Abstract
Vertebral compression fractures (VCFs) may result in a kyphotic deformity which can cause potential systemic complications secondary to respiratory and gastrointestinal dysfunction. The use of implants in the spine for VCF treatment represents a paradigm shift away from cement injection on its own, aiming to combine the analgesic and stabilizing effect of injecting cement into the vertebral body with vertebral height restoration and kyphotic angle correction. Spine implants which can be used for VCF treatment include stents, jacks, PEEK cages and fracture reduction systems. Lumbar spinal stenosis (LSS) with neurogenic intermittent claudication is one of the most commonly occurring spinal conditions, usually affecting people older than 50, which can cause disability and a reducted quality of life. Percutaneous interspinous spacers for the relief of symptoms caused by spinal stenosis can be used in patients who are not surgical candidates. The purpose of this article is to describe the basic concepts of spinal implantation in patients with VCF or spinal stenosis. The role of biomechanics and the different types of implants will be described. Controversies concerning techniques and products will be addressed. Finally, the necessity for an individually tailored approach for the use of different implants in different cases and anatomic locations will be emphasized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the global population ages, a growth in the prevalence of osteoporosis and associated vertebral fractures is expected in the near future [1]. Patients with vertebral compression fractures (VCFs) have a 40% lower survival rate after 8 years when compared to age-matched controls, and this increased risk of mortality is associated with physical frailty due to decreased physical function and resulting weight loss [2,3,4,5]. When compared to those with femoral fractures, patients with VCF have a significantly shorter survival [6]. Vertebral compression fractures may result in a kyphotic deformity which can cause potential systemic complications secondary to respiratory and gastrointestinal dysfunction [7].
In addition to pain and reduced mobility, the kyphosis is caused by VCFs and results in severe mechanical effects that lead to decreased thoracic and abdominal space, increased forces on the anterior vertebral bodies secondary to the anterior shift of the cranio-thoracic center of gravity and a compensatory stance to counter the kyphosis [8,9,10,11]. The clinical consequences of these mechanical effects include decreased pulmonary function, decreased appetite with resultant nutritional impact, frailty and increased future VCF risk as well as secondary chronic back pain due to constant paraspinal muscular contraction [8,9,10,11]. Persistent vertebral deformity has been shown to be associated with increased mortality and new fracture risk [12, 13]. Kyphotic reduction may be associated with optimal spinal alignment, paraspinal muscle relaxation, a more upright posture and reduced pain [14]. The subset analysis of radiologic surgical parameters in the free trial showed that patients with increased correction of kyphotic angle reported a significantly higher improvement in function [15]. Patients in this trial that had better restoration of their kyphotic angle had higher clinical benefits including improvements in pain, function and quality of life [15].
The use of implants in the spine for VCF treatment represents a paradigm shift away from cement injection on its own, aiming to combine the analgesic and stabilizing effect of injecting cement into the vertebral body with vertebral height restoration and kyphotic angle correction. Spine implants which can be used for VCF treatment include stents, jacks, PEEK cages and fracture reduction systems [16,17,18,19].
Lumbar spinal stenosis (LSS) with neurogenic intermittent claudication is one of the most commonly occurring spinal conditions, usually affecting people older than 50, which can cause significant disability and a reduced quality of life [20, 21]. This condition arises from narrowing of the lumbar spinal canal, the lateral recesses or the intervertebral neural foramina due to progressive hypertrophy of any of the surrounding osteocartilaginous and ligamentous elements and may result in compression of neural or vascular contents of the spinal canal at one or more levels.
The purpose of this article is to describe the basic concepts of spinal implantation in patients with VCF or spinal stenosis. The role of biomechanics and the different types of implants will be described. Controversies concerning techniques and products will be addressed. Finally, the necessity for an individually tailored approach for the use of different implants in different cases and anatomic locations will be emphasized.
Vertebral Fractures
Patient Selection
Percutaneous implantation in the spine for the treatment of VCFs should be considered in cases where vertebral height is lost; at present in the literature there is no clear criterion in terms of percentage of vertebral height loss at which insertion of a spinal implant is indicated [7]. Indications for implants in the spine include osteoporotic fractures, traumatic fractures (Magerl A1 to A4 types), primary or metastatic neoplastic vertebral lesions and multiple myeloma [7, 22]. The contraindications are similar to those for standard vertebroplasty and balloon kyphoplasty, including asymptomatic fractures, improvement post-bed rest and conservative therapy, infection, severe coagulopathy and severe cardiorespiratory disease [23,24,25].
