe indicate that about 1% of all CTs are paediatric, while in Japan and the USA up to 6.5% of all CTs are performed on children (Table 1) (Catuzzo et al. 2010; Galanski et al. 2006; Tsushima et al. 2010; Verdun et al. 2008). In the USA, 7.9% of patients below 18 years treated from 2005 to 2007 in selected hospitals had a CT examination. Of these, 44% had at least two CTs and 14% even 3 CTs or more (Dorfman et al. 2011). In the same population, CTs made up 11.9% of all examinations involving ionizing radiation (Dorfman et al. 2011). In Denmark, among patients undergoing CT examinations, the average number of CTs was reported to be 2.6 per patient (Hansen and Jurik 2009). Among paediatric patients, the average number of CTs per patient decreased from 2.6 in 1996 to 2.1 in 2005 (Hansen and Jurik 2009). In contrast, in Japan, the average number of CTs among paediatric CT patients was 3.3 in 2004 (Ghotbi et al. 2006), and 22.9% of the patients undergoing CT in Japan were examined more than once (Ghotbi et al. 2006). From these data, it can be concluded that multiple CTs are only performed in a small subgroup of paediatric patients. Common reasons for multiple CTs are oncologic indications. For example, in Germany for all age groups, 60% of CTs are done with an oncological indication (BfS 2010). For paediatric patients, this proportion is estimated to be roughly one-third (Krille et al. 2011a). Data on the distribution of paediatric examination types have been published for Japan, Switzerland and Germany (Table 2). Most paediatric examinations are done in the very young patients (below 6 years of age) (Galanski et al. 2006; Verdun et al. 2008). The most frequently exposed body part is the head (Galanski et al. 2006; Ghotbi et al. 2006; Verdun et al. 2008). It should be noted that head examinations may include exposure of the eyes and the thyroid due to over-ranging.
For illustration, we analysed individual dose histories for three new-borns examined with CT and conventional X-ray at the University Medical Centre of the Ludwig Maximilians University (LMU), Munich, Germany. In the same examination, the doses to the same organ may vary heavily only because of different scan areas, leaving specific organs completely or partly unexposed (Fig. 2, patient 3). Depending on the machine settings, one single CT examination can easily outweigh numerous conventional X-ray examinations (Fig. 2). On the other hand, with appropriate scanner settings, the organ doses may be only a little higher than those from conventional X-ray examinations. Even if appropriate settings are used, organ doses can sum up to 100 mGy in repeated examinations (Brenner et al. 2001; Dorfman et al. 2011). Doses from paediatric CTs published so far seem to be mainly based on single calculations and often available as effective doses only (Mettler et al. 2008b). Pages and colleagues performed a multicentre study in Belgium. They reported effective doses that ranged from 0.3 to 19.9 mSv for different standard paediatric CT examinations (Pages et al. 2003). More detailed analyses of organ doses from paediatric CT examinations are clearly required.
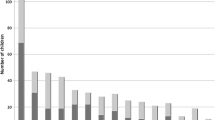
We also analysed the frequency of publications in the British Journal of Radiology and the journal Paediatric Radiology regarding doses from medical imaging for paediatric patients and, in a separate analysis, regarding CTs. Contrary to the declining absolute frequency of publications on dosimetry for adults (Fig. 1), publications about radiation doses in children have been increasing steadily, with a focus on CT (Fig. 3). These findings underline the importance of the topic and are also reflected in the research recommendations of the BEIR VII Report (BEIR 2006) where it is stated that “Epidemiologic studies of such exposures would be particularly useful if they are feasible, particularly: follow-up studies of cohorts of persons receiving CT scans, including children […]”.
The concept of effective dose, though used in the context of radiation protection, cannot be used for assessing site-specific cancer risk (Brenner 2008). To estimate the cancer risk after exposure to ionizing radiation, organ doses are needed instead. Obviously, direct measurements are not possible. Therefore, organ doses either must be measured in a phantom or must be estimated based on assumptions on the distribution of underlying measurements for examinations, equipment settings and the patient’s body.
Compared with adults, children receive higher organ doses from comparable investigations as their bodies are much smaller and the shielding of individual organs is lower (Brenner 2002).
