Abstract
Low back pain is associated with degenerative disc diseases of the spine. Surgical treatment includes fusion and non-fusion types. The gold standard is fusion surgery, wherein the affected vertebral segment is fused. The common complication of fusion surgery is adjacent segment degeneration (ASD). The ASD often leads to revision surgery, calling for a further fusion of adjacent segments. The existing designs of nonfusion type implants are associated with clinical problems such as subsidence, difficulty in implantation, and the requirement of revision surgeries. Various surgical approaches have been adopted by the surgeons to insert the spinal implants into the affected segment. Over the years, extensive biomechanical investigations have been reported on various surgical approaches and prostheses to predict the outcomes of lumbar spine implantations. Computer models have been proven to be very effective in identifying the best prosthesis and surgical procedure. The objective of the study was to review the literature on biomechanical studies for the treatment of lumbar spinal degenerative diseases. A critical review of the clinical and biomechanical studies on fusion spine surgeries was undertaken. The important modeling parameters, challenges, and limitations of the current studies were identified, showing the future research directions.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The spine supports the weight of the body, provides flexibility for daily activities, and protects the nervous system. It is exposed to various physiological loads such as compression/tension, bending and twisting moments, or their combinations [1, 2]. Spine deformation leads to pain, neurological problems, and mobility challenges. Problems due to aging, trauma, and eccentric exercises are also significant concerns [3]. Various studies reported that the most common issue observed in the spine is low back pain (LBP) [3,4,5,6]. It is reported that up to 80% of the human population experiences difficulty doing daily activities due to LBP [3, 7, 8]. A study estimated an overall burden of LBP of 21.8 million disability-adjusted life years (DALYs) due to ergonomic exposures at work [8]. Men (13.5 million DALYs) are more affected than women (8.3 million DALYs) due to LBP [8]. Over the years, many studies reported that the primary causes of LBP are degenerative diseases, including disc herniation, stenosis, etc., which need conservative treatment or surgical interventions for correction (non-conservative treatment) [9, 10]. Non-conservative treatments are broadly classified into fusion (recognized gold standard [11]) and non-fusion surgeries [12]. Various prostheses like pedicle screws (PS), plates, cages, and rods are used to fuse the affected spine segments [12].
The advancement of finite element (FE) analysis tools contributed significantly to investigating the effect of various surgical approaches and prosthesis designs on the clinical outcomes of spine surgeries [13]. Several biomechanical studies on the lumbar spine have been reported for normal and implanted spines under various physiological loading conditions (flexion–extension, axial twisting and lateral bending) [14,15,16,17]. Various studies investigated the performance of spinal implants by analyzing the ranges of motion (ROM) before and after spinal fusion [11,12,13,14]. In contrast, other studies investigated the effects of implant design on stress/strain distributions in the vertebral bodies [15, 16]. The stabilization of the vertebra by fusing the problematic segment leads to the degeneration of the adjacent segments [18, 19]. This will likely lead to revision surgeries (RS), calling for a further fusion of the adjacent segments.
It is reported that non-fusion surgery is a useful approach that can preserve the motion of the segment, but the high rate of failures of existing devices is a matter of concern [20]. Other studies have indicated high stresses and strains in the fused segment, leading to adjacent segment degeneration (ASD) [15, 16]. However, the exact cause of initiation and its propagation has rarely been reported [21, 22]. Several authors specifically mentioned that the pattern of load distribution varies after implantation, but they did not investigate how this pattern affects the adjacent segments [14,15,16,17, 21, 22]. The limitations of simplified geometry and material properties in the earlier FE studies, including spine biomechanics and other musculoskeletal biomechanics, have been partially resolved by the availability of medical images and the advancement of image processing tools [23,24,25,26,27,28].
Over the decades, major developments have been reported in terms of biomechanical studies on the lumbar spine. A couple of recent review articles have presented an overview of lumbar disc replacement from a clinical perspective, emphasizing artificial disc replacement (ADR) [5, 29]. The objective of the study was to review the literature on biomechanical studies for the treatment of degenerative disc diseases (DDD) in the lumbar spine. A brief description of the anatomy and physiological movements of the lumbar spine was presented. The various DDDs and surgical treatments were briefly summarized. A critical review of the clinical and biomechanical studies on spinal fusion surgeries was undertaken. Important modeling parameters, challenges and limitations of the current studies were identified to show the future research directions.
2 Anatomy of lumbar spine
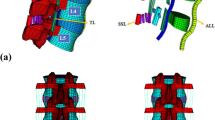
The lumbar spine consists of five vertebrae (L1–L5), having slightly different geometrical shapes from each other. In between each vertebra, there are intervertebral discs (IVD), composed of annulus fibrosus (AF) and jelly-like nucleus pulposus (NP) in the center. IVD acts as a shock absorber and joins the vertebral body together through cartilaginous endplates (CE) [30, 31]. The IVDs are subjected to evenly distributed pressure [32]. Figure 1 represents the three-dimensional (3D) geometry of a lumbar spine (L1–L5) with various components of an IVD, including two bony endplates (BE), two CEs, AF, and NP. There are seven types of ligaments in the spine restricting excessive movements of the spine, especially hyperextension/flexion [33]. The ligaments include the Anterior longitudinal ligament (ALL), posterior longitudinal ligament (PLL), ligamentum flavum (LF), intertransverse ligament (ITL), capsular ligament (CL), interspinous ligament (ISL), and supraspinous ligament (SSL) [1]. Figure 2 represents simplified version of the ligaments of the lumbar spine. These ligaments were modeled using ANSYS (Ansys Inc., Pennsylvania, USA). Each spine has four articular processes on the posterior side, called facet joints (two inferior and two superior to pedicles). Facet joints consist of a fibrous capsule, articular cartilage and synovial fluid [34].
Intact lumbar spine model with the simplified representation of all ligaments. The abbreviations ITL, ALL, LF, FCL, SSL, PLL and ISL represent intertransverse ligament, anterior longitudinal ligament, ligamentum flavum, facet capsular ligament, supraspinous ligament, posterior longitudinal ligament and interspinous ligament, respectively
The smallest physiological motion unit of the spine, which shows major biomechanical features and physiological characteristics, is called a functional spinal unit (FSU). Two adjacent vertebrae, IVD between them, two facet joints, and structures surrounded by the seven ligaments without the muscles constitute an FSU [35]. It is reported that the L3–L4 FSU is prone to a high chance of deformity because it has the highest curvature. Moreover, the line of action of compressive load through the center of gravity of the body passes through the center of IVD at L3–L4 [16, 36]. The flexion–extension, lateral bending toward left or right, and torsional twisting to both sides are the main physiological movements of the lumbar spine, as shown in Fig. 3. Lumbar flexion is the physiological movement where the upper body bends towards the anterior side in the sagittal plane (SP). In comparison, lumbar extension is bending towards the posterior side in the SP. Sideways movement of the upper body in the coronal plane is termed lateral bending. Rotational movement of the spine about an axis normal to the transverse plane is known as the twisting of the spine. Figure 3 shows the physiological movements of the human spine.
3 Lumbar degenerative diseases and surgical treatments
3.1 Degenerative Disc diseases
The primary cause of LBP is DDD which occurs due to aging, obesity, genetics, trauma, improper exercise, and heavy-duty jobs [37]. Any change in the composition, structure and function of the spine is termed as degeneration. If the disc loses its water-absorbing matrix component, it may cause disruption of AF, cracks, and fissures due to dehydration. This finally leads to a loss of biomechanical functions, reduced disc height, and extreme pain in the lower back, affecting elastic properties, shock-absorbing properties, and flexibility of the IVD [29, 38].
Lumbar disc herniation is one of the major DDD which needs surgical interventions for rectification. The disc herniates towards the spinal canal and tries to compress the spinal cord and related nerves, leading to numbness in the lower back and legs. This causes pain and difficulty in daily activities [39]. Lumbar spinal stenosis (LSS) affects those subjects whose spine undergoes frequent fatigue loading and is a common issue even in middle-aged adults [40]. The term usually represents an anatomical reduction in lumbar spinal size [40]. It also leads to narrowing of the spinal canal and ends in sciatica and hypertrophy of the facet joints capsule and LF. The thinning of IVD leads to dislocation of the vertebral body over the inferior one. This condition mainly affects the lumbar region (L5-S1) and is clinically termed Spondylolisthesis [3]. Patients with less severe symptoms may be treated conservatively, but surgery is required when sliding is rapid and/or neurological disorders persist. The success rate of such surgeries varies from country to country, ranging from 10 to 90% [7, 41,42,43,44].
3.2 Surgical treatments
The choice for treating DDD is fusion and non-fusion-based surgical interventions. In fusion surgery, the affected vertebral bodies are fused by using PS, plates, cages, interspinous spacers (ISS), and rods to achieve immediate stabilization and pain relief [45]. The degenerated IVD is removed, and disc space is replaced with different types of cages for correcting the curvatures and foramina height [15, 46,47,48,49,50,51,52]. The major approaches for lumbar interbody fusion (LIF) and insertion of implants are posterior (PLIF), anterior (ALIF), Lateral (LLIF), oblique (OLIF), and transforaminal (TLIF) [3]. The various surgical approaches are shown in Fig. 4. Surgeons choose an appropriate approach according to degeneration position and patient condition. In PLIF, the thecal sac should be moved sideways in order to access the IVD since the approach is through the dorsal side of the body [15]. This has a high-risk factor since any damage to the spinal cord will induce serious injuries. In ALIF and LLIF, most of the abdominal muscles are needed to be incised to reach the IVD [53, 54]. According to several studies, the best approach with minimal risk elements is the TLIF [3, 53,54,55]. In TLIF, the parts of facet joints are removed in order to reach IVD and it does not possess risk elements to the thecal sac or abdominal muscles. Surgical methods require either removing or changing the biomechanics of related ligaments.
The non-fusion surgery technique is an alternative to fusion surgery to preserve the motions of the spine [20, 29, 56]. It is often used in the correction of scoliosis, DDDs and other spinal deformities. ADRs are used to replace the degenerated disc. Complete removal of the disc is not always necessary for a herniated disc; in that case, annular closure devices (ACD) are used to remove the herniated part and block the recurring herniation [57]. Spinal stabilization systems, such as device for intervertebral assisted motion (DIAM) and dynamic neutralization system (DYNESYS) have been developed as an alternative to fusion surgery. For fusion surgeries, the major problem is ASD which may lead to follow-up surgeries [19, 22, 58, 59]. Studies investigating ASDs due to fusion and non-fusion surgeries are limited. Most studies do not present how an ASD is generated and progresses after fusion surgeries.