Imaging evaluation (including standard x-rays, computed tomography and magnetic resonance imaging) performed prior to spine implantation should include assessments for spinal alignment, the presence of any rotation or translation, kyphotic angle, vertebral height loss, bone injury and edema [7, 24]. Spine implants can be placed under epidural anesthesia, sedation or general anesthesia depending on the spinal level and number of vertebrae to be treated [17,18,19,20, 24].
From a technical point of view, introduction of these devices is more complex than standard augmentation techniques and a learning curve should be anticipated for experience and optimal performance (Table 1). The working cannulas for spine implants are of larger diameter than the standard trocars used in vertebroplasty, and therefore, pedicular size is an important factor for a feasible and successful implantation. Furthermore, according to the manufacturer’s guidelines, all the implants are placed in pairs into the vertebral body through a bilateral extra- or transpedicular approach; an exception being the KIVA system which is the only one, according to the manufacturer’s guidelines, that is placed through a unilateral transpedicular approach.
Vertebral Body Stenting (VBS)
Vertebral body stenting (DePuy Synthes, Synthes GmbH, Switzerland) is a minimally invasive percutaneous treatment for vertebral body fractures using an expandable, intrasomatic, titanium device. The system consists of trocars (or guide wires), working sleeves, a drill and blunt plunger, vertebral body balloons with inflation devices and the vertebral body stents. The stents and balloons are available in three sizes; the stent size for a specific vertebral body can be selected based on the preoperative CT. The indications include painful osteoporotic vertebral compression fractures without posterior wall involvement, painful vertebral compression fractures types A1.1, A1.2, A1.3 and A3.1 according to the Magerl AO classification and osteolytic lesions located within the vertebral body with an intact cortical shell.
Vertebral body stenting is performed with the patient in the prone position. Under fluoroscopic control via a bilateral, extra- or transpedicular approach, trocars are inserted in order to create the pathway and position the instruments in a single step. At the time of access, it is important to plan to place the two stents symmetrically toward the midline. Working sleeves are inserted coaxially into the vertebral body, through which, first the drill and then the blunt plunger are inserted to create an access channel for the stents. (There are three grooves toward the distal tip of the plunger that correspond to the three stent lengths.) Once the access is created, a vertebral body balloon catheter of appropriate size is inserted through the working sleeve. (The catheter should be positioned according to the anticipated stent position.) An inflation system is used for bilateral and simultaneous dilatation, and the balloon catheters are then retrieved. The balloon is designed to re-expand the collapsed vertebral body until the height is restored and maintained by the stent (stentoplasty) [2]. The inflation system is connected to the vertebral body stent catheters which are inserted through the sleeves into the vertebral body and deployed. The balloon inflation and subsequent stent deployment create a well-defined cavity permitting the cement to be injected at low pressure [2, 26]. Catheters are then removed and PMMA is injected under real-time x-ray control; the stent is stabilized in situ with cement injection in, and around, the implant (Fig. 1), [2].
A 68-year-old female patient with painful osteoporotic fracture treated with vertebral body stenting. A Lateral fluoroscopy view during the deployment of the stents. B–D Lateral (B, C) and P-A (D) fluoroscopy views during the cement injection. E, F Lateral (E) and P-A (F) fluoroscopy views post-cement injection
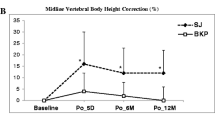
Biomechanical tests comparing stentoplasty to balloon kyphoplasty have demonstrated significant height restoration favoring stenting over standard balloon inflation alone; similarly with regards to pain reduction, clinical trials favored stentoplasty over kyphoplasty [20, 27,28,29,30]. Hartmann et al retrospectively reviewed 18 patients with traumatic thoracolumbar incomplete burst fractures treated with VBS and reported correction of vertebral kyphosis by 3.2° and segmental kyphosis correction by 5° [31]. A systematic search of databases concluded that stentoplasty is comparable to kyphoplasty in terms of kyphosis correction, radiation exposure time and postoperative cement migration; additionally, the technique was found comparable to vertebroplasty in terms of vertebral height restoration and injected bone cement volume [16]. On the other hand, Werner et al, in a two-armed randomized controlled trial between kyphoplasty and stentoplasty for fresh osteoporotic vertebral compression fractures concluded that there is no beneficial effect of vertebral body stenting over balloon kyphoplasty in terms of kyphotic correction, cement leakage, radiation exposure time and neurologic sequelae; however, this study reported significantly higher pressures during stentoplasty’s balloon inflation and more material-related complications [32]. At present there is no evidence in the literature to support the preferential use of VBS over standard vertebroplasty or balloon augmentation.