Cancer risk from ionizing radiation for children is estimated to be higher than for adults. Per unit dose, children are more likely to develop a radiation-induced cancer due to enhanced cell proliferation. Children also have a longer life-span under risk (Brenner 2002). Age, sex and site-specific lifetime cancer models are provided, among others, by BEIR and UNSCEAR based on the data from the atomic bomb survivors studies (BEIR 2006; UNSCEAR 2000b). To calculate the CT-related disease burden, these risk models may be applied to average-age-dependent organ doses from specific CT examination types. It is useful to report additional cancer cases from CT in relation to the background cancer incidence of the general population.
Various estimations of CT-induced cancer incidence or mortality have been performed, as listed in Table 3 (Berrington de González et al. 2009; Brenner et al. 2001; Chodick et al. 2007; de Jong et al. 2006; Einstein et al. 2007; Huang et al. 2009). A direct comparison of the results of these models is limited due to the different models used in estimating the cancer risk, different target populations and partly specialized exposure types. Nevertheless, all publications estimate an increased cancer risk for children after CT. Brenner et al. (2001) estimated the paediatric excess lifetime mortality related to CT: among 600,000 children under the age of 15 who had CT scans of the head or abdomen in 2000 in the USA, 500 will die from cancer as a result of the radiation exposure. This corresponds to an excess lifetime cancer mortality risk of 0.35% (Brenner et al. 2001). In Israel, a study by Chodick et al. (2007) used the same methodology as Brenner but included up to 18-year-old adolescents. The age-specific attributable excess cancer mortality (AEM) dropped from 0.52 (for children below the age of 3) to 0.21% (for adolescents) (Chodick et al. 2007). Consequently, the attributable excess lifetime cancer risk is slightly lower than that reported by Brenner et al. (2001). Another modelling study published by de Jong et al. (2006) investigated the effects of repeated CT examinations on survival in paediatric patients with cystic fibrosis (CF). The frequent repetition of CT scans from childhood to middle age led to an AEM of up to 12%. Thus, the already reduced life expectancy of CF patients was further affected by the CT surveillance. Berrington de González et al. (2009) estimated the age-specific distribution of various CT examinations in the USA for 2007. In their study, the attributable excess cancer incidence (AEI) decreases if the age at exposure is 15 years or older. Studies by Einstein et al. (2007) and Huang et al. (2009) evaluated the lifetime attributable cancer incidence following CT coronary angiography. As they used different baseline assumptions, direct comparison is difficult. However, they both show elevated cancer risks, especially in younger ages. In summary, all modelling studies show increased cancer risks after exposure to CT, reflected in a recent meta-analysis by Stein et al. (2008). The number of additional cancer cases is, however, very low in comparison with the background cancer incidence. As indicated, direct evidence from the studies of CT-exposed children is lacking. Given the inherent uncertainties in the modelling approaches and the differing exposure situations as well as the secular trends in CT usage, studies providing direct risk estimations appear necessary.
One study using data of children and adolescents with medical diagnostic exposures was recently published (Hammer et al. 2009). The cohort consisted of 92,000 children who were followed up for childhood cancer from 1980 to 2006. CT doses were not calculated, and CT examinations were categorically summarized under “high exposures”. No risk increases associated with diagnostic radiation were found in this study. However, the vast majority of children (~83%) in the study were exposed to very low cumulative doses below 100 μSv. The small fraction of highly exposed patients (<3% of the total cohort), including those with CT examinations, also did not show a statistically increased cancer risk (Hammer et al. 2009).
Methods
Feasibility study
In order to investigate the feasibility of an epidemiological study on cancer risk following exposure to ionizing radiation from CT in childhood, all major hospitals in Germany were contacted. We asked for the estimated number of CTs performed in children per year and whether it was possible to provide patient data for a national cohort study. For a subset of hospitals that agreed to participate, detailed information on the absolute numbers of CTs per year, the time period for which data are available, the format and storage type in which the data are available and the most common CT examinations conducted in children was collected. In one hospital (University Medical Centre Mainz), we abstracted data manually from paper records. However, as this approach turned out to be too time-consuming, only electronically available data will be used in the planned study. Additionally, in the University Medical Centre Mainz, we collected data from both the electronic patient records (Radiologic Information System, RIS) and the Picture Archiving and Communication System (PACS). For the latter, specialized software was developed by the study group. Based on the experience obtained, the main study was designed and the required cohort size was calculated.