4 Literature review
4.1 Clinical studies on lumbar spine
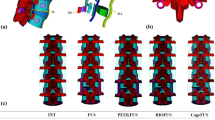
The clinical studies focused on establishing the performance and outcomes of various spinal implants, such as intervertebral cages, pedicle screw-rod systems and ISS under physiological loading conditions. A recent clinical meta-analysis concluded that expandable interbody cages used in minimally invasive (MI) LIF surgery are associated with decreased subsidence rate and operating time [52]. An increase in disc height that equals natural IVD was reported. The best spacer can be chosen according to surgeons’ experience and preferences. Another recent study reported on the outcomes of TLIF surgery at a single level [50]. This clinical study included a retrospective review at a single institution and 252 single-level TLIFs from 2012 to 2018 (152 non-expandable cages and 100 expandable cages). The study observed that TLIFs utilizing expandable cages do not significantly improve clinical outcomes compared with non-expandable cages, but it increases the risk of intraoperative subsidence. The study reported high usage of bone morphogenic protein (BMP) with these cages. More research on expandable cages is thereby necessary for improvement.
An investigation was reported on 215 patients for an average follow-up of 6.7 years to determine the rate of ASD in the case of posterior lumbar fusion surgery [58]. In this study, the rate of ASD was predicted as 16.5% in 5 years and 36.1% in 10 years, but there was no correlation between the lengths of fusion and the rate of ASD. A clinical and radiographic report was published recently on MI-LLIF surgery [60]. In this multi-surgeon retrospective study, 32 patients were implanted with a static polyetheretherketone (PEEK) cage and 57 patients with titanium (Ti) expandable cage. The study showed a significant positive outcome for patients implanted with MI-LLIF expandable cage with no subsidence. A recently published randomized clinical study on 121 patients compared PEEK and allograft spacers used in TLIF surgery [61]. The study showed that TLIF surgery with both cages demonstrated good clinical outcomes regardless of randomization groups. The latest clinical studies presented in this section did not focus on ASD [50, 52, 60, 61].
A randomized controlled trial on ISS (70 patients, 40 Wallis spacer and 37 without Wallis implantation) used for primary lumbar degeneration showed that the spacer implanted after discectomy did not improve outcome at three years [62]. The study concluded that such a device is not enough to prevent ASD. Another randomized trial of lumbar discectomy and ACD implantation (554 patients) showed that the risk of reherniation can be reduced [63]. However, the study reported that the ACD implant failed due to the failure in the bone anchorage portion of the device.
The ADRs are designed to preserve the ROM by accommodating loading and reducing friction and wear. A standard disc has six degrees of freedom (3 in translation and 3 in rotation) [64]. The first lumbar ADR was performed in 1960 using a steel ball by Fernstrom [64]. In the 1980s, Schellnack and Buttner [64] implanted a Charite prosthesis using an anterior approach. ADRs are typically recommended for treating single-level DDD in the lumbar spine for patients aged between 10 to 50 years [65]. The first generation Prodisc-L (Synthes, West Chester, USA), an ADR, was designed in 1989. Prodisc-L received Food and Drug Administration (FDA) approval in the United States in 2006 [66]. This ADR was based on the spherical joint articulation of cobalt chromium molybdenum (CoCrMo) alloy and an ultra-high molecular weight polyethylene (UHMWPE) [66]. Other ADRs, such as Mobi-disc (Zimmer Biomet Inc., USA), Maverik (Medtronic Inc., USA), and M6-L (Spinal Kinetics Inc., USA), were recently developed by incorporating specific design changes and material variations. Lumbar ADRs are inserted through an anterior approach to treat chronic LBP [64]. The outcomes of ADR performed on 11 professional athletes using M6-L prosthesis were reported in Byvaltsev et al. [67]. The study concluded that disc replacement could preserve the ROM of the operated lumbar segment. This can reduce the risk of ASD as it reduces mechanical stress in the operated segment. However, the authors did not report a detailed investigation of ASD. A clinical study involving 19 patients removed the failed ADRs and fused the segments [68]. This study reported that the rate of RS for ADRs is alarming due to its deteriorating effect. Figure 5 shows an example of failed ADR, which has been fused after removing the ADR [68]. As reported in various studies, the major drawbacks of ADRs are subsidence, malpositioning, excessive loads on facets, and an increase in axial rotational instability [69,70,71]. About 6 to 14% of patients underwent RS after ADR [20, 72, 73].
Example of failed ADR and its revision with fusion surgery [68]
4.2 Biomechanical studies on lumbar spine
4.2.1 Finite element modeling
Finite element method stands as a standard tool for evaluating the biomechanical behavior of the spine, analyzing existing implant designs, investigating patient-specific surgical approaches, and designing and developing new implants. Various parameters can be studied independently and in combination by using the FE method [14]. Several studies have investigated the effect of variations in material properties, implant design and surgical approaches on the biomechanical behavior of the lumbar spine [16, 45, 47, 74]. In the early 1980s, Shirazi-Adl et al. [37] developed a 3D FE program and applied it to analyze the L2–L3 FSU. The FE predicted results were compared and validated with in-vitro measurements of disc bulge, endplate bulge and intervertebral disc pressure (IDP) reported in the literature [75, 76]. The concerned experimental studies [75, 76] determined the moment-rotation relation, IDP and disc bulge using a cadaver spine subjected to pure sagittal plane (flexion–extension) moments. Shiraz-Adl et al. [37] reported that flexion moment generated relatively large IDP, with a highest value of 1.52 MPa at 60 Nm. In contrast, suction type pressure (< 0.15 MPa) was predicted under extension moment [37]. In their experimental measurements, Schultz et al. [75] observed a maximum IDP of 700 kPa in flexion for 10 Nm moment. In their comparison, Shiraz-Adl et al. [37] did not report the percentage difference of the validation parameters, possibly due to the fact that the loading condition considered in the FE modeling [37] and experimental measurements [75] are not equivalent (60 Nm versus 10 Nm). However, it was reported that the numerical prediction was in good agreement with the experimental measurements [37]. Furthermore, Shirazi-Adl et al. [37] reported that the disc bulged outward under the flexion moment but inward under the extension moment. Similar observations have been reported by Brown et al. [76] in their experimental investigations of disc bulge. According to Shirazi-Adl et al. [37], a maximum difference of 0.25 mm disc bulge was observed between the numerically predicted values [37] and experimental measurements [76], under the condition of maximum flexion. In general, the comparison of the predicted gross response of the spine with available experimental measurements indicated a satisfactory agreement [37].
The challenges were modeling a realistic geometry of the spine, assigning realistic material properties to the bone elements, and simulating contact at the interface of the facet joints. Shirazi-Adl et al. [37] developed the FE model of L2–L3 FSU based on discrete measurements from a cadaver spine. The homogenous material property of bone was assigned to the FE model. Intermediate values between the cortical and cancellous bone properties were used. Such simplifications in geometry and material properties were possibly due to the unavailability of medical images and/or image-processing tools. To simulate the contact behavior at the interfaces of the articulating facet joints, the study [37] developed a methodology to calculate the perpendicular distances between a set of points on the articulating surfaces of the adjacent vertebrae as a response after each increment of loading. Contact was considered to have occurred at the point under investigation if its perpendicular distance is discovered to be smaller than a specified gap limit.
In the 1990s, Goel et al. [77, 78] investigated FSUs at single and multiple levels using FE models, followed by experimental validation. In these studies, geometric data was acquired from computed tomography (CT) scans of a cadaveric specimen. These FE models on FSU studied ROM, IDP, and facet joint forces of a natural and implanted spine. The ROM included all physiological movements that are responsible for developing IDP and forces on articulating joints [13]. The studies assigned homogenous material properties for cortical bone (12,000 MPa) and cancellous bone (100 MPa). The authors considered a constant thickness for cortical bone. The ligaments were modeled using tension-only cable elements. Authors reported that these assumptions are suitable to mimic a true physiological condition experienced by the ligaments. Assignment of realistic material properties to the soft-tissues was challenging. In an attempt to simulate the composite nature of the IVD, the authors modeled the NP as an incompressible fluid and the AF as fiber-reinforced concrete components with a tension-only option for collagenous fibres completely embedded in a matrix of ground substance with alternating fibre angles of 30°. The challenges experienced in FE modeling of a vertebra, endplates, facets, IVD, and ligaments were due to its anatomical complexity and difficulty in assigning realistic material properties. Later on, the individual part of the vertebra was modeled and analyzed in various FE studies, validated by measurements obtained in experimental studies [16, 45, 79].
An FE study investigated stress distribution on a single fused spine segment (L3–L4) to evaluate the risk of ASD [80]. The study concluded that ASD is a serious issue to investigate for any fused spine segments and surgical approaches. Recently, another study investigated the risk of ASDs and the instrumentation required for RS [81]. In this study, the T12–L5 FE model was constructed and validated. Four different implant replacement constructs were analyzed for ROM and IDP (Fig. 6). The study observed an obvious reduction in ROM in each RS model, approximately in an identical pattern. However, the IDP of the first two constructs was found to be lower than the other two. Jiang et al. [82] presented different grades of proximal ASD after PLIF surgery. This study observed that ASD is related to decreased ROM and increased IDP. However, no comparative FE study on ASD for various surgical approaches has been reported in the literature. Bashkuev et al. [48] analyzed different designs of porous intervertebral cages and observed that proper fusion might occur with good biocompatible material. The porosity of the implant influences osseointegration [48]. In this study [48], solid and hollow cages of two different sizes, ring cages of trapezoidal cross-section, and two T-section cages consisting of two different materials with higher core elastic modulus (E) were investigated. The results showed that cage geometry substantially influences the fusion outcome.
a L1–L5 BPS fixation; b connector-rods at L1–L2; c the bilateral satellite rods with connector rods above L2 and below L3 PS; d the bilateral satellite rods with connector above the L2 and below the L4 PS (with permission from [81])
In another recent biomechanical study, the outcomes of TLIF and OLIF surgical procedures were compared to find the risk of ASD [22]. A 400-N vertical preload and 10-Nm moment was applied on L1 for simulating physiological loading conditions. ROM and IDP were used to evaluate the adjacent segments. It was found that ROM and IDP were similar in TLIF and OLIF, but the IDP was higher than the intact model. Hence, the study indicated that the potential risk of ASD exists. A similar study determined whether an extension of the PS was necessary to predict ASD in LLIF procedures [21]. They performed bone-graft fusion LLIF with bilateral pedicle screw (BPS) fixation at L4–L5, standalone LLIF, LLIF + lateral screws (LS), and PLIF + BPS. The NP and AF (ground substance) were modeled as a homogenous, hyper-elastic material using the Mooney–Rivlin model. The stresses in the cage, screw-bone interface, cage-endplate interface, and IDP were analyzed. The study reported that standalone LLIF has limited stability. There is a potential risk of an increase in the number of patients having ASD with the increase in PS-rod instrumentation. Fan et al. [55] conducted a study to compare the vibrational characteristics of fused lumbar spine through PLIF and TLIF surgical approaches using the FE method. A 40 kg point mass was added on the top of L1 vertebra. The models were subjected to a ± 40 N (5 Hz) sinusoidal vertical load with a 400-N compressive follower preload and simulated for transient dynamic analysis. In the PLIF model, higher contact pressure was observed between the endplates and the cages. Whereas, the TLIF model produced higher stresses on PS. This study also did not consider investigating the risk of ASD. Similar biomechanical studies with TLIF, PLIF, and LLIF surgery were reported, concluding that ASD is a common issue that needs further attention [47, 83,84,85].