Osseofix
Osseofix (ATEC Spine Inc, Carlsbad CA, USA) is an expandable mesh made of titanium which is designed to expand inside the vertebral body and compact the surrounding trabecular bone, restoring vertebral body height and decreasing kyphotic deformity [7]. This implant is intended for compression fractures in the T6-L5 region; it acts as scaffold to facilitate the stabilization and reduction of spinal fractures, while at the same time, creation of bony channels allows for cement interdigitation within the cancellous bone. Osseofix implantation is performed with the patient placed in the prone position. Under fluoroscopy, targeting needles are inserted via both pedicles to the posterior edge of the vertebral body. With sequential exchanges, guide wires are inserted, the targeting needles are removed, and drill sleeves are introduced. A drill the diameter of which corresponds to the diameter of the non-expanded implant guide is inserted over the guide wire into the drill sleeve in order to create an access channel. After drill removal, the sleeve is replaced by the working cannula of the insertion apparatus; the non-expanded implant is inserted, the guide wire removed, and the Osseofix is expanded. (The system has a stop mechanism to prevent excessive expansion.) After removal of the insertion apparatus, cement is injected though the working cannula (Fig. 2).
Upasani et al performed a biomechanical comparison of Osseofix to balloon kyphoplasty reporting that the titanium mesh provided greater height maintenance with a smaller volume of injected cement [33]. Furthermore, Ghofrani et al, performing an in vitro biomechanical investigation with human cadaveric vertebral bodies compared kyphoplasty and titanium mesh with or without cement, concluded that based on the biomechanical data, the titanium mesh with or without cement was similar to balloon kyphoplasty [34].
In the clinical setting, Ender et al used Osseofix to stabilize incomplete osteoporotic thoracolumbar burst fractures in 15 patients, reporting significant pain reduction and radiologic improvement of Cobb’s angle [35]. Eschler et al. combined titanium mesh cages and a transpedicular fixation system with expandable screws for the treatment of unstable thoracolumbar burst fractures in the elderly, reporting substantial pain relief, adequate maintenance of reduction and a low complication rate after cementless fixation [36]. Finally, in a prospective study, Osseofix was used in 32 consecutive patients (46 symptomatic osteoporotic or tumorous fractures) with significant pain reduction, Oswestry Disability Index (ODI) score reduction and kyphotic angle improvement at 12-month follow-up [1]. Pua et al reported a unipedicular approach in 7 patients with satisfactory technical efficacy and pain reduction, concluding that this approach might have the potential for use in the upper thoracic and lower cervical spine levels [37].
Jack Dilators
The Jack dilators (VEXIM Stryker, Balma/France) consist of a rotator hilt, a handle, a connecting tube and the head of the device [38]. These dilators create a parasagittal vertical cleft extending across the entire vertebral height which is then filled with cement, thus providing height restoration through a laminar strut. They are deployed in a controlled cranio-caudal direction, resulting in fracture reduction, with preservation of the surrounding bony trabeculae [17]. Once the fracture has been reduced, the Jack dilator maintains the restoration of the fracture and cement is injected for internal fixation. Indications include mobile spinal fractures that may result from osteoporosis, trauma fractures types A according to the Magerl classification and malignant myeloma or osteolytic lesions.
