Abstract
Olaparib (Lynparza®) is a poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitor approved for first-line maintenance treatment in adults with advanced ovarian cancer who are in complete or partial response to first-line, platinum-based chemotherapy. Originally approved as monotherapy, olaparib is also approved to be administered in combination with bevacizumab in patients whose cancer is associated with homologous recombination deficiency (HRD), defined by either a BRCA1/2 mutation and/or genomic instability. In phase III trials, olaparib monotherapy significantly improved progression-free survival (PFS) relative to placebo (SOLO-1), as did olaparib plus bevacizumab relative to placebo plus bevacizumab (PAOLA-1), in patients with advanced ovarian cancer who had responded to platinum-based chemotherapy. In PAOLA-1, improvements in PFS with olaparib plus bevacizumab were not seen in patients with HRD-negative tumours relative to placebo plus bevacizumab. Both olaparib monotherapy and olaparib in combination with bevacizumab had generally manageable tolerability profiles. Olaparib, alone or in combination with bevacizumab, is a useful option for the first-line maintenance treatment of adults with HRD-positive, advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy.
Plain Language Summary
Oral olaparib (Lynparza®) was originally approved as monotherapy for the first-line maintenance treatment of adults with advanced high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who responded to first-line, platinum-based chemotherapy. Olaparib is also approved to be used in combination with bevacizumab in patients whose cancer is associated with homologous recombination deficiency (HRD), which is characterized by BRCA1/2 mutations or genomic instability. Olaparib reduced the risk of disease progression or death in patients who had received platinum-based chemotherapy without bevacizumab (when olaparib was given as monotherapy) and with bevacizumab (when olaparib was given with bevacizumab). However, this reduction was not seen in patients with HRD-negative tumours who were treated with olaparib plus bevacizumab compared with placebo plus bevacizumab. Both olaparib monotherapy and olaparib in combination with bevacizumab had generally manageable tolerability profiles. Olaparib, alone or in combination with bevacizumab, is a useful option for the first-line maintenance treatment of adults with HRD-positive, advanced ovarian cancer who responded to first-line, platinum-based chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.15139005 |
PARP inhibitor approved as monotherapy and in combination with bevacizumab for the maintenance treatment of advanced ovarian cancer |
Extends PFS in responders to platinum-based chemotherapy with HRD-positive tumours |
Manageable tolerability profile |
1 Introduction
Among all gynaecological cancers, ovarian cancer has the highest mortality rate in the USA [1] and the second highest in the EU and UK (behind cervical cancer) [2]. Due to a lack of clear symptoms earlier in the disease, the majority of patients with ovarian cancer present with advanced disease at the time of diagnosis, and relapse despite aggressive treatment measures such as cytoreductive surgery and platinum-based chemotherapy; their life expectancy beyond treatment is usually short [3]. Minimizing the chances of disease recurrence in those who have responded to primary treatment therefore forms an important facet in the treatment of ovarian cancer. Until recently, bevacizumab [a vascular endothelial growth factor receptor (VEGFR) inhibitor] was the only available option for maintenance treatment following first-line platinum-based chemotherapy in advanced ovarian cancer; it may also be administered alongside chemotherapy in patients with advanced ovarian cancer [4].
High-grade serous ovarian carcinoma (HGSOC) is the most common subtype of epithelial ovarian cancer [5] and is frequently associated with genomic instability, with homologous recombination deficiency (HRD) seen in ≈ 50% of patients with HGSOC [6]. HRD commonly manifests as BRCA1 and/or BRCA2 mutations in these tumours and results in limited functionality in DNA double-strand break repair [7]. As poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors obstruct another key component in DNA repair, they are particularly effective in exacerbating DNA damage in these cells and therefore in promoting their death [8]. PARP inhibitors including olaparib (Lynparza®), niraparib and rucaparib, have accordingly demonstrated effectiveness as maintenance treatment in patients with HRD-positive epithelial ovarian cancer, with improved rates of survival [8].
Olaparib as monotherapy is approved for the first-line maintenance treatment of adults with advanced high-grade epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy, with deleterious or suspected deleterious germline or somatic BRCA1/2 mutations [9, 10]; its use in this indication has been previously discussed [11, 12]. Recently, olaparib has also been approved in the EU [9] and USA [10] to be administered as first-line maintenance treatment in combination with bevacizumab for patients whose cancer is associated with HRD-positive status, which is defined by either a deleterious or suspected BRCA1/2 mutation and/or genomic instability. This article reviews the therapeutic efficacy and tolerability of olaparib in these first-line maintenance indications; discussion of its used in other approved indications is beyond the scope of this review. The pharmacological properties of olaparib are summarized in Table 1.