Mas et al. [13] compared the ROM of a healthy spine with a spine having dynamic fixation. Dynamic fixation refers to the stabilization of the affected level of the spine with flexible structures. The study compared two dynamic fixations, DYNESYS (Zimmer Spine Inc., USA) and DIAM (Medtronic Inc., USA), to find the superior design of the two. DYNESYS system consists of screws (made with TiAlNb alloy of E = 110,000 MPa), space bars/rods (made with polyethylene-terefthalate of E = 1980 MPa), and cord (made with polyethylene-terefthalate of E = 3225 MPa), whereas DIAM is an H-shaped device, made of silicone in the core covered by woven polyester (E = 2100 MPa), implanted between two adjacent spinous processes [13]. The DYNESYS system allows a small amount of lengthening and shortening between two PS. Whereas DIAM is an H-shaped silicone and woven polyester device implanted between two adjacent spinous processes. The study observed that both techniques could preserve movement [13]. However, the DYNESYS system may have longer stability. A study to understand the effect of PS fixation on foramina height and ROM in the lumbar spine was reported [86]. In this study, three rods made up of Ti alloy (Ti6Al4V), UHMWPE, and PEEK were used at L3–L5 levels. The results were compared with an intact model. The study concluded that the Ti6Al4V rod restricts motion more than the other materials. Another FE study investigated how TLIF affects the adjacent segment’s lumbar lordosis [59]. Implantation was done on the L4–L5 region to evaluate the ROM and IDP in L3–L4. Based on the higher stresses observed on the adjacent segment (L3–L4), this study concluded that the pathologic development of ASD is significant. Recently, a study on flexible connecting PEEK rods and DIAM system was reported to compare the stresses of the fusion cage at the L4–L5 level under bending moments [45]. The results were compared with those for rigid fixation using Ti rods. This study observed that the flexible fixation systems might increase the risk of cage subsidence and cage damage but can promote fusion. It was also observed that the risk of screw breakage was low for the rigid rod but higher for the flexible rod.
For the DYNESYS system, Pham et al. [87] found that the rates of operative site infection, pedicle screw loosening, pedicle screw fracture and ASD were 4.3%, 11.7%, 1.6%, and 7.0%, respectively. 11.3% of patients underwent revision surgery. Of the patients who developed ASD due to DYNESYS, 40.6% had to undergo a revision. Although DYNESYS is reported to have a slightly lower incidence of ASD, it has a complication rate similar to published data on lumbar fusion [87]. Boody et al. [88] determined the efficacy of the DIAM spinal stabilization system for the treatment of low back pain in 38 patients. Clinically significant improvements in Oswestry Disability Index were seen in the majority of the patients 2-years post-surgery. However, the implant caused a foreign body reaction with delayed inflammatory complications in two patients. Therefore, the study recommended extended follow-up to evaluate the clinical outcome, at least for 5 to 10 years. The effects of ASD and screw loosening on revision rates following dynamic stabilization are poorly understood. Therefore, further research is needed to determine whether the performance of the stabilization system is superior to that of the fusion procedure [87, 89].
Zhang et al. [74] reported on how the porosity of the additively manufactured (AM) cages affect the performance of TLIF. The study compared the ROM, cage stress, endplate stress, and IDP. It was observed that the ROM of the spine implanted with Ti cages was lower compared to PEEK cages. The study concluded that porous Ti cages could be used instead of solid PEEK cages in TLIF surgery. A study on footprints during TLIF approaches was reported [90]. The FE model of the L3–S1 lumbar segment was used to simulate TLIF constructs in this study. A generic TLIF device and an articulating vertebral interbody TLIF device (AVID) were analyzed. The study reported that the AVID implant allowed better load sharing than the former.
Geometrical and material modelling
The recent FE models are subject-specific, developed from CT scans using image processing software to capture the exact 3D geometry of the spine [4]. Several input parameters are required to generate and solve the FE model of the spine. The following part will discuss the different approaches for geometrical and material modeling used in the spine.
In earlier models, the geometry of the spine was developed based on in-vitro measurements of the cadaveric spine. For example, Shirazi-Adl et al. [37] used the measurement from a cadaveric L2–L3 of a 29-year-old woman to develop a simplified 3D geometry of the spine. Through measurements of the specimen, it was possible to determine the overall outer shape and boundaries of the spine approximately. With the advancement of medical imaging techniques and image processing, it has been possible to develop more accurate 3D geometry of any anatomical parts based on clinical medical images. Several studies developed 3D geometry of the spine using CT data [16, 32, 47, 74, 91]. However, the segmentation between the cortical and cancellous bone of the spine from CT scan data is often challenging. To overcome this issue, the cortical layer was modeled using shell elements. In a recent study, Fang et al. [85] used CT data of a healthy subject to develop spine geometry. The study considered cortical bone as a shell of a thickness of 1 mm that envelopes the inner cancellous core. Various studies assumed a thickness ranging from 0.35 mm to 1.5 mm for segmenting the vertebral body as the outer cortical shell [32, 85, 91, 92]. Other studies considered 3D solid elements to model the cortical and cancellous bone [47, 93].
The earlier models assigned homogenous material properties for the cortical and cancellous bones [47, 85, 91, 94]. Assigning more realistic material properties will improve the FE results. However, the characterization of anisotropic features is complicated. Several subject-specific FE studies have assigned heterogeneous material properties of the bone based on CT gray value [16, 86], which is currently accepted as the gold standard. This technique is based on the assumption that bone is linear elastic and isotropic [95]. The linear attenuation coefficient measurements (CT numbers) are transformed into the system of Hounsfield units (HU) [95]. Using the HU value, element-specific bone apparent density (ρ in g.cm−3) is computed following a linear correlation between them [95, 96]. The elastic modulus (E in MPa) of each bone element is then calculated from the apparent density (ρ), using an equation of the form E = CρD. C and D are constants and their range of values has been reported for different anatomic sites by Morgan et al. [97]. For details on this technique, please refer to the studies cited here [98, 99]. Tables 1 and 2 show various material properties (linear elastic isotropic and transversely isotropic) used in multiple literature to model the spine. A recent study compared eight different well-developed lumbar spine models previously reported in the literature [30]. The study concluded that the variation in material models has negligible influence (1–2%) on the ROM.
Generally, the IVD is modeled separately for NP and AF (annular ground substances and fibers) with a volumetric ratio of 3:7 between AF and NP [100]. However, a study considered only 43% of the total disc volume to model the NP [101]. Recently, linear elastic material formulations have been used to represent the bulk response of AF [35]. Several studies used a small value of E to model the fluid-like behavior of NP [83, 102, 103]. However, other studies considered AF as hyper-elastic and NP as visco-elastic materials [93, 104,105,106]. Various approaches were undertaken to model the AF ground substance, including one-dimensional spring elements [107], homogenous formulation [108], and a continuum approach [109]. Table 3 shows the summary of material properties used to model IVD in various studies.
In a cadaveric study to determine the structural properties of CEs of the lumbar spine, Roberts et al. [110] reported that the CE consists of a thin layer of hyaline cartilage (0.6 mm thickness, variable). Wang et al. [111], in their experimental study on 150 cadaveric lumbar spines, reported that the thickness of CEs can be high as 1 mm. In contrast, another cadaveric study reported CE thickness to be only 0.75 mm [112]. It has been observed that the CE thickness varies between samples. Therefore, a wide range of thicknesses has been considered for modeling the BEs and CEs, separately (0.35 mm, 0.5 mm, 0.8 mm) [74, 81, 85, 91, 92]. Most of these studies have considered the interface between the endplates and the vertebral body as bonded, whereas Schmidt et al. [113] modeled the interfaces as frictionless contact with surface-to-surface contact. More commonly, endplates were modeled using linear elastic and isotropic material properties [77, 86, 114, 115].
The ligaments influence the ROM more than the load distribution in the spine [1, 13, 116]. Various studies used hyper-elastic and piecewise nonlinear elastic material properties for ligaments [105, 106]. Other studies used either linear or nonlinear stress–strain curves or exponential force–displacement curves for assigning ligament properties [79, 117, 118]. These approaches increased the complexity of the FE model, calling for more computational time to solve the models [79, 105, 106, 117, 118]. In contrast, various other FE studies simplified the ligaments as two-node spring or tension-only link elements [16, 35, 74, 86, 102]. Table 4 shows material properties used for ligaments and endplates in various studies.
Studies used different modeling approaches for facet joint interactions (FJI). Ahuja et al. [104] simulated facets through 0.5 mm gap elements. In this study, the coefficient of friction of 0.1 was used to define the sliding contacts of the surfaces. Various authors followed a similar approach [16, 17, 119]. However, other authors assigned frictionless contacts for facets [35, 120]. In a study, facet joints were modeled as gap elements where contact stiffness changes when the gap closes [32]. A couple of studies considered 0.2-mm thickness for the facet cartilage layer [77, 81].
4.2.2 Experimental studies
Over the years, several studies analyzed the functioning and physiological movements of the natural and implanted spine experimentally. An in vivo measurement to find the IDP for various body movements and postures was reported by Wilke et al. [124]. A probe was inserted into the IVD of a living subject. IDP of 2.30 MPa was observed when the subject lifted a 20 kg deadweight with a round back. As stated by the authors, this observation may vary from patient to patient. In another study, Wilke et al. [125] determined the trunk muscle forces during flexion and extension. A follower load (compressive load applied along a path) technique was used in this study in order to represent the upper body weight that follows the curvature of the spine. This subjects the whole lumbar spine to nearly pure compression [126]. The observed values for erector spinae muscle forces were 100 N in standing, 130 N for 15° extension, and 520 N for 30° flexion of the upper body.
