Abstract
Olaparib (Lynparza™) is a first-in-class, orally-active, small molecule, poly (ADP-ribose) polymerase inhibitor that induces synthetic lethality in homozygous BRCA-deficient cells. In the EU, the capsule formulation of olaparib is indicated as monotherapy for the maintenance treatment of adult patients with platinum-sensitive, relapsed, BRCA-mutated (germline and/or somatic), high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in complete or partial response to platinum-based chemotherapy. This approval was based on the results of study 19, a randomized phase II trial in 265 patients with platinum-sensitive, relapsed, high-grade serous ovarian cancer (HGSOC) who had received two or more platinum-based regimens and who had a partial or complete response to their most recent platinum-based regimen. Study 19 met its primary endpoint by demonstrating a significant improvement in progression-free survival in patients receiving olaparib compared with those receiving placebo. Moreover, a preplanned retrospective analysis identified those patients with a BRCA mutation (who comprised one-half of the overall study population) as being the subgroup that derived the greatest clinical benefit from olaparib. Single-agent olaparib was generally well tolerated, with the majority of adverse events being of mild to moderate severity and not requiring interruption of treatment. Fatigue, anaemia and neutropenia were the most frequently reported severe (grade ≥3) adverse events. An as yet unapproved tablet formulation of olaparib that has a lower pill burden than the capsule formulation is currently being investigated in phase III clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Approved as monotherapy for maintenance treatment of patients in response to their most recent platinum-based regimen |
Orally administered and generally well tolerated, making it suitable for long-term maintenance therapy |
Treatment should commence ≤8 weeks after the last dose of platinum-based chemotherapy |
Significantly prolongs progression-free survival, but not overall survival, according to analyses of the pivotal phase II trial that have been performed to date |
1 Introduction
Epithelial ovarian cancer (OC) is the most common cause of death from gynaecological tumours in the Western world [1, 2]. The majority of women with OC present with advanced (stage III or IV) disease; currently, they have a 5-year survival rate of <30 % [3]. OC is a heterogeneous disease, with high-grade serous ovarian cancer (HGSOC) being both the most common (≈60–80 % of cases) and the most aggressive histological subtype [4].
The standard treatment for advanced OC includes both cytoreductive surgery and platinum-based chemotherapy [5]. Initial response rates are high, although most (≈70 %) patients experience recurrence within a 3-year period [5–7]. Re-treatment with a platinum-based doublet is the current treatment of choice for patients who are ‘platinum sensitive’ (i.e. those who relapse ≥6 months after an initial or subsequent course of platinum-based chemotherapy), although this approach is limited by cumulative toxicities and, ultimately, the development of chemoresistance [5, 8]. Patients are considered to be ‘platinum resistant’ if they experience recurrence or progression <6 months after an initial or subsequent course of platinum-based chemotherapy; they are considered to be ‘platinum-refractory’ if they experience recurrence or progression during or <1 month after an initial or subsequent course of platinum-based chemotherapy [5].
Greater understanding of the tumour biology of OC has led to the development of targeted anticancer medications that have the ability to improve tumour responses to platinum-based chemotherapy, thereby alleviating some of the limitations of the latter [3, 9]. The most advanced agents in this regard are bevacizumab, an intravenously-administered vascular endothelial growth factor inhibitor [10], and olaparib (Lynparza™), a first-in-class, orally-active, small molecule, poly (ADP-ribose) polymerase (PARP) inhibitor [11–13]. Regarding the latter, DNA damage is repaired via six primary pathways: four addressing single-strand breaks [base excision repair (BER), nucleotide excision repair, mismatch repair and translesional synthesis] and two addressing double-stranded breaks [homologous recombination (HR) and non-homologous end-joining) in an interactive and interdependent manner. PARP is an important component of the BER pathway; PARP inhibition, by blocking BER, leads to the formation of double-stranded DNA breaks, which cannot be accurately repaired in HR-deficient cells, such as cancer cells harbouring homozygous BRCA mutations, but can in HR-proficient cells, such as non-cancer cells in BRCA mutation carriers (which are heterozygous for the mutation and therefore produce sufficient functional BRCA proteins) [12, 14–16]. As such, PARP inhibition leading to the selective death of HR-deficient tumour cells provides the first clinical example of the concept of ‘synthetic lethality’ [17, 18]. Approximately one-half of patients with HGSOC may have HR-deficient tumour cells due to germline or somatically acquired BRCA1 or BRCA2 mutations, epigenetic inactivation of BRCA1, or BRCA mutation-independent defects in the HR repair pathway [6, 19]; approximately one-quarter have germline (≈17 %) or somatic (≈6 %) BRCA mutations [6, 20].