With the patient prone, under fluoroscopic control and transpedicular access a trocar is used to determine the path to the vertebral body and to optimally position the implant. With sequential exchanges, a guide wire is inserted, the trocar is removed, and a reamer/working cannula is introduced, and drilled into the vertebral body. The implant’s site is then cleaned with a template and a cannula plug is inserted aiming to stop any potential bleeding; this cannula plug has a radiopaque marker which visualizes the implant’s depth in order to position the contralateral implant accordingly. An implant expander is then inserted into each prepared path, and cement is injected via cement pushers placed inside the expander (Fig. 3).
An 81-year-old male patient with painful traumatic fracture treated with the SpineJack system 7 days post-injury. A CT scan sagittal reconstruction of the T12 vertebral fracture. B, C Lateral (B) and P-A (C) fluoroscopy view during the deployment of the SpineJack system. D, E P-A (D) and lateral (E) fluoroscopy view post-cement injection. F CT scan sagittal reconstruction of the T12 vertebral fracture post-vertebral augmentation
Sietsma et al performed a controlled in vitro biomechanical evaluation comparing SpineJack to balloon kyphoplasty; both procedures restored height, while strength and stiffness were partially restored without any significant differences; however, less cement was used in the SpineJack arm [17]. A retrospective review of 218 patients (236 levels treated) with osteoporotic VCFs reported significant pain reduction, ODI score reduction, increase of anterior and central body height and correction of Cobb’s angle with cement leakages noted only in 12/218 patients [38]. Li et al in a retrospective review of 16 patients with solitary thoracolumbar osteoporotic VCFs reported long-term pain relief and vertebral body height—spinal alignment restoration [39]. In a recent article comparing balloon kyphoplasty and the SpineJack, Noriega et al reported that SpineJack has a shorter intervention time, produced comparable improvements in pain and function and had a statistically significant improvement in vertebral height and vertebral body angle [40].
KIVA System
The KIVA system (Benvenue Medical, Santa Clara, CA/USA) consists of a PEEK-OPTIMA spiraled coiled implant loaded with 15% barium sulfate rendering it visible under fluoroscopy [19]. It is indicated for the reduction and treatment of spinal fractures in the thoracic and/or lumbar spine from T6-L5. The KIVA system is inserted with the patient in the prone position under fluoroscopic guidance; it is the only implant which, according to the manufacturer’s guidelines, can be placed with a unilateral transpedicular approach. A trocar is inserted through the pedicle to the vertebral body’s posterior wall. A guide pin is coaxially inserted and over the pin the working cannula is introduced into the vertebral body. Through the working cannula, a nitinol coil is deployed over which the PEEK polymer cage is introduced. After removal of the nitinol coil the peek cage is filled with cement (Fig. 4).
A T1 weighted, sequence at sagittal level illustrating low signal intensity at an osteoporotic L3 vertebral fracture B, C lateral (B) and P-A (C) fluoroscopy view during the deployment of the KIVA system inside the vertebral body. D Computed tomography axial scan post-cage deployment and cement injection
Korovessis et al. compared KIVA to balloon kyphoplasty in a randomized trial of 168 patients reporting significant Gardner angle reduction and lower cement leakage rates in the KIVA arm [41]. Anselmeti et al reported the first use of the implant in a neoplastic setting and later prospectively evaluated 40 patients with painful spinal malignancy treated with the KIVA system reporting median pain reduction of 9 Visual Analogue Scale units [42, 43]. Korovessis et al. compared KIVA to balloon kyphoplasty in cancer patients with osteolytic spinal metastases, reporting no difference in pain relief; however, in the KIVA arm, there was no cement leakage [44]. The KAST (Kiva Safety and Effectiveness Trial) study was a pivotal, multicenter, randomized control trial that successfully established non-inferiority of the KIVA system against balloon kyphoplasty for the treatment of osteoporotic VCFs in terms of pain reduction, function improvement and device-related severe complications [18]. An economic analysis of the KAST study concluded that the KIVA system in a hospital setting is cost-saving over balloon kyphoplasty, mainly due to a reduced risk of the development of adjacent level fractures [45]. Otten et al. compared the KIVA system to balloon kyphoplasty with matched pairs (26 patients for each procedure), reporting significantly better pain improvement at 6-month follow-up and lower mean operation time in the KIVA arm, with similar vertebral height restoration but with statistically significantly fewer new fractures after vertebral augmentation [46].