A cadaveric study compared the changes in the foraminal area between indirect decompression (insertion of an anterior lumbar interbody spacer during ALIF) and direct decompression (foraminotomy) [53]. The study also compared the measured foraminal areas with that of the intact condition. The results indicated that the interbody spacer provides superior decompression through ALIF. In a cadaveric study, Lai et al. [106] quantified the biomechanical stability of the lumbar spine after implanting an interbody spacer using LLIF surgery with and without various supplemental fixations (Fig. 7). The different implantations used in this study were interbody implant without supplemental fixation, LLIF plus unilateral pedicle screw (UPS) fixation, LLIF plus BPS, LS and lateral plates (LLIF plus LP), LLIF plus interspinous plate (IP), and LLIF plus LP and IP. The study observed a significant reduction in ROM for LLIF plus BPS and LLIF plus LP plus IP constructs.
a Intact lumbar spine; b LLIF cages alone; c LLIF plus LP; d LLIF plus UPS; e LLIF plus BPS [127]
A cadaveric study compared the stability of cadaveric lumbar segments implanted with either standalone cages or cages with supplementary BPS [128]. The study observed that stress-shielding is not significant when cages with supplementary BPS (multi-construct devices) are implanted for stabilization. However, the study did not analyze the failure of fused segments and adjacent segments. Hence, the long-term performance of standalone cage is unknown. A cadaveric biomechanical study investigated spinal ROM for five different conditions; intact lumbar spine, spinous process fixation device CD Horizon SPIRE spinal system (Medtronic Inc., Germany), PS fixation and two working prototypes [84]. The two working prototypes were “Facfix” (facet fixation type) and “Latplat” (used along with screws to fix spinous processes). The facet fixation prototype was reported to have more stability under all physiological loadings. Considering ROM, the result indicated that the facet fixation device is not superior to BPS fixation. A similar study for ALIF with various fixation options in static and vibrational conditions also reported that the BPSs are advantageous when compared with ISS [129].
Several biomechanical studies on LLIF focussed on one or two levels of implantation. Apart from LLIF and PLIF, other studies investigated the performance of interbody fusion cages implanted using TLIF [46, 50]. One such study compared the performances of three different shapes of cages and their positioning by TLIF [46]. The designs investigated in this study were kidney-shaped, articulating type and bullet-shaped interbody cages. The study could not establish the superiority of any design over the other. The footprint locations of cages are considered implant locations and the observations were independent of these locations.
Due to the limitations, such as disease transmission, high sample variability, decay and fatigue, of cadaveric and animal specimens, synthetic biomimetic spine models have been used as an alternative [130]. Repeated testing on the synthetic spine does not alter the biomechanics. These models provide the option of non-destructive testing (NDT) between many different procedures without carry-over effects. Synthetic models pose small intra-specimen variability and zero biohazards. Recently, an experimental study used an artificial intact spine model to measure the ROM of the full lumbar spine [131]. The study observed that the measured ROM data was comparable to published data from cadaveric studies. In general, synthetic biomimetic models are cost-effective alternatives for cadaveric spine models. Several studies conducted biomechanical testing on artificial models for validation purposes [132,133,134].
Due to the longer incision and associated complexities of standard surgical procedures, MI surgical techniques have been gaining popularity in treating DDD [135, 136]. The advantages of MI surgery include reduced trauma to the muscles and soft tissues, minimal blood loss, and faster recovery. In MI fusion surgery, expandable intervertebral cages are used instead of static cages [52, 137]. Cannestra et al. [137] evaluated the stability of different intervertebral cages inserted at the L4–L5 IVD space using an MI plus TLIF technique. Specimens were implanted with either medial–lateral (expandable) Ti6Al4V cage or a conventional (non-expandable) PEEK cage with UPS and BPS. The study observed that the performance of the expandable device is similar to the conventional cages. Another cadaveric study observed that additional sagittal corrections of lordosis might be possible with expandable Ti6Al4V cages [138]. Mantell et al. [138] compared an expandable lateral cage with a static TLIF cage in an in-vitro cadaveric study of spondylolisthesis. The L4–L5 segment of a cadaveric spondylolisthesis specimen was implanted with the cages and analyzed for ROM. In this study, Caliber-L (Globus Medical Inc., USA) expandable cage, and Sustain-O (Globus Medical Inc., USA) static TLIF cage were used. The study observed that an expandable cage with UPS fixation provides stability equivalent to a TLIF cage with BPS. However, the collapse of endplates was evident with an expandable cage. A study measured the ROM of the L1–L5 spine implanted with expandable cages. Twelve cadaveric specimens were operated on using the TLIF approach (L1–L5) [139]. This study observed that there was no significant difference between static and expandable cages for the reduction in ROM. A similar cadaveric study was conducted using seven L2–L5 specimens. The device used in the study was a Wave-D expandable cage (Medtronic Inc., USA) [140]. The study observed a significant reduction in ROM while using Wave-D. A recent study observed damage to the endplate, subsidence, and loss of intervertebral and neuroforaminal height with recurrence of symptoms due to overexpansion of expandable cage, especially in patients with poor bone quality [51]. Despite the promise, several studies reported no superior outcome of expandable cages over static ones [51, 138, 139, 141]. A recent study observed that only anterior disc height was statistically significant, and additional supplemental fixation with PS cannot significantly promote sagittal alignment [142].
A standalone expandable device Varilift-L (Wenzel Spine Inc., USA) has been introduced recently [143, 144]. This device does not need any supplemental fixation for fusing the affected segments. It is reported clinically that the standalone device has a low incidence of revision, better fusion and effective relief of symptoms [144]. Another study reported the advantages of using the same standalone expandable cage [145]. This study compared a MI posteriorly inserted vertebral cage with a standalone design. It has been observed that a compromise in terms of vertebral fusion exists in standalone cages. However, the device requires further biomechanical investigations to validate its standalone performance. No other standalone devices are available in the literature to date.
Recently, an annular closure device (ACD) that can be implanted on IVD to close the hole after microdiscectomy has been proposed [57]. A microdiscectomy is required to remove the herniated part of an IVD. The implanted ACD helps to stop recurring herniation [146]. Several clinical studies attempted to establish the performance of ACD in the lumbar region [63, 147, 148]. Clinical studies observed breakage of Ti bone-anchor in patients [39, 148]. The part that closes the hole is woven Dacron (polyethyleneterephthalate). The ungluing of Dacron is observed in several patients [39, 148].
5 Discussion
The study reviewed the literature to establish the effect of various surgical approaches and implant designs on the performance of spinal implants used in lumbar fusion surgeries. An interbody fusion can stabilize the spine. The cages are reported to preserve the disc height [1]. Although static cages are commonly used, expandable cages have drawn considerable attention in recent times [52]. Pedicle screws and rod systems and bone grafts are used for PLF, PLIF and TLIF surgical approaches. A PS and rod system is often required to maintain segmental stability [86]. Several studies performed separate and comparative investigations of the various surgical approaches (PLIF, ALIF, TLIF, OLIF, etc.). The clinical results support the TLIF method more because it is less invasive compared to other approaches. In the fusion techniques, the adjacent segments are the most crucial parts to investigate. When two levels of the lumbar vertebral body are fused, the adjacent IVD, vertebral body, facet joints, and related parts experience degenerations, which lead to RS in many patients [38]. It is commonly reported that ASD is a significant cause of failure of fusion surgery. Most of the clinical studies referred to in the literature review section proposed a concern of failure due to the deterioration of adjacent segments [21, 80,81,82]. Other literature has presented the ASD issue along with other problems such as cage subsidence, screw breakage, and high stress/strain values [48, 55, 83, 84, 149].
The FE modeling has been widely used in biomechanical studies since it can effectively predict the realistic behavior of implant-bone structures [98]. By incorporating the FE techniques, researchers can predict the short- and long-term performance of prostheses implanted on the lumbar spine or any other part of the body [138]. The FE studies encompassed the different types of geometrical models of the lumbar spine (simplified cylindrical and actual geometry based on CT scan data) [77, 150]. Studies also reported a range of material models (linear elastic isotropic, hyperelastic, viscoelastic, and poroelastic) for the IVD, vertebral body, posterior bone and ligaments. Hyperelastic, poroelastic, and viscoelastic models have been variably used to assign material properties for AF and NP. However, linear elastic models are common in various studies. The material properties of bone were assumed as homogenous in earlier studies, whereas CT data-based heterogeneous distributions of linear elastic and isotropic bone properties were used in recent studies [16, 86].
For the validation and verification of FE models, studies adopted different criteria. Some of the studies used ROM of the lumbar spine reported in the literature. On the other hand, other studies considered stresses and strains as the parameter for comparison [16, 35]. Limiting values of the strains were reported for the cortical and cancellous bone enabling direct comparison before and after implantation [97, 151]. To develop a trustworthy FE model of the spine, the thickness of the endplates, material properties and IVD volumes are very important. The improvement of image processing technology has made the development of patient-specific FE models possible prior to clinical interventions. A comparative study between various surgical approaches to predict the occurrence and progression of ASD is a future scope.
The advancements in imaging technology and modeling techniques aid the selection of the best surgical approaches, identify the critical modeling parameters, analyze design variations of implants, and can help indecision making of a surgical planning. For manufacturing a complex geometry, additive manufacturing (AM) techniques play a significant role. Patient-specific implants can be manufactured by adopting the AM technique [152]. For complex surgeries, decision-making and manufacturing of the implantare important. FE modeling and AM technology are useful in this aspect. The recent developments in expandable cages, standalone cages and annular closure devices are promoting MI surgery in the lumbar spine [135, 136, 153]. In MI surgery, a tiny incision is required to approach the internal organs, reducing the loss of blood. As the incision is small, it also leads to fast healing of the wound. The size of the prosthesis should be suitable for insertion through the small incision in the MI approach [135].
For any fusion cages, its performance is analyzed by comparing the percentage decrease in ROM between pre- and post-surgery along with the magnitude of stress-shielding observed. ASD should also be considered as a parameter to predict the successful performance of cages or any other spinal implants. Such performance-related comparisons are rarely observed in the literature. Various studies focussed on stresses and strains distributions for the intact and implanted spine to predict the success rate of fusion surgery using various implants. Studies investigating bone remodeling and its impact on adjacent discs are limited. In order to model the IVD realistically, consideration should be given to relate the local extracellular matrix (ECM) composition and organization to its mechanical behavior. The volume fraction of the compositions of the AF and NP (water, fixed charged density, collagen and ground substance) should be considered, as this influence the properties of AF and NP directly. For predicting the degeneration of the adjacent disc over time, changes in biochemical composition due to cell activity should also be considered [154].The changes in bone density and degeneration of discs using a coupled mechanoregulatory algorithm to investigate the behavior of IVD were reported very rarely [154]. The limitation of such model is that it is patient-specific. This makes it more complex to generalize the output of any biomechanical and clinical studies. In such cases, more patients and lengthy clinical follow-up will be required. Recently, new devices like standalone expandable cages and expandable screws have been proposed and studied in the literature [102, 155]. The expandable cages are designed to reduce neurological complications, enable better lordosis restoration, and improve ease of insertion [59]. However, they are expensive. There exists a major gap in studies relating to these new devices and the risk of ASD these devices may pose. Apart from these, there is no literature on standalone expandable cage design that can be implanted using the TLIF approach. More research to develop such a device is necessary.