This article briefly summarizes the pharmacological properties of olaparib and, in line with the approved use of the drug in the EU [21], focuses on the efficacy and tolerability of monotherapy for the maintenance treatment of adult patients with platinum-sensitive, relapsed, BRCA-mutated, HGSOC who are in complete or partial response to platinum-based chemotherapy.
2 Pharmacodynamic Properties
Olaparib is a potent inhibitor of PARP-1, the most important and best understood of the 17 PARP family members [22]. In several cell lines, including ovarian A2780 cancer cells, olaparib inhibited 50 % of PARP-1 activity at concentrations of 6–8 nmol/L; >90 % inhibition occurred at a concentration of ≈100 nmol/L [13]. In a phase I clinical trial [23], PARP in the mononuclear cells of patients with advanced solid tumours, including ovarian cancers, was inhibited >90 % with olaparib dosages of ≥60 mg twice daily.
Olaparib displayed antitumour activity as a single agent in a BRCA-mutated human ovarian cancer xenograft model [24]. Additional preclinical data, which suggest that HR-proficient ovarian cancer cells can be sensitized to olaparib by combining the drug with an agent that inhibits HR, such as 17-allylamino-17-demethoxygeldanamycin [25] or suberoylanilide hydroxamic acid [26], are also consistent with the concept of synthetic lethality [25, 26] (Sect. 1). Although carboplatin showed better single agent efficacy than olaparib in the aforementioned xenograft model, the best treatment response was seen with a combination of olaparib and carboplatin [24].
Olaparib demonstrated antitumour activity as monotherapy in several phase I or II multicentre trials in women with relapsed, germline BRCA-mutated, advanced OC (n = 17–193) [23, 27–30]. The objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines ranged from 31 to 41 % in heavily pretreated patients who received olaparib 400 mg twice daily in phase II studies [28–30]. Moreover, responses were observed in patients considered to be platinum-sensitive (e.g. 38 [28] and 60 [30] %) as well as those considered to be platinum-resistant (e.g. 30 [28] and 33 [30] %). Olaparib 400 mg twice daily also showed antitumour activity (ORR of 24 %) among a cohort of 46 patients with non-BRCA-mutated OC who were included in one phase II study; all 11 responders had HGSOC [30].
3 Pharmacokinetic Properties
Table 1 summarizes the pharmacokinetic parameters of olaparib, based on the capsule formulation, which has been used in the clinical studies discussed in Sects. 2 and 4. Absorption of olaparib is rapid; similarly, elimination of the drug, mainly via the urine and faeces, is relatively rapid (Table 1).
Systemic exposure to olaparib increased in a less than dose-proportional fashion following administration of doses >100 mg in a phase I study in patients with advanced solid tumours, including those with OC carrying a BRCA mutation [23].
Cytochrome P450 (CYP) 3A4/5 are the isoenzymes primarily responsible for the metabolic clearance of olaparib; in the absence of relevant (drug-drug interaction) data, it is recommended that coadministration of olaparib with strong or moderate CYP3A inhibitors or inducers be avoided [21]. Moreover, olaparib may inhibit CYP3A4 in vitro; caution should be exercised if the drug is coadministered with a CYP3A4 substrate, particularly one with a narrow therapeutic margin [21].
Olaparib is a substrate for, and may also be an inhibitor of, the efflux transporter P-glycoprotein (P-gp) in vitro; caution should be exercised if the drug is coadministered with a statin, as exposure to the latter may be increased [21].
4 Therapeutic Efficacy
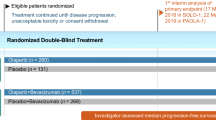
The efficacy of olaparib maintenance monotherapy in women with platinum-sensitive, relapsed, HGSOC, including those with a BRCA mutation, who are in response to platinum-based chemotherapy has been assessed in study 19, a randomized, double-blind, placebo-controlled, multicentre, phase II trial [8, 31].