Percutaneous Vertebral Implants
With current evidence it is clear that percutaneous vertebroplasty and balloon kyphoplasty are more efficient than conservative therapy for the management of painful fractures, prolonging survival and preventing morbidity in these patients [23]. The disadvantage of these implants is their significantly higher cost when compared to that of standard vertebroplasty or balloon kyphoplasty. Although only approximately one third of VCF patients are treated with implants, their costs constitute 70% of the total budget for vertebral augmentation [7, 47, 48]. Currently, there is no evidence to support the preferential use of one device over the others nor over kyphoplasty or vertebroplasty [7, 17, 19, 20, 23, 24].
Percutaneous Interspinous Spacers for Spinal Stenosis
Diagnosis of spinal stenosis is achieved by means of clinical history and examination as well as the use of MRI or CT. Electromyography is used to confirm the presence of radiculopathy, differentiating it from neuropathy.
If conservative therapy fails, surgical decompression results in similar outcomes to earlier intervention [49]. However, surgery typically requires general anesthesia, and thus, it may not always be feasible in the growing elderly population with neurogenic intermittent claudication (NIC) [50].
Recently, interspinous implants, requiring minimal open surgery, have been proposed in Table 2 [51, 52]. Early small-randomized controlled trials showed favorable outcomes at 4 years in patients with NIC treated with the X-Stop IPS compared to conservative therapy [53, 54]. Subsequent clinical series are characterized by more variable clinical outcome rates and higher reoperation rates [54, 55]. More recently, totally percutaneous devices have been introduced (Fig. 5) [56, 57].
Percutaneous interspinous spacer at L4–L5 level (Lobster®, TechlaMed Florence/Italy). A Introduction of the first access needle. B Introduction of the dilators. C The largest dilator with the two tips properly positioned between the spinous process. D The 8-mm probe is introduced through the dilator between the spinous process. E–G Once the size of the probe properly fits, the corresponding size device is then introduced. H The spacer is kept on place by opening the wings. I–J Correct position of the delivered implant in AP and lateral view
The percutaneous procedure is performed under local anesthesia and mild sedation. Under AP and lateral fluoroscopy, a series of sizing trocars (typically 8-10-12-14-16mm devices) are introduced and advanced toward the interspinous space; allowing selection of the most appropriate device size to achieve optimal decompression. When the correct size access needle has been identified, it is removed and a corresponding measurement device introduced. The cylindrical implant is positioned and then superior and inferior wings deployed to clasp the spinous processes above and below in order to fix it securely in place [57]. Several non-randomized prospective studies have assessed the efficacy of totally percutaneous interspinous spacers, demonstrating pain relief, reduced disability and significant increases in spinal canal and foraminal cross-sectional areas at the treated level [57,58,59,60,61].
On the other hand, a 2-year double-blind randomized controlled trial comparing interspinous process devices versus conventional surgical decompression concluded that there was no confirmation of any device advantage over surgery; moreover in the device treatment arm reoperation rate was higher and back pain was slightly more intense compared to the decompression treatment arm [62]. Furthermore, a cost-utility analysis of interspinous process devices versus conventional surgical decompression for lumbar spinal stenosis concluded that indirect device-mediated decompression is highly unlikely to be cost-effective compared with bony decompression in this group of patients with intermittent neurogenic claudication [63].
A meta-analysis evaluating the efficacy and safety of interspinous devices compared with open decompression surgery in treating lumbar spinal stenosis concluded that the device group was governed by better effects (in terms of perioperative blood loss, hospitalization time and operation time which were shorter/lower) and a lower postoperative complication rate than the surgical group; however, due to a higher reoperation rate in the percutaneously treated patients, the meta-analysis failed to conclude that the devices could replace surgery as the gold standard, but may be a viable alternative in treating lumbar spinal stenosis [64]. A systematic review and meta-analysis of 19 published trials (17) concerning surgical effectiveness for lumbar spinal stenosis concluded that interspinous devices are slightly more effective than decompression plus fusion for disability but that they resulted in significantly higher reoperation rates when compared to decompression alone [65]. A systematic review of the Cochrane database for lumbar spinal stenosis surgical options including 5 trials (737 participants) concerning interspinous spacer versus lumbar decompression with or without fusion reports moderate quality evidence showing no superior benefit of one technique over the other in terms of pain reduction and life quality improvement [66].