2 Therapeutic Efficacy of Olaparib
Two randomized, double-blind, placebo-controlled, multinational phase III trials assessed the efficacy of first-line maintenance therapy with oral olaparib, as monotherapy (SOLO-1; n = 391 [13]) and as combination therapy with bevacizumab (PAOLA-1; n = 806 [14]), in women newly diagnosed with advanced (International Federation of Gynecology and Obstetrics stage III or IV) high-grade serous or endometerioid ovarian cancer, primary peritoneal cancer, and/or fallopian tube cancer (Fig. 1).
Clinical trial designs of the SOLO-1 [13] and PAOLA-1 [14] studies. Oral olaparib was administered at a dosage of 300 mg twice daily in both studies. In PAOLA-1, bevacizumab was administered at a dose of 15 mg per kilogram of body weight every 3 weeks for up to 15 months. Both studies are currently ongoing. HR hazard ratio, NR not reached
Supplementary file1 (MP4 8457 kb)
Eligible patients (aged ≥ 18 years) had received first-line, platinum-based chemotherapy (6–9 cycles; ≥ 4 cycles in patients who had discontinued chemotherapy earlier due to non-haematological toxicity) without bevacizumab [13] or with bevacizumab [14], to which they had a complete or partial clinical response [13, 14]. In SOLO-1, patients with stage III disease had previously received cytoreductive surgery before or during but before the end of chemotherapy, and those with stage IV disease had undergone biopsy or cytoreductive surgery (upfront or interval) [13]. SOLO-1 participants also had a confirmed or suspected germline or somatic BRCA1 and/or BRCA2 mutation at baseline [13]. PAOLA-1 enrolled patients regardless of BRCA mutation status and also included patients with other non-mucinous epithelial ovarian cancers, provided that they had a deleterious germline BRCA1 or BRCA2 mutation [14]. Patients who had received radiotherapy within 3 weeks [13] or 6 weeks [14] of the studies were excluded. In SOLO-1, those with BRCA1 or BRCA2 mutations not considered detrimental and those previously treated with a PARP inhibitor were also excluded [13].
In both studies, participants were randomly assigned in a 2:1 ratio to receive oral olaparib 300 mg twice daily or placebo, starting from 3 to 9 weeks after completing platinum-based chemotherapy [13, 14]. In SOLO-1, patients who had no evidence of disease after 2 years discontinued treatment, while those with evidence of disease were permitted to continue treatment [13]. In PAOLA-1, olaparib or placebo administration continued for up to 2 years; all participants also received intravenous bevacizumab (15 mg per kilogram of body weight) from the start of chemotherapy, and continued to receive it after randomization into their respective treatment arms every 3 weeks for up to 15 months in total [14]. Patients in both studies discontinued study treatment if they experienced objective radiological disease progression (as per RECIST 1.1) or unacceptable tolerability issues [13, 14]. After discontinuation of the trial intervention, patients could receive other treatments at the investigators’ discretion [13, 14].
The primary endpoint in both studies was investigator-assessed progression-free survival (PFS), defined according to investigators’ assessments as the time from baseline to objective disease progression or death; estimates in PFS were calculated using the Kaplan–Meier method [13, 14]. In both studies, PFS, second PFS and overall survival were assessed hierarchically (in that order) [13, 14]. Patients were assessed for disease progression via computerized tomography (CT) or magnetic resonance imaging (MRI) every 12 weeks for up to 3 years, and every 24 weeks thereafter, in SOLO-1 [13], and every 24 weeks [or at planned visits every 12 weeks if there was evidence of clinical progression or elevation of cancer antigen 125 (CA-125)] in PAOLA-1 [14].
2.1 Monotherapy
At baseline, the majority of SOLO-1 participants (78%) had completed six cycles of platinum-based chemotherapy [13]. Most patients (82%) had a complete response to chemotherapy; 18% had a partial response. Almost all patients (96%) had serous ovarian cancer, and all but three had a germline BRCA1 or BRCA2 mutation; two patients were BRCA wild type but had somatic BRCA mutations and one had a germline BRCA variant of undefined significance. Most patients had stage III disease (83%; 17% had stage IV disease) and the primary tumour location was in the ovaries, fallopian tube and peritoneum in 85%, 8% and 6% of patients, respectively (unconfirmed in 1%). The majority of patients had upfront surgery (62% of olaparib and 65% of placebo recipients); 36% of olaparib and 33% of placebo recipients received interval cytoreductive surgery [13].