A study design for investigation of the late effects of exposure to ionizing radiation from CT in childhood
Based on the results of the feasibility study, details for the main study were laid down, as mentioned previously in brief (Krille et al. 2011b). Patients will be recruited in several major hospitals starting from the date when electronic data became available but not before 1980. The end of the inclusion period will be 31 December 2010 resulting in a study period of up to 30 years.
The study population consists of patients who underwent at least one hospital-based CT examination before the age of 15 years, starting from 1980 onwards in one of the participating hospitals. All patients must reside in Germany for cancer follow-up. Furthermore, patients with a cancer diagnosis before or up to 6 months after the date of the first CT will be excluded. This minimum latency period was chosen to exclude those cancers which, due to the short time span between exposure and cancer diagnosis, cannot be caused by the CT-related radiation exposure. Sensitivity analyses will be performed for different latency periods according to the statistical analysis plan.
Data abstraction will be done from two sources: RIS, the Radiologic Information System, and PACS, the Picture Archiving and Communication System. The electronic patient record database, RIS, stores all information needed to recruit patients for the cohort, with SQL as the most common database structure. It includes variables such as name, address, date of birth, date of exposure and the type of examination as well as indication and result. In most clinics, RIS was implemented by commercial providers, with partly individualized solutions. This fact hampers a standardized approach retrieving RIS data for the cohort study.
The second clinical database is the PACS, which stores and distributes the images of the examinations. This database type was introduced in most hospitals around the year 2000 or later. PACSs generally work with the internationally standardized format DICOM (Digital Imaging and Communications in Medicine) (NEMA 2011). Examinations and their images are managed within this format. Additionally, technical data and patient data are stored as meta-information embedded within the image file. The structure of the PACS as an image archive does not allow abstracting this information directly. Instead, the images must be retrieved in order to access the meta-information. We modified a specialized software called PerMoS developed by the Centre de Recherché Henri Tudor in Luxembourg to retrieve data from PACS (Jahnen et al. 2011). This software acts as a DICOM node. It runs automatic DICOM queries for a given list of patients at a specified frequency and in a defined time window. The images will be deleted after extracting the meta-information from the DICOM header. On demand, the data can be transferred to the dedicated study server via HTTPS (Hypertext Transfer Protocol Secure). The locally stored data will be deleted after upload. The study server combines the information from every single image to a dataset for one single CT examination. On this basis, individual effective doses and organ doses can be calculated.
Once the cohort has been assembled in the participating hospitals, all patients will be followed for incident cancer cases through linkage with the GCCR (German Childhood Cancer Registry), which is known to have a high level of completeness and nationwide coverage (Kaatsch and Spix 2010). Individual data from the GCCR and from the departments of radiology include the full name, addresses, date of birth, last names and any history of changes in residence where applicable. Both databases will be encrypted with an identical algorithm, allowing statistical linkage without the need for plain text information. Using this procedure, incident cancer patients can be identified and data protection can be assured.
The parameters to estimate radiation exposure will be derived from the PACS. In general, patient height and weight are neither available in RIS nor in PACS. For the present study, average body measurements will be taken from population-based age and calendar-year-specific distributions. This information will be used by the software package CT-Expo to calculate effective doses and organ doses (Stamm and Nagel 2002). Additionally, new conversion factors for newer CT scanners and additional phantoms of the body will be developed, and comparisons will be made for validation. For the time period without detailed exposure information, published reference values or those derived from other hospitals within the study must be used. In an additional analysis, the frequency of CTs per patient as a surrogate variable for exposure will be used.
The indication to perform a CT scan may be associated with the outcome of interest (cancer) as well as with the exposure. Patients with early symptoms of cancer have a high risk of developing cancer (by definition) and will also have an increased probability of receiving CT examinations for cancer diagnosis. To address this problem, the first 6 months after CT exposure will contribute neither to the analysis nor to the calculation of person-years under risk.