Conclusion
Vertebral compression fractures and kyphosis are associated with increased mortality and new fracture risk. The use of implants in the spine for the treatment of vertebral compression fractures is a paradigm shift away from the injection of cement on its own. Implants aim to restore vertebral height and improve the kyphotic angle. In cases with moderate to severe vertebral height reduction, the use of implants to treat fractures can provide long-term restoration and correction of kyphosis. Biomechanical and clinical comparative studies versus standard augmentation (i.e., percutaneous vertebroplasty or balloon kyphoplasty) thus far reported non-inferiority of spine implants with a reduced volume of injected cement; however, thus far there are no studies demonstrating superiority of one device over the other.
Comparison of percutaneous interspinous spacers with conventional surgical techniques (bony decompression with or without fusion) reports non-inferiority in terms of pain reduction and life quality improvement with a lower postoperative complication rate and hospitalization duration, however, at a higher financial cost. In addition, there is a higher reoperation rate in the percutaneously treated patients. Percutaneous interspinous spacers for the relief of symptoms caused by spinal stenosis can be an attractive and viable alternative in patients who are not surgical candidates; however, they cannot yet be considered the primary treatment.
References
Ender SA, Gradl G, Ender M, Langner S, Merk HR, Kayser R. Osseofix® system for percutaneous stabilization of osteoporotic and tumorous vertebral compression fractures - clinical and radiological results after 12 months. Rofo. 2014;186(4):380–7.
Heini PF, Teuscher R. Vertebral body stenting / stentoplasty. Swiss Med Wkly. 2012;142:w13658.
Lau E, Ong K, Kurtz S, et al. Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am. 2008;90(7):1479–86.
Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporosis Int. 2000;11(7):556–61.
Kado DM, Duong T, Stone KL, et al. Incident vertebral fractures and mortality in older women: A prospective study. Osteoporosis Int. 2003;14(7):589–94.
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556–61.
Muto M, Marcia S, Guarnieri G, Pereira V. Assisted techniques for vertebral cementoplasty: Why should we do it? Eur J Radiol. 2015;84(5):783–8.
Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, Ziegler R, Leidig-Bruckner G. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8(3):261–7.
Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int. 2005;16(8):1004–10.
Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27–31.
Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR, Study of Osteoporotic Fractures. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med. 2009;150(10):681–7.
Hasserius R, Karlsson MK, Nilsson BE, et al. Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int. 2003;14:61–8.
Delmas PD, Genant HK, Crans GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: Results from the MORE trial. Bone. 2003;33:522–32.
Papanastassiou ID, Phillips FM, Van Meirhaeghe J, et al. Comparing effects of kyphoplasty, vertebroplasty, and non-surgical management in a systematic review of randomized and non-randomized controlled studies. Eur Spine J. 2012;21(9):1826–43.
Van Meirhaeghe J, Bastian L, Boonen S, Ranstam J, Tillman JB, Wardlaw D. on behalf of the FREE investigators: a randomized trial of balloon kyphoplasty and non-surgical management for treating acute vertebral compression fractures: Vertebral body kyphosis correction and surgical parameters. Spine. 2013;38:971–83.
Martín-López JE, Pavón-Gómez MJ, Romero-Tabares A, Molina-López T. Stentoplasty effectiveness and safety for the treatment of osteoporotic vertebral fractures: a systematic review. Orthop Traumatol Surg Res. 2015;101(5):627–32.
Sietsma MS, Hosman AJ, Verdonschot NJ, Aalsma AM, Veldhuizen AG. Biomechanical evaluation of the vertebral jack tool and the inflatable bone tamp for reduction of osteoporotic spine fractures. Spine. 2009;34(18):E640–4.
Tutton SM, Pflugmacher R, Davidian M, Beall DP, Facchini FR, Garfin SR. KAST Study: The Kiva system as a vertebral augmentation treatment-a safety and effectiveness trial: a randomized, noninferiority trial comparing the kiva system with balloon Kyphoplasty in treatment of osteoporotic vertebral compression fractures. Spine. 2015;40(12):865–75.