Initial findings from SOLO-1 demonstrated that olaparib monotherapy was effective as first-line maintenance treatment in women with newly diagnosed advanced ovarian cancer (Table 2) [13]. At the time of the first interim analysis, 47% of olaparib and 27% of placebo recipients completed treatment at 2 years (10% and 2% continued treatment beyond 2 years). The median PFS was not yet reached in the olaparib group and was 13.8 months in the placebo group; estimates based on investigator-assessed PFS data showed a 70% improvement in the risk of disease progression or death in 3 years with olaparib relative to placebo (primary endpoint; Table 2), with a predicted PFS rate of 60% (vs 27%). Sensitivity analyses of PFS further supported these findings where estimates were similar when assessed by blinded independent central review PFS data [69 vs 35%; hazard ratio (HR) 0.28, 95% CI 0.20–039; p < 0.001], and the median PFS was 36 months longer than with placebo when evaluated for potential attrition bias. The risk of second disease progression or death in 3 years was estimated to be improved by 50% with olaparib compared with placebo (second PFS rate 75 vs 60%) [Table 2] [13].
Findings from subgroup analyses indicated that PFS was improved with olaparib regardless of baseline surgery outcome, chemotherapy response, BRCA mutation subtype or disease state [13]. Compared with placebo, olaparib decreased the risk of disease progression or death by 69% and 63% in patients with previous upfront and interval surgery, 56% and 66% in those with residual and no residual disease post-surgery, 66% and 69% in those with complete and partial response to chemotherapy, and 59% and 80% in patients with BRCA1 and BRCA2 mutations [15]. Olaparib also reduced the risk of disease progression or death relative to placebo by 66% (investigator-assessed median PFS 39.0 months vs 11.1 months with placebo) in higher-risk patients (i.e. those with stage IV disease; stage III disease that was inoperable, or had residual disease after primary debulking surgery; or stage III disease and had undergone interval surgery) and by 67% (median PFS not reached vs 21.9 months) in lower-risk patients (i.e. those with stage III disease without residual disease after primary debulking surgery) [16].
While median overall survival was not reached at the time of the first interim analysis in both the olaparib and placebo treatment arms (Table 2), the overall survival rate at 3 years was estimated to be 84% and 80%, respectively [13]. Olaparib improved the time to the first subsequent therapy or death (TFST) by 70% relative to placebo (Table 2). The time to second subsequent therapy or death (TSST) was improved by 55% relative to placebo (median TSST with olaparib not reported vs 40.7 months with placebo; HR 0.45, 95% CI 0.32–0.63) and, at 3 years, the rate of freedom from the use of a second subsequent therapy and death was 74% with olaparib and 56% with placebo [13].
Health-related quality of life (HR-QoL) was not noticeably affected by olaparib compared with placebo [13]. The mean Trial Outcome Index score on the Functional Assessment of Cancer Therapy–Ovarian Cancer (FACT-O) questionnaire was generally maintained from baseline with olaparib, and the estimated between-group difference of the adjusted mean change from baseline (− 3.00 points; 95% CI − 4.78 to − 1.22) was not considered clinically significant [13, 17]. Post-hoc analyses indicated that, relative to placebo, olaparib significantly (p < 0.001) increased quality-adjusted PFS (adjusted based on EuroQoL 5D-5L questionnaire scores; between-group difference 12.2 months) and time without significant symptoms of toxicity (defined as grade ≥ 2 nausea, vomiting or fatigue; between-group difference 12.9 months) [17].
Findings from a second interim analysis indicate that patients continued to experience therapeutic benefits with olaparib monotherapy after five years of treatment [18, 19]. Investigator-assessed PFS with olaparib was consistent with what was observed in the first interim analysis, with a 67% reduction in risk of disease progression or death (Table 2). The annual proportions of progression-free patients with olaparib were estimated to be 87.7–48.3% over the 5-year period (vs 51.4–20.5% with placebo) [18]. Recurrence-free survival outcomes (with recurrence defined as new lesions by imaging) showed a similar trend to those of PFS. While the median recurrence-free survival with olaparib was not reached at the time of analysis (vs 15.3 months with placebo; HR 0.37, 95% CI 0.27–0.52), the annual proportion of patients being recurrence free ranged from 91.0 to 51.9% with olaparib and 58.0–21.8% with placebo. Disease progression or death occurred in 45% of olaparib and 76% of placebo recipients, and recurrences were reported in 42% and 73% of patients, respectively [18]. After 5 years, 42% and 17% of olaparib and placebo recipients at higher risk and 56% and 25% of lower-risk patients were progression-free [19].
2.2 Combination Therapy with Bevacizumab
Over half of all PAOLA-1 participants (53%) had no evidence of disease at baseline (following cytoreductive surgery and chemotherapy); complete and partial clinical responses to chemotherapy were seen in 20% and 27% of patients [14]. Most patients (96%) had serous ovarian cancer and 30% had deleterious tumor BRCA mutations. Most patients had stage III disease (70%; 30% had stage IV), and the primary tumour location was in the ovaries, fallopian tube and peritoneum in 86%, 6% and 8% of patients, respectively. In the olaparib plus bevacizumab and placebo plus bevacizumab groups, 50% and 51% of patients received upfront cytoreductive surgery and 42% and 41% received interval cytoreductive surgery; 7% and 8% did not undergo surgery [14].