6 Conclusions
An overview of various biomechanical and clinical studies on the intact and implanted lumbar spine has been presented. Various geometrical and material modeling parameters, surgical approaches and loading and boundary conditions used in biomechanical studies of the spine have been reviewed. The computational models stand as a valuable tool for patient-specific preclinical studies. Among all the fusion surgical approaches, TLIF is the preferred one since it does not affect the abdominal muscles or spinal cord. Future research should be directed toward investigating the progression of ASD post-fusion surgery. Mechanoregulatory-based algorithms may be integrated with the FE modeling to predict the progression of ASD. Further development of standalone expandable cages and comparative studies on the performance of expandable cages and standard cages are required. Various cage geometries have been clinically used so far. However, the long-term effect of such cage geometries on the adjacent segment biomechanics is not well-understood. Studies on bone ingrowth in the porous cages used in fusion surgeries will be beneficial in identifying the best cage design. Despite several articles proposing a concern on ASD, detailed investigation of its pattern and propagation over time is limited. The comparison of intensity and chances of ASD between different surgeries has rarely been reported. Further research on the generation and propagation of ASD is warranted.
Abbreviations
- LBP:
-
Low Back Pain
- PS:
-
Pedicle Screw
- FE:
-
Finite Element
- ROM:
-
Ranges of Motion
- RS:
-
Revision Surgeries
- ASD:
-
Adjacent Segment Degeneration
- ADR:
-
Artificial Disc Replacement
- DDD:
-
Degenerative Disc Diseases
- IVD:
-
Intervertebral Disc
- AF:
-
Annulus Fibrosus
- NP:
-
Nucleus Pulposus
- CE:
-
Cartilaginous Endplate
- BE:
-
Bony Endplate
- 3D:
-
Three dimensional
- ALL:
-
Anterior Longitudinal Ligament
- PLL:
-
Posterior Longitudinal Ligament
- LF:
-
Ligamentum Flavum
- ITL:
-
Inter Transverse Ligament
- CL:
-
Capsular Ligament
- ISL:
-
Inter Spinous Ligament
- SSL:
-
Supra Spinous Ligament
- FSU:
-
Functional Spinal Unit
- SP:
-
Sagittal Plane
- LSS:
-
Lumbar Spinal Stenosis
- ISS:
-
Inter Spinous Spacers
- LIF:
-
Lumbar Interbody Fusion
- PLIF:
-
Posterior Lumbar Interbody Fusion
- ALIF:
-
Anterior Lumbar Interbody Fusion
- LLIF:
-
Lateral Lumbar Interbody Fusion
- OLIF:
-
Oblique Lumbar Interbody Fusion
- TLIF:
-
Transforaminal Lumbar Interbody Fusion
- ACD:
-
Annular Closure Device
- DIAM:
-
Device for Intervertebral Assisted Motion
- DYNESYS:
-
Dynamic Neutralization System
- MIS:
-
Minimally Invasive Surgery
- BMP:
-
Bone Morphogenic Protein
- PEEK:
-
Polyetherether ketone
- FDA:
-
Food and Drug Association
- UHMWPE:
-
Ultra High Molecular Weight Polyethylene
- IDP:
-
Intra Discal Pressure
- CT:
-
Computed Tomography
- E:
-
Elastic modulus
- BPS:
-
Bilateral Pedicle Screw
- UPS:
-
Unilateral Pedicle Screw
- AVID:
-
Articulating Vertebral Interbody Device
- IP:
-
Interspinous Plate
- NDT:
-
Non Destructive Testing
- AM:
-
Additive Manufacturing
- DALYs:
-
Disability-Adjusted Life Years
References
Panjabi MM, White 3rd AA (1980) Basic biomechanics of the spine. Neurosurgery 7(1):76–93. https://doi.org/10.1227/00006123-198007000-00014
Newell N, Little JP, Christou A et al (2017) Biomechanics of the human intervertebral disc: a review of testing techniques and results. J Mech Behav Biomed Mater 69:420–434. https://doi.org/10.1016/j.jmbbm.2017.01.037
Dakwar E, Deukmedjian A, Ritter Y et al (2016) Chapter 16 - Spinal Pathology, conditions, and deformities: surgical intervention, in pathology and intervention in Musculoskeletal rehabilitation (Second Edition). In: Magee DJ et al (ed) W.B. Saunders, pp 584–611
Filippiadis DK, Marcia S, Ryan A et al (2018) New implant-based technologies in the Spine. Cardiovasc Intervent Radiol 41(10):1463–1473. https://doi.org/10.1007/s00270-018-1987-z
Sueki D, Carr E and Barcohana B (2013) Chapter 17 - lumbar spine disc replacement. In: Maxey L and Magnusson J, (eds) Rehabilitation for the postsurgical orthopedic patient, 3rd edn. pp 335–360. https://doi.org/10.1016/B978-0-323-07747-7.00017-4
Knezevic NN, Candido KD, Vlaeyen JWS et al (2021) Low back pain. The Lancet 398(10294):78–92. https://doi.org/10.1016/S0140-6736(21)00733-9
Daniell JR, Osti OL (2018) Failed Back surgery syndrome: a review article. Asian Spine J 12(2):372–379. https://doi.org/10.4184/asj.2018.12.2.372
Fatoye F, Gebrye T, Odeyemi I (2019) Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int 39(4):619–626. https://doi.org/10.1007/s00296-019-04273-0
Schroeder GD, Vaccaro AR, Divi SN et al (2021) 2021 Position statement from the international society for the advancement of spine surgery on cervical and lumbar disc replacement. Int J Spine Surg 15(1):37. https://doi.org/10.14444/8004
Cerciello T, Romano M, Bifulco P et al (2011) Advanced template matching method for estimation of intervertebral kinematics of lumbar spine. Med Eng Phys 33(10):1293–1302. https://doi.org/10.1016/j.medengphy.2011.06.009
Austevoll IM, Ebbs E (2022) Fusion Is not a safeguard to prevent revision surgery in lumbar spinal stenosis. JAMA Netw Open 5(7):e2223812–e2223812. https://doi.org/10.1001/jamanetworkopen.2022.23812
Kaner T, Ozer AF (2013) Dynamic stabilization for challenging lumbar degenerative diseases of the spine: a review of the literature. Adv Orthop 2013:753470. https://doi.org/10.1155/2013/753470
Más Y, Gracia L, Ibarz E et al (2017) Finite element simulation and clinical follow-up of lumbar spine biomechanics with dynamic fixations. PLoS ONE 12(11):e0188328. https://doi.org/10.1371/journal.pone.0188328
Zhang QH, Teo EC (2008) Finite element application in implant research for treatment of lumbar degenerative disc disease. Med Eng Phys 30(10):1246–1256. https://doi.org/10.1016/j.medengphy.2008.07.012
Fantigrossi A, Galbusera F, Raimondi MT et al (2007) Biomechanical analysis of cages for posterior lumbar interbody fusion. Med Eng Phys 29(1):101–109. https://doi.org/10.1016/j.medengphy.2006.02.007
Talukdar RG, Mukhopadhyay KK, Dhara S et al (2021) Numerical analysis of the mechanical behaviour of intact and implanted lumbar functional spinal units: Effects of loading and boundary conditions. Proc IMechE Part H: J Eng Med 235(7):792–804. https://doi.org/10.1177/09544119211008343
Sanjay D, Kumar N, Chanda S (2021) Stress-strain distribution in intact L4–L5 vertebrae under the influence of physiological movements: a finite element (FE) investigation. IOP Conf Ser: Mater Sci Eng 1206(1):012024. https://doi.org/10.1088/1757-899x/1206/1/012024
Hashimoto K, Aizawa T, Kanno H et al (2019) Adjacent segment degeneration after fusion spinal surgery—a systematic review. Int Orthop 43(4):987–993. https://doi.org/10.1007/s00264-018-4241-z
Eck JC, Humphreys SC, Hodges SD (1999) Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop (Belle Mead NJ) 28(6):336–340
David T (2007) Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE artificial disc in 106 patients. Spine (Phila Pa 1976) 32(6):661–666. https://doi.org/10.1097/01.brs.0000257554.67505.45
Liang Z, Cui J, Zhang J et al (2020) Biomechanical evaluation of strategies for adjacent segment disease after lateral lumbar interbody fusion: is the extension of pedicle screws necessary? Research Square. https://doi.org/10.21203/rs.2.17339/v2
Wang B, Hua W, Ke W et al (2019) Biomechanical evaluation of transforaminal lumbar interbody fusion and oblique lumbar interbody fusion on the adjacent segment: a finite element analysis. World Neurosurg 126:e819–e824. https://doi.org/10.1016/j.wneu.2019.02.164
Lacroix D, Prendergast PJ (2002) A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech 35(9):1163–1171. https://doi.org/10.1016/s0021-9290(02)00086-6
Isaksson H, Wilson W, van Donkelaar CC et al (2006) Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J Biomech 39(8):1507–1516. https://doi.org/10.1016/j.jbiomech.2005.01.037
Liu X, Niebur GL (2007) Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech Model Mechanobiol 7(4):335. https://doi.org/10.1007/s10237-007-0100-3
Ghosh R, Chanda S, Chakraborty D (2020) The influence of macro-textural designs over implant surface on bone on-growth: a computational mechanobiology based study. Comput Biol Med 124:103937. https://doi.org/10.1016/j.compbiomed.2020.103937
Ghosh R, Chanda S, Chakraborty D (2021) Qualitative predictions of bone growth over optimally designed macro-textured implant surfaces obtained using NN-GA based machine learning framework. Med Eng Phys 95:64–75. https://doi.org/10.1016/j.medengphy.2021.08.002
Damm N, Rockenfeller R, Gruber K (2020) Lumbar spinal ligament characteristics extracted from stepwise reduction experiments allow for preciser modeling than literature data. Biomech Model Mechanobiol 19(3):893–910. https://doi.org/10.1007/s10237-019-01259-6
Sandhu FA, Dowlati E, Garica R (2020) Lumbar Arthroplasty: past, present, and future. Neurosurgery 86(2):155–169. https://doi.org/10.1093/neuros/nyz439
Dreischarf M, Zander T, Shirazi-Adl A et al (2014) Comparison of eight published static finite element models of the intact lumbar spine: predictive power of models improves when combined together. J Biomech 47(8):1757–1766. https://doi.org/10.1016/j.jbiomech.2014.04.002