Ender SA, Wetterau E, Ender M, Kühn JP, Merk HR, Kayser R. Percutaneous stabilization system osseofix® for treatment of osteoporotic vertebral compression fractures - clinical and radiological results after 12 months. PLoS ONE. 2013;8(6):e65119.
Kalichman L, Cole R, Kim DH, Li L, Suri P, Guermazi A, et al. Spinal stenosis prevalence and association with symptoms: The Framingham study. The Spine Journal. 2009;9:545–50.
Battie MC, Jones CA, Schopflocher DP, Hu RW. Health-related quality of life and comorbidities associated with lumbar spinal stenosis. The Spine Journal. 2012;12:189–95.
Vanni D, Galzio R, Kazakova A, Pantalone A, Grillea G, Bartolo M, Salini V, Magliani V. Third-generation percutaneous vertebral augmentation systems. J Spine Surg. 2016;2(1):13–20.
Filippiadis DK, Marcia S, Masala S, Deschamps F, Kelekis A. Percutaneous Vertebroplasty and Kyphoplasty: current status, new developments and old controversies. Cardiovasc Intervent Radiol. 2017. https://doi.org/10.1007/s00270-017-1779-x.
Tsoumakidou G, Too CW, Koch G, Caudrelier J, Cazzato RL, Garnon J, et al. CIRSE Guidelines on Percutaneous Vertebral Augmentation. Cardiovasc Intervent Radiol. 2017;40(3):331–42.
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40(8):1141–6.
Aparisi F. Vertebroplasty and kyphoplasty in vertebral osteoporotic fractures. Semin Musculoskelet Radiol. 2016;20(4):382–91.
Fürderer S, Anders M, Schwindling B, et al. Vertebral body stenting. A method for repositioning and augmenting vertebral compression fractures. Orthopade. 2002;31:356–61.
Klezl Z, Majeed H, Bommireddy R, et al. Early results after vertebral body stenting for fractures of the anterior column of the thoracolumbar spine. Injury. 2011;42:1038–42.
Diel P, Röder C, Perler G, et al. Radiographic and safety details of vertebral body stenting: results from a multicenter chart review. BMC Musculoskelet Disord. 2013;14:233.
Werner CM, Osterhoff G, Schlickeiser J, et al. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am. 2013;95:577–84.
Hartmann F, Griese M, Dietz SO, Kuhn S, Rommens PM, Gercek E. Two-year results of vertebral body stenting for the treatment of traumatic incomplete burst fractures. Minim Invasive Ther Allied Technol. 2015;24(3):161–6.
Werner CM, Osterhoff G, Schlickeiser J, Jenni R, Wanner GA, Ossendorf C, Simmen HP. Vertebral body stenting versus kyphoplasty for the treatment of osteoporotic vertebral compression fractures: a randomized trial. J Bone Joint Surg Am. 2013;95(7):577–84.
Upasani VV, Robertson C, Lee D, Tomlinson T, Mahar AT. Biomechanical comparison of kyphoplasty versus a titanium mesh implant with cement for stabilization of vertebral compression fractures. Spine. 2010;35(19):1783–8.
Ghofrani H, Nunn T, Robertson C, Mahar A, Lee Y, Garfin S. An evaluation of fracture stabilization comparing kyphoplasty and titanium mesh repair techniques for vertebral compression fractures: is bone cement necessary? Spine. 2010;35(16):E768–73.
Ender SA, Eschler A, Ender M, Merk HR, Kayser R. Fracture care using percutaneously applied titanium mesh cages (OsseoFix®) for unstable osteoporotic thoracolumbar burst fractures is able to reduce cement-associated complications–results after 12 months. J Orthop Surg Res. 2015;10:175.
Eschler A, Ender SA, Schiml K, Mittlmeier T, Gradl G. Bony healing of unstable thoracolumbar burst fractures in the elderly using percutaneously applied titanium mesh cages and a transpedicular fixation system with expandable screws. PLoS ONE. 2015;10(2):e0117122.
Pua U, Quek LH, Ng LC. Central stentoplasty: technique for unipedicular single midline vertebral body stent implantation. Cardiovasc Intervent Radiol. 2014;37(3):810–4.