PAOLA-1 findings indicate that olaparib in combination with bevacizumab provided greater therapeutic benefit than bevacizumab alone [14]. At the initial interim analysis, the median investigator-assessed PFS was significantly longer with olaparib plus bevacizumab than with placebo plus bevacizumab, reducing the risk of progression or death by 41% (primary endpoint; Table 2). When assessed via blinded independent central review, the median PFS and HR were consistent with investigator-assessed PFS (26.1 months with olaparib plus bevacizumab vs 18.3 months with placebo plus bevacizumab; HR 0.63, 95% CI 0.51–0.77). Olaparib plus bevacizumab and placebo plus bevacizumab recipients were estimated to have a 79% and 80% rate of freedom from disease progression and death at 18 months (HR 0.86; 95% CI 0.69–1.09). The TFST was improved by 41% with olaparib plus bevacizumab relative to placebo plus bevacizumab (Table 2) [14]. At the final analysis of second PFS (occurring a year after the initial interim analysis), second PFS was significantly improved with olaparib plus bevacizumab (Table 2) as was the TSST (median 38.2 months vs 31.5 months; HR 0.75, 95% CI 0.64–0.95; p = 0.0115) [abstract] [20].
In predefined subgroup analyses, PFS findings favoured olaparib plus bevacizumab over placebo plus bevacizumab in patients with HRD-positive tumours (defined by tumour HRD score ≥ 42 or a tumour BRCA mutation) [14]. The median PFS with olaparib plus bevacizumab in these patients was improved by 67% relative to placebo plus bevacizumab (37.2 months vs 17.7 months; HR 0.33, 95% CI 0.25–0.45). Patients with HRD-positive, but BRCA mutation-negative tumours also benefited from olaparib plus bevacizumab therapy (median PFS 28.1 months vs 16.6 months; HR 0.43, 95% CI 0.28–0.66). However, PFS did not appear to be improved in patients with HRD-negative tumours or those with an unknown HRD tumour status, with comparable median PFS observed between the two treatment groups in these patients (median PFS 16.9 months vs 16.0 months; HR 0.92, 95% CI 0.75–1.35) [14]. At the time of final analysis of the second PFS, the HR for second PFS between the olaparib plus bevacizumab versus placebo plus bevacizumab groups in HRD-negative patients was 1.04 (95% CI 0.77−1.42); HRs were 0.53 (95% CI 0.34−0.83) in patients with BRCA mutations, 0.56 (95% CI 0.41−0.77) in HRD-positive patients, and 0.60 (95% CI 0.38−0.96) in HRD-positive patients without BRCA mutations (abstract) [20].
In an exploratory analysis using a range of homologous recombination repair (HRR) gene panels to assess PFS in patients with tumour mutations, HRR mutations (except for those in BRCA) did not appear to be predictive of the extent of PFS benefit with respect to olaparib plus bevacizumab therapy, with consistent findings seen across subgroups of differing HRR mutations [21]. Another exploratory analysis indicated that investigator-assessed PFS was improved with olaparib plus bevacizumab relative to placebo plus bevacizumab in both higher-risk patients (i.e. stage III patients with upfront surgery and residual disease or had received neoadjuvant chemotherapy; stage IV patients) and lower-risk patients (i.e. stage III patients with upfront surgery and no residual disease) [HR in higher-risk patients 0.60, 95% CI 0.46−0.74; lower-risk patients 0.46, 95% CI 0.30−0.72] [22].
There were no clinically significant changes in HR-QoL in each treatment arm [14]. The adjusted mean change from baseline in mean global health status-quality of life score was − 1.33 points (95% CI − 2.47 to − 0.19) in assessed olaparib plus bevacizumab recipients (n = 498; baseline HR-QoL score 68.6 points) and − 2.89 points (95% CI − 4.52 to − 1.26) in placebo plus bevacizumab recipients (n = 246; baseline score 67.1 points). The estimated between-group difference of 1.56 points (95% CI − 0.42 to 3.55) was also not considered to be of clinical significance [14].
3 Tolerability of Olaparib
Oral olaparib, administered with [14] or without [23] bevacizumab, had a generally manageable tolerability profile in adults with advanced, high-grade serous or endometerioid ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. In SOLO-1 [23] and PAOLA-1 [14], the median duration of treatment was 24.6 months and 13.9 months in the olaparib and placebo groups, respectively [23] and 17.3 months and 15.6 months in the olaparib plus bevacizumab and placebo plus bevacizumab groups, respectively [14]. No new safety signals were reported after a median follow-up duration of ≈ 5 years [18].