Briefly, eligible patients were aged ≥18 years and had recurrent ovarian or fallopian tube cancer, or primary peritoneal cancer, with high-grade (grade 2 or 3) serous features or a serous component. They were required to have: (1) completed at least two previous courses of platinum-containing therapy, (2) demonstrated platinum sensitivity to the penultimate line of platinum-containing therapy and (3) shown an objective response to the most recent line of platinum-containing therapy, according to RECIST or Gynecologic Cancer Intergroup cancer antigen 125 criteria. Those recruited were randomized to receive olaparib 400 mg twice daily (capsules) or placebo within 8 weeks after completion of the last dose of platinum-based chemotherapy; study medications were continued until progression in the absence of unacceptable toxicity [8, 31].
Knowledge of BRCA mutation status was not necessary for study entry, but was established retrospectively for 254 (96 %) of the 265 trial participants. A prespecified exploratory analysis of all efficacy endpoints was done according to BRCA mutation status; patients who harboured a deleterious, or suspected deleterious, germline or somatic BRCA mutation were included in the BRCA-mutation subgroup (n = 136), while patients with no known or reported BRCA mutation and those with BRCA variants of unknown significance were included in the wild-type BRCA subgroup (n = 118). The BRCA-mutation and wild-type BRCA subgroups were generally well matched for demographic and baseline characteristics [8].
Compared with placebo, olaparib as maintenance monotherapy significantly increased investigator-assessed progression-free survival (PFS), the primary endpoint of the trial [31]. According to the preplanned, retrospective analysis [8], the magnitude of the improvement over placebo in median PFS in the BRCA-mutation subgroup (6.9 months) was larger than that in the wild-type BRCA subgroup (1.9 months) and the overall study population (3.6 months) (Table 2). The hazard ratio for PFS in the BRCA-mutation subgroup based on blinded independent central review [0.22 (95 % CI 0.12–0.40); p < 0.0001) [8] was consistent with the investigator assessment (see Table 2).
Retrospective, exploratory analyses were conducted to determine the time to first subsequent therapy or death (TFST) as well as the time to second subsequent therapy or death (TSST) [31]. The aim of TFST was to assess PFS with additional maturity [at the time of the PFS analysis, 153 progression events had occurred (in 57.7 % of patients)]. The intention of TSST was to provide information about the treatment benefit beyond progression. Both analyses were performed in patients who had received at least one dose of study medication [31]. Compared with placebo, olaparib significantly prolonged TFST and TSST, regardless of BRCA mutation status (Table 2).
Overall survival (OS) did not differ significantly between olaparib and placebo recipients in the overall study population or the BRCA-mutation and wild-type BRCA subgroups, based on the second interim analysis of this secondary endpoint, which was performed at 58 % maturity (i.e. after 58 % of the patients had died) (Table 2). There was, however, no evidence of a survival detriment among patients with a BRCA mutation who received olaparib [8]. A further, final analysis of OS is planned at ≈85 % maturity [8].
Disease-related symptoms and health-related quality of life endpoints were also assessed; however, no statistically significant or clinically relevant differences were noted between olaparib and placebo recipients in the overall study population or the BRCA-mutation and wild-type BRCA subgroups [8].
Among the eight olaparib and 10 placebo recipients with a somatic BRCA mutation in the BRCA-mutation subgroup, three and six experienced progression events and four and six died [8].
5 Tolerability
Olaparib as maintenance monotherapy was generally well tolerated in women with platinum-sensitive, relapsed, HGSOC, including those with a BRCA mutation, who were in response to platinum-based chemotherapy in study 19 [31]. The majority of adverse events were of mild to moderate severity and did not require interruption of the treatment [31].
In the overall population, the most frequently reported adverse events of any grade in olaparib recipients were nausea (71 vs. 36 % for placebo), fatigue (52 vs. 39 %), vomiting (34 vs. 14 %), diarrhoea (27 vs. 24 %), abdominal pain (25 vs. 27 %), anaemia (21 vs. 5 %), headache (21 vs. 13 %), constipation (21 vs. 11 %) and decreased appetite (21 vs. 13 %) [8]. The most frequently reported severe adverse events (grade ≥3) in olaparib recipients were fatigue (7 vs. 3 % for placebo), anaemia (5 vs. <1 %) and neutropenia (4 vs. <1 %) [8]. Of the five olaparib recipients who experienced severe neutropenia, three experienced a grade 4 adverse event [8]. Small intestinal obstruction, the most common serious adverse event, occurred in 2 (1 %) of 136 olaparib-treated patients versus 3 (2 %) of 128 placebo-treated patients [8].