Indication and result of the CT examination will be recoded whenever available. Patients will be grouped according to their indications. The examinations’ indications and results are available as unstructured text-based information only. To make this information accessible, a semiautomatic approach will be used: a random subset will be analysed by a medically skilled reviewer. The categorized indications and results will then be entered in a programme for text analysis (Averbis Extraction Platform) (Daumke et al. 2010). The software used will perform morphological analyses to assign standardized terms, which are taken from an extended version of RadLex in combination with ICD-10 (DMDI 2011; RSNA 2011). Based on the distributions of the standardized terms, rules for automated classification will be derived. The accuracy of the automated classification will be analysed by cross-validations. Imprecise assignments as well as random samples will be reviewed manually, and the resulting information again entered into the machine’s learning process. After reaching a high degree of accuracy, all remaining indications and results will be entered in the software. Based on this procedure, patients diagnosed with illnesses leading to a priori higher mortality risks or higher cancer risks will be flagged and analysed separately.
Standard statistical analysis for cohort studies will be used: person-years under risk will be calculated starting from the 6 month after the first known CT examination until either the 15th birthday, the date of cancer diagnosis or 31 December 2010, whichever occurs first. Date of death will not be obtained for the members of the cohort as childhood mortality is very low and mortality follow-up is costly in Germany. To take mortality into account, the person-years based on the mortality rates of children in Germany will be discontinued.
We will perform both an external comparison (standardized incidence ratio (SIR)) and an internal (dose–response) analysis. In the external analysis, cancer incidence within the cohort will be compared with that of the underlying population. For the dose–response analysis, the cumulative doses for organs and the absolute number of CT examinations per person will be used. A detailed analysis plan will be prepared prior to analysis.
The possible size of the cohort was estimated based on the results of the feasibility study. For this, the average number of paediatric CTs per hospital type was calculated. The estimated fraction of CTs performed for prevalent cancers and the use of multiple CTs in the same individuals were estimated and a crude number of children available for the study were calculated. As a result, we expect to include about 85,000 eligible patients below the age of 15 without cancer at the time of exposure. These individuals will contribute about 800,000 person-years, yielding an expected number of about 110 spontaneous cancer cases, including 38 children with leukaemia, during the period of follow-up. Thus, the cohort will have sufficient power to detect an SIR of 1.3 for all cancers and 1.5 for leukaemia (with α = 0.05 and β = 0.2). Even if the final cohort is somewhat smaller, SIRs of similar magnitude can be found (Table 4).
Discussion
Computed tomography has become a major resource in modern radiology and medicine. Besides the obvious benefits of improved diagnostics, an increased cancer risk after exposure to CT has been estimated in several publications based on current radiation risk models (BEIR 2006; UNSCEAR 2000b). However, epidemiological studies on CT and paediatric cancer risk have not been conducted so far.
We conducted a feasibility study to assess options for an epidemiological study and used this experience to finalize the study design. The cohort members will be recruited retrospectively from medical databases of German radiology departments. Individual radiation doses will be reconstructed, and incident cancers in the cohort will be identified from the nationwide German Childhood Cancer Registry. This approach allows deriving SIR (standardized incidence ratios) and dose–response relationships, albeit with limited statistical power for the latter.
By deriving cancer risk estimates for specific age groups and types of CT examinations, it is expected to gain an improved understanding about the underlying dose–response relationships after exposure to low levels of ionizing radiation. It should be noted that direct estimates of radiation effects in the low-dose range are needed to complement risk estimations based on extrapolations from the Life Span Study of atomic bomb survivors in Japan, and to add information on the shape of the dose–response curve below about 100 mGy.
Any radiation-associated risk from CT needs to be viewed in perspective. Patients with a severe illness often show high disease-associated risks, and CT diagnosis is likely to offer major benefits (Prokop 2008). There may be other instances where the situation is less clear, particularly when other imaging modalities are available. In any case, CT-associated risks are probably better understood when reported in comparison with the baseline risks. For example, a reported attributable lifetime cancer incidence risk of 1/143 from a single angiogram performed in a 20-year-old woman will be judged differently if contrasted with a natural lifetime cancer incidence risk of 1/3. The corresponding lifetime cancer risk increase associated with the angiogram is 2% (Lucan 2010).