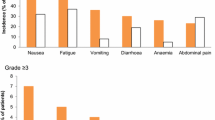
SOLO-1 and PAOLA-1 findings indicate that the tolerability profile of olaparib was generally similar with or without bevacizumab. Most patients in both studies (≥ 98% of those receiving olaparib and ≥ 92% of those not receiving olaparib) experienced adverse events (AEs) of any grade [14, 23]; 39% and 18% of olaparib and placebo recipients in SOLO-1 , and 57% and 51% of olaparib plus bevacizumab and placebo plus bevacizumab recipients in PAOLA-1, experienced grade ≥ 3 AEs. The most common any-grade AEs (incidence ≥ 30% with olaparib) occurring in more olaparib than placebo recipients in SOLO-1 were nausea, fatigue or asthenia, vomiting, anemia and diarrhea [23]; those occurring in more olaparib plus bevacizumab than placebo plus bevacizumab recipients in PAOLA-1 were fatigue or asthenia, nausea, and anemia (Fig. 2) [14]. Hypertension, which has been associated with bevacizumab therapy [24], was also among the most frequent type of AEs in PAOLA-1 and occurred in more placebo than olaparib recipients (Fig. 2) [14]. The most common (incidence > 5%) grade ≥ 3 AEs in the olaparib group in SOLO-1 were anemia and neutropenia [23], and the most common in the olaparib plus bevacizumab group in PAOLA-1 were hypertension, anemia and neutropenia (Fig. 2) [14].
Most common adverse events (incidence of ≥ 20% for any-grade adverse events, or > 5% for grade ≥ 3 adverse events, in any treatment arm) in SOLO-1 (olaparib group n = 260, placebo group n = 130) [23] and PAOLA-1 (olaparib plus bevacizumab group n = 535, placebo plus bevacizumab group n = 267) [14]. AEs adverse events, BEV bevacizumab, OLA olaparib, PL placebo, θ = 0% (for grade ≥ 3 AEs)
Serious AEs (SAEs) occurred in 21% and 12% of olaparib and placebo recipients in SOLO-1 [23] and in 31% in both treatment arms of PAOLA-1 [14]; the most common (incidence > 5%) were anemia (6.5 vs 0% [23]; 6 vs < 1% [14]) and hypertension (not reported in SOLO-1; 9 vs 13% [14]). No AE resulted in death during treatment or within 30 days after discontinuing treatment in SOLO-1 [23]; in PAOLA-1, fatal AEs occurred in one olaparib recipient (aplastic anemia and pneumonia) and four placebo recipients (myocardial infarction in two patients; intestinal perforation and dyspnea in one patient each) [14]. Among patients receiving olaparib in SOLO-1 [23] and PAOLA-1 [14], 12% (vs 2% of patients not receiving olaparib) and 20% (vs 6%) discontinued treatment due to an AE, 52% (vs 17%) and 54% (vs 24%) had AE-related dose interruptions, and 28% (vs 3%) and 41% (vs 7%) had AE-related dose reductions.
Data collected only from when bevacizumab was administered with olaparib or placebo (i.e. during ≤ 15 months after randomization) in PAOLA-1 are consistent with these findings [14]. The median duration of treatment with bevacizumab from randomization in the olaparib and placebo groups was 11.0 (range 0.7–21.4) months and 10.6 (range 0.7–17.1) months. Grade ≥ 3 AEs occurred in 49% and 43% of the olaparib plus bevacizumab and placebo plus bevacizumab groups, and SAEs in 24% of both groups. AEs led to dose interruption, dose reduction and treatment discontinuation in 50% (vs 19% of placebo plus bevacizumab recipients), 39% (vs 6%) and 15% (vs 4%) of olaparib plus bevacizumab recipients, respectively. Fatal AEs were reported in no olaparib plus bevacizumab recipient and two placebo plus bevacizumab recipients (causative AEs not specified) [14].
In PAOLA-1, myelodysplastic syndromes, acute myeloid leukemia, or aplastic anemia occurred in six olaparib plus bevacizumab recipients and one placebo plus bevacizumab recipient [14]. In SOLO-1, acute myeloid leukemia was reported in three olaparib and no placebo recipients, all of which occurred more than 30 days after the end of olaparib therapy [23]. New primary cancers were reported in five olaparib and no placebo recipients in SOLO-1 [23] and in seven olaparib plus bevacizumab and three placebo plus bevacizumab recipients in PAOLA-1 [14]. Pneumonitis or interstitial lung disease each occurred in five olaparib and no placebo recipients in SOLO-1 [23]; in PAOLA-1 [14], grade 1 or 2 pneumonitis, interstitial lung disease, or bronchiolitis occurred in six olaparib plus bevacizumab and no placebo plus bevacizumab recipients.