Calisse J, Rohlmann A, Bergmann G (1999) Estimation of trunk muscle forces using the finite element method and in vivo loads measured by telemeterized internal spinal fixation devices. J Biomech 32(7):727–731. https://doi.org/10.1016/s0021-9290(99)00052-4
Rohlmann A, Burra NK, Zander T et al (2007) Comparison of the effects of bilateral posterior dynamic and rigid fixation devices on the loads in the lumbar spine: a finite element analysis. Eur Spine J 16(8):1223–1231. https://doi.org/10.1007/s00586-006-0292-8
Goel VK, Ramirez SA, Kong W et al (1995) Cancellous bone Young’s modulus variation within the vertebral body of a ligamentous lumbar spine—application of bone adaptive remodeling concepts. J Biomech Eng 117(3):266–271. https://doi.org/10.1115/1.2794180
Sharma M, Langrana NA, Rodriguez J (1995) Role of ligaments and facets in lumbar spinal stability. Spine 20(8):887–900
Shin DS, Lee K, Kim D (2007) Biomechanical study of lumbar spine with dynamic stabilization device using finite element method. Comput Aided Des 39(7):559–567. https://doi.org/10.1016/j.cad.2007.03.005
Dolan P, Adams M (2001) Recent advances in lumbar spinal mechanics and their significance for modelling. Clin Biomech (Bristol, Avon) 16(Suppl 1):S8–S16. https://doi.org/10.1016/S0268-0033(00)00096-6
Shirazi-Adl A, Ahmed AM, Shrivastava SC (1986) A finite element study of a lumbar motion segment subjected to pure sagittal plane moments. J Biomech 19(4):331–350. https://doi.org/10.1016/0021-9290(86)90009-6
An HS, Masuda K (2006) Relevance of in vitro and in vivo models for intervertebral disc degeneration. J Bone Joint Surg Am 88(Suppl 2):88–94. https://doi.org/10.2106/jbjs.E.01272
Kienzler JC, Rey S, Wetzel O et al (2021) Incidence and clinical impact of vertebral endplate changes after limited lumbar microdiscectomy and implantation of a bone-anchored annular closure device. BMC Surg 21(1):19. https://doi.org/10.1186/s12893-020-01011-3
Genevay S, Atlas SJ (2010) Lumbar spinal stenosis. Best Pract Res Clin Rheumatol 24(2):253–265. https://doi.org/10.1016/j.berh.2009.11.001
Goel SA, Modi HN (2018) Reoperations following lumbar spinal canal stenosis. Indian J Orthop 52(6):578–583. https://doi.org/10.4103/ortho.IJOrtho_380_17
Cornefjord M, Byröd G, Brisby H et al (2000) A long-term (4- to 12-year) follow-up study of surgical treatment of lumbar spinal stenosis. Eur Spine J 9(6):563–570. https://doi.org/10.1007/s005860000161
Lehr AM, Delawi D, van Susante JLC et al (2021) Long-term (> 10 years) clinical outcomes of instrumented posterolateral fusion for spondylolisthesis. Eur Spine J 30(5):1380–1386. https://doi.org/10.1007/s00586-020-06671-6
Grotle M, Småstuen MC, Fjeld O et al (2019) Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 9(8):e028743. https://doi.org/10.1136/bmjopen-2018-028743
Fan W, Guo L-X, Zhang M (2021) Biomechanical analysis of lumbar interbody fusion supplemented with various posterior stabilization systems. Eur Spine J 30(8):2342–2350. https://doi.org/10.1007/s00586-021-06856-7
Comer G, Behn A, Ravi S et al (2015) A biomechanical comparison of shape design and positioning of transforaminal lumbar interbody fusion cages. Glob Spine J 6. https://doi.org/10.1055/s-0035-1564568
Xu H, Ju W, Xu N et al (2013) Biomechanical comparison of transforaminal lumbar interbody fusion with 1 or 2 cages by finite-element analysis. Oper Neurosurg 73(2):ons198-ons205. https://doi.org/10.1227/01.neu.0000430320.39870.f7
Bashkuev M, Checa S, Postigo S et al (2015) Computational analyses of different intervertebral cages for lumbar spinal fusion. J Biomech 48(12):3274–3282. https://doi.org/10.1016/j.jbiomech.2015.06.024
Loenen ACY, Peters MJM, Bevers RTJ et al (2022) Early bone ingrowth and segmental stability of a trussed titanium cage versus a polyether ether ketone cage in an ovine lumbar interbody fusion model. Spine J 22(1):174–182. https://doi.org/10.1016/j.spinee.2021.07.011
Stickley C, Philipp T, Wang E et al (2021) Expandable cages increase the risk of intraoperative subsidence but do not improve perioperative outcomes in single level transforaminal lumbar interbody fusion. Spine J 21(1):37–44. https://doi.org/10.1016/j.spinee.2020.08.019
Lewandrowski K-U, Ferrara L, Cheng B (2020) Expandable interbody fusion cages: an editorial on the surgeon's perspective on recent technological advances and their biomechanical implications. Int J Spine Surg 14(s3):S56. https://doi.org/10.14444/7127
Calvachi-Prieto P, McAvoy MB, Cerecedo-Lopez CD et al (2021) Expandable versus static cages in minimally invasive lumbar interbody fusion: a systematic review and meta-analysis. World Neurosurg 151:e607–e614. https://doi.org/10.1016/j.wneu.2021.04.090
Odeh K, Rosinski A, Nguyen J et al (2020) Anterior lumbar interbody fusion may provide superior decompression of the foraminal space compared with direct foraminotomy: biomechanical cadaveric study. World Neurosurg 135:e71–e76. https://doi.org/10.1016/j.wneu.2019.10.139
Reis MT, Reyes PM, Bse et al (2016) Biomechanical evaluation of lateral lumbar interbody fusion with secondary augmentation. J Neurosurg: Spine SPI 25(6):720–726. https://doi.org/10.3171/2016.4.SPINE151386
Fan W, Guo LX, Zhao D (2021) Posterior lumbar interbody fusion versus transforaminal lumbar interbody fusion: finite element analysis of the vibration characteristics of fused lumbar spine. World Neurosurg 150:e81–e88. https://doi.org/10.1016/j.wneu.2021.02.094
Goel VK, Kiapour A, Faizan A et al (2007) Finite element study of matched paired posterior disc implant and dynamic stabilizer (360° motion preservation system). Sas j 1(1):55–61. https://doi.org/10.1016/sasj-2006-0008-rr
Choy WJ, Phan K, Diwan AD et al (2018) Annular closure device for disc herniation: meta-analysis of clinical outcome and complications. BMC Musculoskelet Disord 19(1):290. https://doi.org/10.1186/s12891-018-2213-5
Ghiselli G, Wang J, Bhatia N et al (2004) Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg. Am Vol 86-A:1497–1503. https://doi.org/10.2106/00004623-200407000-00020
Zhao X, Du L, Xie Y et al (2018) Effect of lumbar lordosis on the adjacent segment in transforaminal lumbar interbody fusion: a finite element analysis. World Neurosurg 114:e114–e120. https://doi.org/10.1016/j.wneu.2018.02.073
Li YM, Frisch R, Huang Z et al (2019) Comparative effectiveness of adjustable lordotic expandable versus static lateral lumbar interbody fusion devices: two-year clinical and radiographic outcomes. Neurosurgery 66. https://doi.org/10.1093/neuros/nyz310_822
Villavicencio AT, Nelson EL, Rajpal S et al (2021) Prospective, randomized, double-blinded clinical trial comparing PEEK and allograft spacers in patients undergoing transforaminal lumbar interbody fusion surgeries. Spine J. https://doi.org/10.1016/j.spinee.2021.06.005
Gu H, Chang Y, Zeng S et al (2018) Wallis interspinous spacer for treatment of primary lumbar disc herniation: three-year results of a randomized controlled trial. World Neurosurg 120:e1331–e1336. https://doi.org/10.1016/j.wneu.2018.09.086
Kienzler JC, Klassen PD, Miller LE et al (2019) Three-year results from a randomized trial of lumbar discectomy with annulus fibrosus occlusion in patients at high risk for reherniation. Acta Neurochir (Wien) 161(7):1389–1396. https://doi.org/10.1007/s00701-019-03948-8
Vital JM, Boissière L (2014) Total disc replacement. Orthop Traumatol: Surg Res 100(1, Supplement):S1–S14. https://doi.org/10.1016/j.otsr.2013.06.018
Vicars R, Hall R, Hyde PJ (2017) 7.14 Wear: Total intervertebral disc prostheses☆, in comprehensive biomaterials II, P. Ducheyne, Editor, Elsevier: Oxford 246–264
Bertagnoli R, Habbicht H (2008) The ProDisc-L lumbar prosthesis. Int Surg 3(4):209–213. https://doi.org/10.1007/s11610-007-0042-6
Byvaltsev VA, Kalinin AA, Aliyev MA et al (2021) Clinical-Instrumental results and analysis of functional activity restoration in professional athletes after lumbar total disk replacement. World Neurosurg 151:e1069–e1077. https://doi.org/10.1016/j.wneu.2021.05.066
Kitzen J, Schotanus MGM, van Kuijk SMJ et al (2020) Long-term clinical outcome of the Charité III total lumbar disc replacement. Eur Spine J 29(7):1527–1535. https://doi.org/10.1007/s00586-020-06308-8
van den Eerenbeemt KD, Ostelo RW, van Royen BJ et al (2010) Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J 19(8):1262–1280. https://doi.org/10.1007/s00586-010-1445-3
Jacobs W, Van der Gaag NA, Tuschel A et al (2012) Total disc replacement for chronic back pain in the presence of disc degeneration. Cochrane Database of Syst Reviews (9) Art. No.: CD008326. https://doi.org/10.1002/14651858.CD008326.pub2
Siepe CJ, Zelenkov P, Sauri-Barraza JC et al (2010) The fate of facet joint and adjacent level disc degeneration following total lumbar disc replacement: a prospective clinical, X-ray, and magnetic resonance imaging investigation. Spine (Phila Pa 1976) 35(22):1991–2003. https://doi.org/10.1097/BRS.0b013e3181d6f878
Lu S-b, Hai Y, Kong C et al (2015) An 11-year minimum follow-up of the Charite III lumbar disc replacement for the treatment of symptomatic degenerative disc disease. Eur Spine J 24(9):2056–2064. https://doi.org/10.1007/s00586-015-3939-5
Laugesen LA, Paulsen RT, Carreon L et al (2017) Patient-reported outcomes and revision rates at a mean follow-up of 10 years after lumbar total disc replacement. Spine (Phila Pa 1976) 42(21):1657–1663. https://doi.org/10.1097/brs.0000000000002174