One major limitation of the proposed study is that any radiation exposure of cohort members obtained outside the participating hospitals is not included, leading to an underestimation of the total dose. However, 63% of all paediatric CTs are performed in university medical centres and only 33% in other hospitals. In Germany, about 4% of paediatric CTs are done in private radiological offices (Galanski et al. 2006). By focussing on university hospitals, we expect to include the majority of paediatric CT exposures in the study. In relation to CT, the radiation dose from most other radiological examinations is small.
Based on the power calculations performed, the cohort will have sufficient power to detect 30–50% risk increases for cancer and leukaemia. It should be noted that it is unlikely that the relative risk for CT-induced cancer will be as high as 1.3–1.5. However, the study will contribute to a larger European study coordinated by IARC, with substantially more power (EC CORDIS 2011). Further pooling with other international studies is desirable. First results from the German study will be available by the end of 2013.
References
BEIR (2006) Health risks from exposure to low levels of ionizing radiation (BEIR VII-Phase 2). The National Academies Press, Washington, DC
Berrington de González A, Mahesh M, Kim KP, Bhargavan M, Lewis R, Mettler F, Land C (2009) Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169:2071–2077
BfS (2008) Umweltradioaktivität und Strahlenbelastung im Jahr 2007. Bundesregierung Deutschland, Berlin
BfS (2010) Umweltradioaktivität und Strahlenbelastung im Jahr 2009. Bundesregierung Deutschland, Berlin
Brenner D (2002) Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol 32:228–231
Brenner D (2008) Effective dose: a flawed concept that could and should be replaced. Br J Radiol 81:521–523
Brenner DJ, Hall EJ (2007) Computed tomography—An increasing source of radiation exposure. N Engl J Med 357:2277–2284
Brenner DJ, Elliston CD, Hall EJ, Berdon WE (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol 176:289–296
Brix G, Nekolla E, Griebel J (2005) Radiation exposure of patients from diagnostic and interventional X-ray procedures. Facts, assessment and trends. Radiology 45:340–349
Catuzzo P, Aimonetto S, Zenone F, Fanelli G, Marchisio P, Meloni T, Pasquino M, Tofani S (2010) Population exposure to ionising radiation from CT examinations in Aosta Valley between 2001 and 2008. Br J Radiol 83:1042–1051
Chodick G, Ron E, Ronckers C, Shalev V (2007) Excess lifetime cancer mortality risk attributable to radiation exposure from computed tomography examinations in children. Isr Med Assoc J 8:584–587
Daumke P, Enders F, Simon K, Poprat M, Markó K (2010) Semantic annotation of clinical text—the averbis annotation editor. In: Proceedings of the 55th conference of the German society of medical informatics, biometry and epidemiology. GMS, Düsseldorf
de Jong PA, Mayo JR, Golmohammadi K, Nakano Y, Lequin MH, Tiddens HA, Aldrich J, Coxson HO, Sin DD (2006) Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med 173:199–203
DMDI (2011) ICD-10-GM Version 2011. http://www.dimdi.de. 1 Jan 2011
Dorfman AL, Fazel R, Einstein AJ, Applegate KE, Krumholz HM, Wang Y, Christodoulou E, Chen J, Sanchez R, Nallamothu BK (2011) Use of medical imaging procedures with ionizing radiation in children: a population-based study. Arch Pediatr Adolesc Med 165:458–464
EC CORDIS (2011) Epidemiological study to quantify risks for paediatric computerized tomography and to optimise doses. (EPI-CT). http://cordis.europa.eu. 25 Mar 2011
Einstein AJ, Henzlova MJ, Rajagopalan S (2007) Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. J Am Med Assoc 298:317–323
Galanski M, Nagel HD, Stamm G (2006) Paediatrische CT-expositionspraxis in der BRD: ergebnisse 2005/06. Medizinische Hochschule Hannover, Hannover