4 Dosage and Administration of Olaparib
The recommended dosage for olaparib (as both monotherapy and combination therapy with bevacizumab) is 300 mg twice daily, with or without food in the USA [10] and at least 1 h after food (and preferably refraining from eating for ≤ 2 h after administration) in the EU [9]. In the EU, olaparib treatment is recommended to be initiated no later than 8 weeks after the final dose of platinum-based chemotherapy [9]. Olaparib should be continued until disease progression, unacceptable toxicity, or completion of 2 years of treatment; patients with evidence of disease at 2 years, who in the opinion of the treating healthcare provider can derive further benefit from continuous olaparib therapy, may continue treatment beyond 2 years [9, 10]. When administered in combination with olaparib, bevacizumab should be administered at 15 mg/kg once every 3 weeks for a total of 15 months, including the period given with chemotherapy and given as maintenance [9, 10].
Dose reduction or treatment interruption should be considered to manage adverse reactions to olaparib [9, 10]. In the EU and USA, the recommended dose reduction is to 250 mg twice daily; 200 mg twice daily is recommended in patients requiring further dose reduction [9, 10]. Local prescribing information should be consulted for details regarding contraindications, special warnings and precautions, and use in special patient populations.
5 Place of Olaparib in the Management of Advanced Ovarian Cancer
Primary treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer typically begins with debulking surgery and is followed with adjuvant systemic chemotherapy in most patients [25]. US National Comprehensive Cancer Network (NCCN) guidelines [25] and joint guidelines from the European Society of Medical Oncology (ESMO) and European Society of Gynaecological Oncology (ESGO) [26] recommend administering bevacizumab alongside platinum-based chemotherapy after cytoreductive surgery, although this is no longer recommended in the UK by the National Institute for Health and Care Excellence (NICE) due to the relatively high cost of bevacizumab [27]. Prior to the development of the PARP inhibitors olaparib and niraparib, bevacizumab monotherapy was the only available first-line maintenance treatment option in patients who had achieved a complete or partial response to chemotherapy in combination with bevacizumab; there are no data supporting bevacizumab as first-line maintenance treatment in those who received chemotherapy without bevacizumab [25]. Bevacizumab monotherapy remains a maintenance treatment option for complete or partial responders to chemotherapy plus bevacizumab with wild-type BRCA or unknown BRCA mutation status [25].
Olaparib and niraparib are first-line maintenance treatment options in patients who had responded to platinum-based chemotherapy [25, 26, 28]. Current NCCN, NICE and American Society of Clinical Oncology (ASCO) guidelines indicate that olaparib as first-line maintenance therapy should only be administered to patients with a BRCA mutation, and olaparib in combination with bevacizumab to HRD-positive patients (i.e. those with a BRCA mutation and/or genomic instability) [25, 28,29,30]. In NCCN guidelines, niraparib is recommended in patients with a BRCA mutation if previously treated with bevacizumab plus chemotherapy, and in all patients regardless of HRD or BRCA mutation status if not previously treated with bevacizumab [25]. NICE guidelines recommend niraparib regardless of HRD or BRCA mutation status [31].
Olaparib was effective as first-line maintenance treatment in advanced ovarian cancer in phase III clinical trials, both as monotherapy and in combination with bevacizumab (Sect. 2). Olaparib monotherapy significantly extended the median PFS relative to placebo without negatively impacting quality of life in patients who had received chemotherapy alone, as did olaparib plus bevacizumab relative to placebo plus bevacizumab in those who received chemotherapy with bevacizumab. Improvements in PFS were observed regardless of baseline characteristics, such as surgical outcome, chemotherapy response, disease state and BRCA mutation status. PAOLA-1 subgroup analyses indicated that patients with HRD-positive tumours, but not those with HRD-negative tumours, derived benefits from adding olaparib to bevacizumab therapy (Sect. 2.2). Longer-term findings from SOLO-1 suggest that patients who had responded to olaparib after 2 years of treatment continued to experience benefits for up to 5 years (Sect. 2.1). The tolerability profile of olaparib as both monotherapy and in combination with bevacizumab was generally manageable and similar to what was observed in previous olaparib monotherapy studies [11, 12]. Hypertension was common in both treatment groups in PAOLA-1, which is expected due to its association with bevacizumab. The tolerability profile of olaparib as monotherapy and in combination with bevacizumab remained consistent after a median follow-up duration of ≈ 5 years (Sect. 3).
Further data relating to the use of olaparib monotherapy or olaparib in combination with bevacizumab in advanced ovarian cancer will be valuable, especially given the immaturity of some data in these trials (e.g. overall survival in SOLO-1 and PAOLA-1, longer-term PFS findings in PAOLA-1). While improvements in PFS were seen regardless of BRCA mutation status, almost all SOLO-1 participants had germline (deleterious or suspected deleterious) BRCA1/2 mutations; germline or somatic BRCA mutation statuses were not disclosed for PAOLA-1 [14]. Further olaparib studies in patients with somatic BRCA mutations would therefore be useful. Moreover, the subgroups in the PAOLA-1 exploratory analysis assessing the effect of HRR mutations on PFS were reportedly small; analyses specifically powered to assess the differences in olaparib efficacy across HRR mutations would be required to establish the exploratory findings.