Zhang Z, Li H, Fogel GR et al (2018) Finite element model predicts the biomechanical performance of transforaminal lumbar interbody fusion with various porous additive manufactured cages. Comput Biol Med 95:167–174. https://doi.org/10.1016/j.compbiomed.2018.02.016
Schultz AB, Warwick DN, Berkson MH et al (1979) Mechanical properties of human lumbar spine motion segments—part i: responses in flexion, extension, lateral bending, and torsion. J Biomech Eng 101(1):46–52. https://doi.org/10.1115/1.3426223
Brown T, Hansen RJ, Yorra AJ (1957) Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs; a preliminary report. J. Bone Joint Surg Am 39-a(5):1135–1164
Goel VK, Monroe BT, Gilbertson LG et al (1995) Interlaminar shear stresses and laminae separation in a disc. Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine (Phila Pa 1976) 20(6):689–698
Goel VK, Kong W, Han JS et al (1993) A combined finite element and optimization investigation of lumbar spine mechanics with and without muscles. Spine (Phila Pa 1976) 18(11):1531–1541
Zander T, Rohlmann A, Burra NK et al (2006) Effect of a posterior dynamic implant adjacent to a rigid spinal fixator. Clin Biomech (Bristol, Avon) 21(8):767–774. https://doi.org/10.1016/j.clinbiomech.2006.04.001
Yan J-Z, Qiu G-X, Wu Z-H et al (2011) Finite element analysis in adjacent segment degeneration after lumbar fusion. Int J Med Robot Comput Assist Surg 7(1):96–100. https://doi.org/10.1002/rcs.374
Tan Q-c, Liu Z-x, Zhao Y et al (2021) Biomechanical comparison of four types of instrumentation constructs for revision surgery in lumbar adjacent segment disease: a finite element study. Comput Biol Med 134:104477. https://doi.org/10.1016/j.compbiomed.2021.104477
Jiang S, Li W (2019) Biomechanical study of proximal adjacent segment degeneration after posterior lumbar interbody fusion and fixation: a finite element analysis. J Orthop Surg Res 14. https://doi.org/10.1186/s13018-019-1150-9
Xu H, Tang H, Guan X et al (2013) Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Oper Neurosurg 72(1):ons21-ons26. https://doi.org/10.1227/NEU.0b013e3182742a69
Hartmann F, Nusselt T, Maier G et al (2019) Biomechanical testing of different posterior fusion devices on lumbar spinal range of motion. Clin Biomech 62:121–126. https://doi.org/10.1016/j.clinbiomech.2019.01.012
Fang G, Lin Y, Wu J et al (2020) Biomechanical comparison of stand-alone and bilateral pedicle screw fixation for oblique lumbar interbody fusion surgery—a finite element analysis. World Neurosurg 141:e204–e212. https://doi.org/10.1016/j.wneu.2020.05.245
Biswas JK, Rana M, Majumder S et al (2018) Effect of two-level pedicle-screw fixation with different rod materials on lumbar spine: a finite element study. J Orthop Sci 23(2):258–265. https://doi.org/10.1016/j.jos.2017.10.009
Pham MH, Mehta VA, Patel NN et al (2016) Complications associated with the Dynesys dynamic stabilization system: a comprehensive review of the literature. Neurosurg Focus 40(1):E2. https://doi.org/10.3171/2015.10.Focus15432
Boody BS, Smucker JD, Sasso W et al (2020) Evaluation of DIAM™ Spinal Stabilization System for lower lumbar disc degenerative disease: a randomized, prospective, single-site study. J Orthop 21:171–177. https://doi.org/10.1016/j.jor.2020.03.025
Rienmüller AC, Krieg SM, Schmidt FA et al (2019) Reoperation rates and risk factors for revision 4 years after dynamic stabilization of the lumbar spine. Spine J 19(1):113–120. https://doi.org/10.1016/j.spinee.2018.05.025
Faizan A, Kiapour A, Kiapour AM et al (2014) Biomechanical analysis of various footprints of transforaminal lumbar interbody fusion devices. J Spinal Disord Tech 27(4):E118-127. https://doi.org/10.1097/BSD.0b013e3182a11478
Xu M, Yang J, Lieberman IH et al (2017) Lumbar spine finite element model for healthy subjects: development and validation. Comput Methods Biomech Biomed Engin 20(1):1–15. https://doi.org/10.1080/10255842.2016.1193596
Denozière G, Ku DN (2006) Biomechanical comparison between fusion of two vertebrae and implantation of an artificial intervertebral disc. J Biomech 39(4):766–775. https://doi.org/10.1016/j.jbiomech.2004.07.039
Schmidt H, Heuer F, Drumm J et al (2007) Application of a calibration method provides more realistic results for a finite element model of a lumbar spinal segment. Clin Biomech (Bristol, Avon) 22(4):377–384. https://doi.org/10.1016/j.clinbiomech.2006.11.008
Zhong ZC, Chen SH, Hung CH (2008) Load- and displacement-controlled finite element analyses on fusion and non-fusion spinal implants. Proc IMechE Part H: J Eng Med 223(2):143–157. https://doi.org/10.1243/09544119JEIM476
Taddei F, Pancanti A, Viceconti M (2004) An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med Eng Phys 26(1):61–69. https://doi.org/10.1016/S1350-4533(03)00138-3
Rho JY, Hobatho MC, Ashman RB (1995) Relations of mechanical properties to density and CT numbers in human bone. Med Eng Phys 17(5):347–355. https://doi.org/10.1016/1350-4533(95)97314-F
Morgan EF, Bayraktar HH, Keaveny TM (2003) Trabecular bone modulus–density relationships depend on anatomic site. J Biomech 36(7):897–904. https://doi.org/10.1016/S0021-9290(03)00071-X
Pal B, Gupta S (2020) The Relevance of biomechanical analysis in joint replacements: a review. J Inst Eng (India): Ser C 101(5):913–927. https://doi.org/10.1007/s40032-020-00611-5
Helgason B, Perilli E, Schileo E et al (2008) Mathematical relationships between bone density and mechanical properties: a literature review. Clin Biomech (Bristol, Avon) 23(2):135–146. https://doi.org/10.1016/j.clinbiomech.2007.08.024
Moramarco V, Pérez del Palomar A, Pappalettere C et al (2010) An accurate validation of a computational model of a human lumbosacral segment. J Biomech 43(2):334–342. https://doi.org/10.1016/j.jbiomech.2009.07.042
Baroud G, Nemes J, Heini P et al (2003) Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J 12(4):421–426. https://doi.org/10.1007/s00586-002-0512-9
Sanjay D, Bhardwaj JS, Kumar N et al (2022) Expandable pedicle screw may have better fixation than normal pedicle screw: preclinical investigation on instrumented L4–L5 vertebrae based on various physiological movements. Med Biol Eng Comput. https://doi.org/10.1007/s11517-022-02625-w
Li J, Shang J, Zhou Y et al (2015) Finite element analysis of a new pedicle screw-plate system for minimally invasive transforaminal lumbar interbody fusion. PLoS ONE 10(12):e0144637. https://doi.org/10.1371/journal.pone.0144637
Ahuja S, Moideen AN, Dudhniwala AG et al (2020) Lumbar stability following graded unilateral and bilateral facetectomy: a finite element model study. Clin Biomech 75. https://doi.org/10.1016/j.clinbiomech.2020.105011
Park WM, Kim K, Kim YH (2013) Effects of degenerated intervertebral discs on intersegmental rotations, intradiscal pressures, and facet joint forces of the whole lumbar spine. Comput Biol Med 43(9):1234–1240. https://doi.org/10.1016/j.compbiomed.2013.06.011
Little JP, Adam CJ (2015) Geometric sensitivity of patient-specific finite element models of the spine to variability in user-selected anatomical landmarks. Comput Methods Biomech Biomed Engin 18(6):676–688. https://doi.org/10.1080/10255842.2013.843673
Schmidt H, Shirazi-Adl A, Galbusera F et al (2010) Response analysis of the lumbar spine during regular daily activities–a finite element analysis. J Biomech 43(10):1849–1856. https://doi.org/10.1016/j.jbiomech.2010.03.035
Casaroli G, Galbusera F, Jonas R et al (2017) A novel finite element model of the ovine lumbar intervertebral disc with anisotropic hyperelastic material properties. PLoS ONE 12(5):e0177088. https://doi.org/10.1371/journal.pone.0177088
Eberlein∗ R, Holzapfel† GA, Schulze-Bauer‡ CAJ (2001)An anisotropic model for annulus tissue and enhanced finite element analyses of intact lumbar disc bodies.Comput Methods Biomech Biomed Engin 4(3):209–229.https://doi.org/10.1080/10255840108908005
Roberts S, Menage J, Urban JPG (1989) Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 14(2):166–174
Wang Y, Battié MC, Boyd SK et al (2011) The osseous endplates in lumbar vertebrae: thickness, bone mineral density and their associations with age and disk degeneration. Bone 48(4):804–809. https://doi.org/10.1016/j.bone.2010.12.005
Berg-Johansen B, Han M, Fields AJ et al (2018) Cartilage endplate thickness variation measured by ultrashort echo-time MRI Is associated with adjacent disc degeneration. Spine 43(10):E592–E600
Schmidt H, Heuer F, Wilke HJ (2009) Which axial and bending stiffnesses of posterior implants are required to design a flexible lumbar stabilization system? J Biomech 42(1):48–54. https://doi.org/10.1016/j.jbiomech.2008.10.005
Zhong ZC, Wei SH, Wang JP et al (2006) Finite element analysis of the lumbar spine with a new cage using a topology optimization method. Med Eng Phys 28(1):90–98. https://doi.org/10.1016/j.medengphy.2005.03.007
Wu Y, Cisewski S, Sachs BL et al (2013) Effect of cartilage endplate on cell based disc regeneration: a finite element analysis. Mol Cell Biomech 10(2):159–182
Li QY, Kim H-J, Son J et al (2017) Biomechanical analysis of lumbar decompression surgery in relation to degenerative changes in the lumbar spine – validated finite element analysis. Comput Biol Med 89:512–519. https://doi.org/10.1016/j.compbiomed.2017.09.003
Ayturk UM, Puttlitz CM (2011) Parametric convergence sensitivity and validation of a finite element model of the human lumbar spine. Comput Methods Biomech Biomed Engin 14(8):695–705. https://doi.org/10.1080/10255842.2010.493517