Ghotbi N, Ohtsuru A, Ogawa Y, Morishita M, Norimatsu N, Namba H, Moriuchi H, Uetani M, Yamashita S (2006) Pediatric CT scan usage in Japan: results of a hospital survey. Radiat Med 24:560–567
Hammer GP, Seidenbusch MC, Schneider K, Regulla DF, Zeeb H, Spix C, Blettner M (2009) A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res 171:504–512
Hansen J, Jurik AG (2009) Analysis of current practice of CT examinations. Acta Oncol 48:295–301
Huang B, Law MW, Mak HK, Kwok SP, Khong PL (2009) Pediatric 64-MDCT coronary angiography with ECG-modulated tube current: radiation dose and cancer risk. Am J Roentgenol 193:539–544
ICRP (2007) The 2007 recommendations of the internal commission on radiological protection. 103. Elsevier, Oxford
Jahnen A, Kohler S, Hermen J, Tack D, Back C (2011) Automatic computed tomography patient dose calculation using DICOM header metadata. Radiat Prot Dosim 147:317–320
Kaatsch P, Spix C (2010) Jahresbericht des deutschen kinderkrebsregisters 2009. Deutsches Kinderkrebsregister, Mainz
Krille L, Scholz P, Mirizt G, Blettner M (2011a) KiCT: cancer risk after exposure with computed tomographies in childhood: descriptive analysis of the feasibility study. In: Proceedings of the 56th conference of the German society of medical informatics, biometry and epidemiology. GMS, Düsseldorf
Krille L, Jahnen A, Mildenberger P, Schneider K, Weisser G, Zeeb H, Blettner M (2011b) Computed tomography in children: multicenter cohort study design for the evaluation of cancer risk. Eur J Epidemiol 26:249–250
Lucan SC (2010) Reporting of cancer risk from CT scans is misleading. Am Fam Physician 82:1312–1313
Mettler FA Jr, Thomadsen BR, Bhargavan M, Gilley DB, Gray JE, Lipoti JA, McCrohan J, Yoshizumi TT, Mahesh M (2008a) Medical radiation exposure in the U.S. in 2006: preliminary results. Health Phys 95:502–507
Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M (2008b) Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248:254–263
NCRP (2009) Ionizing radiation exposure of the population of the United States—NCRP REPORT No. 160. NCRP Publications Bethesda, Maryland, 3 Mar 2009
NEMA (2011) Digital imaging and communications in medicine (DICOM). http://medical.nema.org/. 1 Jan 2011
Pages J, Buls N, Osteaux M (2003) CT doses in children: a multicentre study. Br J Radiol 76:803–811
Prokop M (2008) Radiation dose in computed tomography. Risks and challenges. Radiology 48:229–242
RSNA (2011) RadLex: A lexicon for uniform indexing and retrieval of radiology Information resources. http://www.rsna.org/radlex/. 1 May 2011
Stamm G, Nagel HD (2002) CT-expo—a novel program for dose evaluation in CT. Fortschr Rontg 174:1570–1576
Stein SC, Hurst RW, Sonnad SS (2008) Meta-analysis of cranial CT scans in children. A mathematical model to predict radiation-induced tumors. Pediatr Neurosurg 44:448–457
Tsushima Y, Taketomi-Takahashi A, Takei H, Otake H, Endo K (2010) Radiation exposure from CT examinations in Japan. BMC Med Imaging 10:24
UNSCEAR (2000a) Report to the general assembly, Annex D: medical radiation exposures. United Nations, New York
UNSCEAR (2000b) Sources and effects of ionizing radiation (volume II: effects). United Nations, Vienna
Verdun FR, Gutierrez D, Vader JP, Aroua A, Alamo-Maestre LT, Bochud F, Gudinchet F (2008) CT radiation dose in children: a survey to establish age-based diagnostic reference levels in Switzerland. Eur Radiol 18:1980–1986
Acknowledgments
The feasibility study was and the main cohort study will be funded by the German Federal Ministry of Education and Research under the grant numbers 02NUK007D and 02NUK016A, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krille, L., Zeeb, H., Jahnen, A. et al. Computed tomographies and cancer risk in children: a literature overview of CT practices, risk estimations and an epidemiologic cohort study proposal. Radiat Environ Biophys 51, 103–111 (2012). https://doi.org/10.1007/s00411-012-0405-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-012-0405-1