Findings from a population-adjusted indirect comparison using pooled data from SOLO-1 and PAOLA-1 suggest that olaparib plus bevacizumab is more beneficial in improving PFS relative to olaparib monotherapy in patients with BRCA mutations [32]. Head-to-head studies between olaparib monotherapy and combination therapy with bevacizumab may better clarify the differences in therapeutic benefit; however, the differences in cost and potential AE risk must be taken into account when evaluating the suitability of one treatment over the other. In the future, direct comparative studies between olaparib and niraparib may be helpful when deliberating which may be more suitable to administer where their indications overlap.
Cost-effectiveness analyses based on SOLO-1 suggest that olaparib monotherapy is generally a cost-effective treatment option relative to active surveillance. In a US-based analysis over a lifetime horizon, olaparib treatment was associated with 3.63 life-years (LYs) and 2.93 quality-adjusted LYs (QALYs) gained relative to active surveillance, with incremental costs of $US42,032 per LY gained and $US51,986 per QALY gained; incremental cost-effectiveness ratios (ICERs) remained below $US100,000 per LY across a range of assessed scenarios [33]. Similarly, in an Italian study, olaparib monotherapy was estimated to have gained 2.87 LYs and 2.41 QALYs relative to active surveillance over a 50-year time horizon in the base-case scenario, with an ICER of €9515 per LY gained, an incremental cost-utility ratio (ICUR) of €11,345 per QALY gained, and an incremental net monetary benefit of €12,104 [34]. The completion of SOLO-1, and therefore the availability of complete and mature data, will permit more robust cost effectiveness estimates. In the UK, NICE recommends first-line olaparib plus bevacizumab maintenance therapy for use within the Cancer Drugs Fund pending the collection of further clinical evidence that will address uncertainties and overestimates of cost-effectiveness [30]. As with SOLO-1, the completion of PAOLA-1 will permit most robust estimates of cost effectiveness.
In conclusion, olaparib, alone and in combination with bevacizumab, is a useful option for the first-line maintenance treatment of adult patients with (respectively) BRCA-mutated and HRD-positive, advanced epithelial ovarian, fallopian tube or primary peritoneal cancer who are in complete or partial response to first-line, platinum-based chemotherapy.
Data Selection Olaparib: 264 records identified
Duplicates removed | 48 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 119 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 63 |
Cited efficacy/tolerability articles | 12 |
Cited articles not efficacy/tolerability | 22 |
Search Strategy: EMBASE, MEDLINE and PubMed from 2018 to present. Previous Adis Drug Evaluation published in 2018 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data. Key words were olaparib, Lynparza, epithelial ovarian cancer, peritoneal cancer, fallopian tube cancer, maintenance therapy. Records were limited to those in English language. Searches last updated 2 Sep 2021 | |
References
American Cancer Society. Key statistics for ovarian cancer. 2021. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed 2 Sept 2021.
Carioli G, Malvezzi M, Bertuccio P, et al. European cancer mortality predictions for the year 2021 with focus on pancreatic and female lung cancer. Ann Oncol. 2021;32(4):478–87.
Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14.
Genentech. Avastin® (bevacizumab): US prescribing information. 2020. https://www.accessdata.fda.gov/. Accessed 2 Sept 2021.
Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–96.
The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Frey MK, Pothuri B. Homologous recombination deficiency (HRD) testing in ovarian cancer clinical practice: a review of the literature. Gynecol Oncol Res Pract. 2017;4:4.
Cook SA, Tinker AV. PARP inhibitors and the evolving landscape of ovarian cancer management: a review. BioDrugs. 2019;33(3):255–73.
European Medicines Agency. Lynparza® (olaparib): EU summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 2 Sept 2021.
AstraZeneca. Lynparza® (olaparib): US prescribing information. 2020. https://accessdata.fda.gov/. Accessed 2 Sept 2021.
Frampton JE. Olaparib: a review of its use as maintenance therapy in patients with ovarian cancer. BioDrugs. 2015;29(2):143–50.
Heo Y-A, Dhillon S. Olaparib tablet: a review in ovarian cancer maintenance therapy. Targeted Oncol. 2018;13(6):801–8.
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–505.
Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–28.
DiSilvestro P, Colombo N, Scambia G, et al. Efficacy of maintenance olaparib for patients with newly diagnosed advanced ovarian cancer with a BRCA mutation: subgroup analysis findings from the SOLO1 trial. J Clin Oncol. 2020;38(30):3528–37.
Colombo N, Bradley W, Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: subgroup analysis by risk in the phase III SOLO1 study [abstract no. 392]. Int J Gynecol Cancer. 2020;30(Suppl 4):A76–7.
Friedlander M, Moore KN, Colombo N, et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol. 2021;22(5):632–42.