Schmidt H, Galbusera F, Rohlmann A et al (2012) Effect of multilevel lumbar disc arthroplasty on spine kinematics and facet joint loads in flexion and extension: a finite element analysis. Eur Spine J 21 Suppl 5(Suppl 5):S663–674. https://doi.org/10.1007/s00586-010-1382-1
McCutchen CW (1962) The frictional properties of animal joints. Wear 5(1):1–17. https://doi.org/10.1016/0043-1648(62)90176-X
Williams JR, Natarajan RN, Andersson GB (2007) Inclusion of regional poroelastic material properties better predicts biomechanical behavior of lumbar discs subjected to dynamic loading. J Biomech 40(9):1981–1987. https://doi.org/10.1016/j.jbiomech.2006.09.022
Chen G, Schmutz B, Epari D et al (2009) A new approach for assigning bone material properties from CT images into finite element models. J Biomech 43:1011–1015. https://doi.org/10.1016/j.jbiomech.2009.10.040
Kang K-T, Koh Y-G, Son J et al (2017) Biomechanical evaluation of pedicle screw fixation system in spinal adjacent levels using polyetheretherketone, carbon-fiber-reinforced polyetheretherketone, and traditional titanium as rod materials. Compos B Eng 130:248–256. https://doi.org/10.1016/j.compositesb.2017.07.052
Guo LX, Fan W (2017) The effect of single-level disc degeneration on dynamic response of the whole lumbar spine to vertical vibration. World Neurosurg 105:510–518. https://doi.org/10.1016/j.wneu.2017.06.008
Wilke H J, Neef P, Caimi M et al (1999) New in vivo measurements of pressures in the intervertebral disc in daily life. Spine (Phila Pa 1976) 24(8):755–762. https://doi.org/10.1097/00007632-199904150-00005
Wilke HJ, Rohlmann A, Neller S et al (2003) ISSLS prize winner: a novel approach to determine trunk muscle forces during flexion and extension: a comparison of data from an in vitro experiment and in vivo measurements. Spine (Phila Pa 1976) 28(23):2585–2593. https://doi.org/10.1097/01.Brs.0000096673.16363.C7
Rohlmann A, Neller S, Claes L et al (2001) Influence of a follower load on intradiscal pressure and intersegmental rotation of the lumbar spine. Spine (Phila Pa 1976) 26(24):E557–561. https://doi.org/10.1097/00007632-200112150-00014
Lai O, Chen Y, Chen Q et al (2021) Cadaveric biomechanical analysis of multilevel lateral lumbar interbody fusion with and without supplemental instrumentation. BMC Musculoskelet Disord 22(1):280. https://doi.org/10.1186/s12891-021-04151-6
Doulgeris JJ, Aghayev K, Gonzalez-Blohm SA et al (2015) Biomechanical comparison of an interspinous fusion device and bilateral pedicle screw system as additional fixation for lateral lumbar interbody fusion. Clin Biomech 30(2):205–210. https://doi.org/10.1016/j.clinbiomech.2014.10.003
Shen H, Chen Y, Liao Z et al (2021) Biomechanical evaluation of anterior lumbar interbody fusion with various fixation options: finite element analysis of static and vibration conditions. Clin Biomech 84. https://doi.org/10.1016/j.clinbiomech.2021.105339
Wang T, Ball JR, Pelletier MH et al (2014) Biomechanical evaluation of a biomimetic spinal construct. J Exp Orthop 1(1):3. https://doi.org/10.1186/s40634-014-0003-z
Diangelo D, Hoyer D, Chung C (2019) Biomechanical evaluation of a full-length (T12-S) synthetic lumbar spine model. MOJ Appl Bionics Biomech 3. https://doi.org/10.15406/mojabb.2019.03.00106
Camisa W, Leasure JM, Buckley JM (2014) Biomechanical validation of a synthetic lumbar spine. Spine J 14(11):S129–S130. https://doi.org/10.1016/j.spinee.2014.08.318
Bohl MA, McBryan S, Newcomb AGUS et al (2019) Range of motion testing of a novel 3D-printed synthetic spine model. Glob Spine J 10(4):419–424. https://doi.org/10.1177/2192568219858981
Bohl MA, Mooney MA, Repp GJ et al (2018) The barrow biomimetic spine: fluoroscopic analysis of a synthetic spine model made of variable 3D-printed materials and print parameters. Spine (Phila Pa 1976) 43(23):E1368–e1375. https://doi.org/10.1097/brs.0000000000002715
Azar FM (2020) Minimally Invasive surgery: is less more? Orthop Clin North Am 51(3):xiii–xiv. https://doi.org/10.1016/j.ocl.2020.04.001
Martens F, Lesage G, Muir JM et al (2018) Implantation of a bone-anchored annular closure device in conjunction with tubular minimally invasive discectomy for lumbar disc herniation: a retrospective study. BMC Musculoskelet Disord 19(1):269. https://doi.org/10.1186/s12891-018-2178-4
Cannestra AF, Peterson MD, Parker SR et al (2016) MIS Expandable interbody spacers: a literature review and biomechanical comparison of an expandable MIS TLIF with conventional TLIF and ALIF. Spine (Phila Pa 1976) 41 Suppl 8:S44–49. https://doi.org/10.1097/brs.0000000000001465
Mantell M, Cyriac M, Haines CM et al (2016) Biomechanical analysis of an expandable lateral cage and a static transforaminal lumbar interbody fusion cage with posterior instrumentation in an in vitro spondylolisthesis model. J Neurosurg Spine 24(1):32–38. https://doi.org/10.3171/2015.4.Spine14636
Mica MC, Voronov LI, Carandang G et al (2017) Biomechanics of an expandable lumbar interbody fusion cage deployed through transforaminal approach. Int J Spine Surg 11(4):24. https://doi.org/10.14444/4024
Soriano-Baron H, Newcomb AGUS, Malhotra D et al (2018) Biomechanical analysis of an expandable lumbar interbody spacer. World Neurosurg 114:e616–e623. https://doi.org/10.1016/j.wneu.2018.03.041
Qandah NA, Klocke NF, Synkowski JJ et al (2015) Additional sagittal correction can be obtained when using an expandable titanium interbody device in lumbar Smith-Peterson osteotomies: a biomechanical study. Spine J 15(3):506–513. https://doi.org/10.1016/j.spinee.2014.10.010
Bhatia NN, Lee KH, Bui CN et al (2012) Biomechanical evaluation of an expandable cage in single-segment posterior lumbar interbody fusion. Spine (Phila Pa 1976) 37(2):E79–85. https://doi.org/10.1097/BRS.0b013e3182226ba6
Emstad E, Del Monaco DC, Fielding LC et al (2015) The VariLift(®) interbody fusion system: expandable, standalone interbody fusion. Med Devices (Auckland, N.Z.) 8:219–230. https://doi.org/10.2147/MDER.S84715
Neely WF, Fichtel F, del Monaco DC et al (2016) Treatment of symptomatic lumbar disc degeneration with the VariLift-L Interbody fusion system: retrospective review of 470 cases. Int J Spine Surg 10:15. https://doi.org/10.14444/3015
Calvo-Echenique A, Cegoñino J, Perez del Palomar A (2019) Is there any advantage of using stand-alone cages? A numerical approach. Biomed Eng Online 18(1):63. https://doi.org/10.1186/s12938-019-0684-8
Thomé C, Klassen PD, Bouma GJ et al (2018) Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J 18(12):2278–2287. https://doi.org/10.1016/j.spinee.2018.05.003
Strenge KB, DiPaola CP, Miller LE et al (2019) Multicenter study of lumbar discectomy with Barricaid annular closure device for prevention of lumbar disc reherniation in US patients: a historically controlled post-market study protocol. Medicine 98(35):e16953
Kursumovic A, Bouma G, Miller L et al (2020) Clinical implications of vertebral endplate disruptions after lumbar discectomy: 3-year results from a randomized trial of a bone-anchored annular closure device. J Pain Res 13:669–675. https://doi.org/10.2147/JPR.S226480
Fan W, Guo L-X (2018) The role of posterior screw fixation in single-level transforaminal lumbar interbody fusion during whole body vibration: a finite element study. World Neurosurg 114:e1086–e1093. https://doi.org/10.1016/j.wneu.2018.03.150
Shirazi-Adl A, Ahmed AM, Shrivastava SC (1986) Mechanical response of a lumbar motion segment in axial torque alone and combined with compression. Spine (Phila Pa 1976) 11(9):914–927. https://doi.org/10.1097/00007632-198611000-00012
Morgan EF, Unnikrisnan GU, Hussein AI (2018) Bone Mechanical properties in healthy and diseased states. Annu Rev Biomed Eng 20:119–143. https://doi.org/10.1146/annurev-bioeng-062117-121139
Burnard JL, Parr WCH, Choy WJ et al (2020) 3D-printed spine surgery implants: a systematic review of the efficacy and clinical safety profile of patient-specific and off-the-shelf devices. Eur Spine J 29(6):1248–1260. https://doi.org/10.1007/s00586-019-06236-2
Kremer MA, Alferink J, Wynsma S et al (2019) Expandable spacers provide better functional outcomes than static spacers in minimally invasive transforaminal lumbar interbody fusion. J Spine Surg 5(3):315–319
Rijsbergen Mv, van Rietbergen B, Barthelemy V et al (2018) Comparison of patient-specific computational models vs. clinical follow-up, for adjacent segment disc degeneration and bone remodelling after spinal fusion. PLoS One 13(8):e0200899. https://doi.org/10.1371/journal.pone.0200899
Gao M, Lei W, Wu Z et al (2011) Biomechanical evaluation of fixation strength of conventional and expansive pedicle screws with or without calcium based cement augmentation. Clin Biomech (Bristol, Avon) 26(3):238–244. https://doi.org/10.1016/j.clinbiomech.2010.10.008
Acknowledgements
The first author is thankful for the Institute Fellowship provided by the Ministry of Education, Government of India, for his Ph.D. Research at Indian Institute of Engineering Science and Technology (IIEST) Shibpur, Howrah -711103, West Bengal, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pradeep, K., Pal, B. Biomechanical and clinical studies on lumbar spine fusion surgery: a review. Med Biol Eng Comput 61, 617–634 (2023). https://doi.org/10.1007/s11517-022-02750-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02750-6