Twice as many olaparib than placebo recipients had dose interruptions (36 vs. 16 %) or reductions (42 vs. 22 %) because of adverse events (most frequently nausea, fatigue and vomiting) [8]. Discontinuations due to adverse events occurred in approximately three times as many olaparib recipients compared with placebo recipients (5.1 vs. 1.6 %) [8].
The adverse event profile of olaparib in the BRCA-mutation subgroup was consistent with that in the overall study population, with, for example, fatigue, anaemia and neutropenia being the most common severe adverse events (Fig. 1).
Commonly occurring adverse events [i.e. incidence among olaparib-treated patients of >20 % (all grades) or ≥2 % (grade ≥3)] in the BRCA-mutation subgroup in a pivotal phase II study [8]. Ø = incidence of 0 %
6 Dosage and Administration
In the EU, the recommended dosage of olaparib capsules for maintenance monotherapy is 400 mg twice daily [21]. Treatment should be commenced no later than 8 weeks after completion of the last dose of platinum-based chemotherapy; it is recommended that maintenance therapy be continued until progression of the underlying disease. Treatment may be interrupted and/or the dosage can be reduced (first to 200 mg twice daily and finally to 100 mg twice daily) in order to manage adverse events [21].
Local prescribing information should be consulted for full details of contraindications, special warnings and precautions, and drug interactions relating to the use of olaparib.
7 Current Status of Olaparib in the Treatment of Ovarian Cancer
In the EU, the capsule formulation of olaparib (400 mg twice daily) is indicated as monotherapy for the maintenance treatment of adult patients with platinum-sensitive, relapsed, BRCA-mutated (germline and/or somatic) high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy. BRCA mutation status should be determined by an experienced laboratory using a validated test method [21].
In the US, in contrast to the EU, the capsule formulation of olaparib (400 mg twice daily) is not indicated as maintenance therapy. Rather, it is indicated as monotherapy in patients with deleterious or suspected deleterious germline BRCA-mutated advanced OC, as detected by an FDA-approved test (BRACAnalysis CDx™), who have completed at least three prior lines of chemotherapy [32]. This approval was based on the results of a single-arm, open-label, pivotal phase II study in which 34 % of 137 patients with germline BRCA-mutated advanced OC who had received three or more prior lines of chemotherapy experienced an objective response for a median duration of 7.9 months [32].
A tablet formulation of olaparib that requires fewer pills to be taken per day compared with the capsule formulation has been developed with the aim of improving patient convenience and compliance [13]. The comparative bioavailability, (antitumour) efficacy and tolerability of the two formulations has been assessed in studies in patients with advanced solid tumours [33–35]. According to the most recent study, in 62 patients with relapsed OC or primary peritoneal cancer and a BRCA mutation, a tablet dosage of 300 mg twice daily showed acceptable tolerability and, compared with the recommended capsule dosage (400 mg twice daily; Sect. 6), demonstrated similar efficacy, but with a much reduced total daily pill burden (4 vs. 16) [35].
The efficacy and tolerability of the tablet formulation of olaparib (300 mg twice daily) as maintenance monotherapy is being investigated in two ongoing, randomized, double-blind, placebo-controlled, multicentre, phase III trials in patients with BRCA-mutated HGSOC or high-grade endometrioid cancer who are in response to platinum-based chemotherapy (SOLO 1 and 2) [36]. SOLO 1 participants have newly diagnosed, advanced disease and have responded to first-line platinum therapy, whereas SOLO 2 participants have completed at least two prior lines of platinum therapy. The primary endpoint of both trials is PFS (blinded independent central review of RECIST data); primary analyses will be performed at ≈60 % maturity [36].
Data selection sources:
Relevant medical literature (including published and unpublished data) on olaparib was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) (searches last updated 7 April 2015), bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Olaparib, AZD2281, Lynparza
Study selection: Studies in patients with relapsed, high-grade serous ovarian cancer, including those carrying a BRCA mutation, who received olaparib. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Cancer Research UK. Cancer mortality for common cancers. 2011. http://www.cancerresearchuk.org. Accessed 11 Dec 2014.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol. 2012;23(Suppl 10):x118–27.
European Medicines Agency. Lynparza. International non-proprietary name: Olaparib. European public assessment report. 2014. http://www.ema.europa.eu. Accessed 4 Mar 2015.
Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Wiedemeyer WR, Beach JA, Karlan BY. Reversing platinum resistance in high-grade serous ovarian carcinoma: targeting BRCA and the homologous recombination system. Front Oncol. 2014;4:34. doi:10.3389/fonc.2014.00034.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61.
Khalique S, Hook JM, Ledermann JA. Maintenance therapy in ovarian cancer. Curr Opin Oncol. 2014;26(5):521–8.
Garcia A, Singh H. Bevacizumab and ovarian cancer. Ther Adv Med Oncol. 2013;5(2):133–41.
Chen Y, Zhang L, Hao Q. Olaparib: a promising PARP inhibitor in ovarian cancer therapy. Arch Gynecol Obstet. 2013;288(2):367–74.
Shaw HM, Hall M. Emerging treatment options for recurrent ovarian cancer: the potential role of olaparib. Onco Targets Ther. 2013;6:1197–206.
Lheureux S, Oza AM. Olaparib for the treatment of ovarian cancer. Expert Opin Orphan Drugs. 2014;2(5):497–508.
Basu B, Sandhu SK, de Bono JS. PARP inhibitors: mechanism of action and their potential role in the prevention and treatment of cancer. Drugs. 2012;72(12):1579–90.
De Lorenzo SB, Patel AG, Hurley RM, et al. The elephant and the blind men: making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front Oncol. 2013;3:228. doi:10.3389/fonc.2013.00228.
Lee JM, Ledermann JA, Kohn EC. PARP inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25(1):32–40.
Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785–90.
Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19:1381–8.
Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;8:17. doi:10.1186/1471-2407-8-17.
Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
AstraZeneca AB. Lynparza 50 mg hard capsules: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 11 Mar 2015.
Sessa C. Update on PARP1 inhibitors in ovarian cancer. Ann Oncol. 2011;22(Suppl 8):viii72–6.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34.
Kortmann U, McAlpine JN, Xue H, et al. Tumor growth inhibition by olaparib in BRCA2 germline-mutated patient-derived ovarian cancer tissue xenografts. Clin Cancer Res. 2011;17(4):783–91.
Choi YE, Battelli C, Watson J, et al. Sublethal concentrations of 17-AAG suppress homologous recombination DNA repair and enhance sensitivity to carboplatin and olaparib in HR proficient ovarian cancer cells. Oncotarget. 2014;5(9):2678–87.
Konstantinopoulos PA, Wilson AJ, Saskowski J, et al. Suberoylanilide hydroxamic acid (SAHA) enhances olaparib activity by targeting homologous recombination DNA repair in ovarian cancer. Gynecol Oncol. 2014;133(3):599–606.
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2014;33(3):244–50.
Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51.
Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9.
Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61.
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92.
AstraZeneca Pharmaceuticals LP. Lynparza™ (olaparib) capsules, for oral use: US prescribing information. 2014. http://www.accessdata.fda.gov. Accessed 11 Mar 2015.
Molife LR, Forster MD, Krebs M, et al. A phase I study to determine the comparative bioavailability of two different oral formulations of the PARP inhibitor, olaparib (AZD2281), in patients with advanced solid tumors (abstract no. 2599). J Clin Oncol. 2010;28(15 Suppl).
Molife LR, Mateo J, McGoldrick T, et al. Safety and efficacy results from two randomized expansions of a phase I study of a tablet formulation of the PARP inhibitor, olaparib, in ovarian and breast cancer patients with BRCA1/2 mutations (abstract no. 3048). J Clin Oncol. 2012;30(Suppl).
Mateo J, Friedlander M, Sessa C, et al. Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation (abstract no. 801). Eur J Cancer. 2013;49:S161.
Moore KN, Di Silvestro P, Lowe ES, et al. SOLO1 and SOLO2: randomized phase III trials of olaparib in patients (pts) with ovarian cancer and a BRCA1/2 mutation (BRCAm) (abstract no. TPS5616). J Clin Oncol. 2014;32(5 Suppl).
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. James Frampton is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: M. Hall, Department of Medical Oncology, Mount Vernon Cancer Centre, Northwood, UK; M. Markman, Cancer Treatment Centers of America, Philadelphia, Pennsylvania, USA.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Olaparib: A Review of Its Use as Maintenance Therapy in Patients with Ovarian Cancer. BioDrugs 29, 143–150 (2015). https://doi.org/10.1007/s40259-015-0125-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-015-0125-6