Banerjee S, Moore K, Colombo N, et al. Maintenance olaparib for patients (pts) with newly diagnosed, advanced ovarian cancer (OC) and a BRCA mutation (BRCAm): 5-year (y) follow-up (f/u) from SOLO1 [abstract no. 811MO]. Ann Oncol. 2020;31(Suppl. 4):S613.
Bradley W. Maintenance olaparib for patients with newly diagnosed, advanced ovarian cancer and a BRCA mutation: 5-year follow-up from SOLO1 [abstract no. 10520]. In: SGO Annual Meeting on Women's Cancer. 2021.
González Martin A, Tazi Y, Heitz F, et al. Maintenance olaparib plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced high-grade ovarian carcinoma (HGOC): final analysis of second progression-free survival (PFS2) in the phase III PAOLA-1/ENGOT-ov25 trial [abstract no. LBA33]. Ann Oncol. 2020;31(Suppl 4):S1163–4.
Pujade-Lauraine E. Homologous recombination repair mutation gene panels (excluding BRCA) are not predictive of maintenance olaparib plus bevacizumab efficacy in the first-line PAOLA-1/ENGOT-ov25 trial [abstract no. 10543]. In: SGO Annual Meeting on Women's Cancer. 2021.
Harter P, Petran D, Scambia G, et al. Efficacy of maintenance olaparib plus bevacizumab by biomarker status in clinical higher- and lower-risk patients with newly diagnosed, advanced ovarian cancer in the PAOLA-1 trial [abstract no. IGCS20_1207]. Int J Gynecol Cancer. 2020;30(Suppl 3):A13–4.
Colombo N, Moore K, Scambia G, et al. Tolerability of maintenance olaparib in newly diagnosed patients with advanced ovarian cancer and a BRCA mutation in the randomized phase III SOLO1 trial. Gynecol Oncol. 2021. https://doi.org/10.1016/j.ygyno.2021.07.016.
Li M, Kroetz DL. Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol Ther. 2018;182:152–60.
Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(2):191–226.
Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30(5):672–705.
National Institute for Health and Care Excellence. Bevacizumab in combination with paclitaxel and carboplatin for first-line treatment of advanced ovarian cancer. 2013. https://www.nice.org.uk/. Accessed 2 Sept 2021.
Tew WP, Lacchetti C, Ellis A, et al. PARP inhibitors in the management of ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38(30):3468–93.
National Institute for Health and Care Excellence. Olaparib for maintenance treatment of BRCA mutation-positive advanced ovarian, fallopian tube or peritoneal cancer after response to first-line platinum-based chemotherapy. 2019. https://www.nice.org.uk/. Accessed 2 Sept 2021.
National Institute for Health and Care Excellence. Olaparib plus bevacizumab for maintenance treatment of advanced ovarian, fallopian tube or primary peritoneal cancer. 2021. https://www.nice.org.uk/. Accessed 2 Sept 2021.
National Institute for Health and Care Excellence. Niraparib for maintenance treatment of advanced ovarian, fallopian tube and peritoneal cancer after response to first-line platinum-based chemotherapy. 2021. https://www.nice.org.uk/. Accessed 2 Sept 2021.
Vergote IB, Moore KN, Hettle R, et al. Population adjusted indirect comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 studies of olaparib with or without bevacizumab, bev alone and placebo in the maintenance treatment of women with newly diagnosed stage III/IV ovarian cancer with BRCA mutation [abstract no. 35]. Gynecol Oncol. 2020;159(Suppl 1):19–20.
Muston DRG, McLaurin K, Hettle R, et al. Cost-effectiveness analysis of olaparib as a maintenance monotherapy for patients with newly diagnosed advanced ovarian cancer and a BRCA1/2 mutation: a United States payer perspective [abstract no. 298]. Gynecol Oncol. 2020;159(Suppl 1):140–1.
Armeni P, Borsoi L, Fornaro G, et al. Cost-effectiveness and net monetary benefit of olaparib maintenance therapy versus no maintenance therapy after first-line platinum-based chemotherapy in newly diagnosed advanced BRCA1/2-mutated ovarian cancer in the Italian National Health Service. Clin Ther. 2020;42(7):1192-209.e12.
Acknowledgements
During the peer review process the manufacturer of olaparib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
Julia Paik is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: S. Nishio, Obstetrics and Gynecology, Kurume University School of Medicines, Fukuoka, Japan; I. Ray-Coquard, Department of Medical Oncology, Centre Léon Bérard, Lyon, France; P. Ghatage, Division of Gynecologic Oncology, Tom Baker Cancer Centre, Calgary, Canada.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paik, J. Olaparib: A Review as First-Line Maintenance Therapy in Advanced Ovarian Cancer. Targ Oncol 16, 847–856 (2021). https://doi.org/10.1007/s11523-021-00842-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00842-1