Fan J, Shen Y, Zhang N, Ren Y, Cai W, Yu L, Wu N, Yin G. Evaluation of surgical outcome of Jack vertebral dilator kyphoplasty for osteoporotic vertebral compression fracture-clinical experience of 218 cases. J Orthop Surg Res. 2016;11(1):56.
Li D, Huang Y, Yang H, Chen Q, Sun T, Wu Y, Li X. Jack vertebral dilator kyphoplasty for treatment of osteoporotic vertebral compression fractures. Eur J Orthop Surg Traumatol. 2014;24(1):15–21.
Noriega DC, Ramajo RH, Lite IS, Toribio B, Corredera R, Ardura F, Krüger A. Safety and clinical performance of kyphoplasty and SpineJack(®) procedures in the treatment of osteoporotic vertebral compression fractures: a pilot, monocentric, investigator-initiated study. Osteoporos Int. 2016;27(6):2047–55.
Korovessis P, Vardakastanis K, Repantis T, Vitsas V. Balloon kyphoplasty versus KIVA vertebral augmentation–comparison of 2 techniques for osteoporotic vertebral body fractures: a prospective randomized study. Spine. 2013;38(4):292–9.
Anselmetti GC, Tutton SM, Facchini FR, Miller LE, Block JE. Percutaneous vertebral augmentation for painful osteolytic vertebral metastasis: a case report. Int Med Case Rep J. 2012;5:13–7.
Anselmetti GC, Manca A, Tutton S, Chiara G, Kelekis A, Facchini FR, Russo F, Regge D, Montemurro F. Percutaneous vertebral augmentation assisted by PEEK implant in painful osteolytic vertebral metastasis involving the vertebral wall: experience on 40 patients. Pain Physician. 2013;16(4):E397–404.
Korovessis P, Vardakastanis K, Vitsas V, Syrimpeis V. Is Kiva implant advantageous to balloon kyphoplasty in treating osteolytic metastasis to the spine? Comparison of 2 percutaneous minimal invasive spine techniques: a prospective randomized controlled short-term study. Spine. 2014; 39(4): E231-9.
Beall DP, Olan WJ, Kakad P, Li Q, Hornberger J. Economic Analysis of Kiva VCF Treatment System Compared to Balloon Kyphoplasty Using Randomized Kiva Safety and Effectiveness Trial (KAST) Data. Pain Physician. 2015;18(3):E299–306.
Otten LA, Bornemnn R, Jansen TR, Kabir K, Pennekamp PH, Wirtz DC, Stuwe B, Pflugmacher R. Comparison of balloon kyphoplasty with the new Kiva® VCF system for the treatment of vertebral compression fractures. Pain Physician. 2013;16(5):E505–12.
Guglielmi G, Andreula C, Muto M, Gilula LA. Percutaneous vertebroplasty: indications, contraindications, technique, and complications. Acta Radiol. 2005;46(3):256–68.
Muto M, Perrotta V, Guarnieri G, et al. Vertebroplasty and kyphoplasty: friends or foes? Radiol Med. 2008;113(8):1171–84.
Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis: Conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424–35 discussion 1435–1426.
Kondrashov DG, Hannibal M, Hsu KY, Zucherman JF. Interspinous process decompression with the x-stop device for lumbar spinal stenosis: A 4-year follow-up study. J Spinal Disord Tech. 2006;19:323–7.
Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20(3):255–61.
Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30(12):1351–8.
Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: Application of the x stop device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463–71.
Epstein NE. A review of interspinous fusion devices: High complication, reoperation rates, and costs with poor outcomes. Surgical neurology international. 2012;3:7.
Stromqvist BH, Berg S, Gerdhem P, Johnsson R, Moller A, Sahlstrand T, et al. X-stop versus decompressive surgery for lumbar neurogenic intermittent claudication: Randomized controlled trial with 2-year follow-up. Spine. 2013;38:1436–42.
Nardi P, Cabezas D, Rea G, Pettorini BL. Aperius PercLID stand alone interspinous system for the treatment of degenerative lumbar stenosis: experience on 152 cases. J Spinal Disord Tech. 2010;23(3):203–7.
Marcia S, Hirsch JA, Chandra RV, Marras M, Piras E, Anselmetti GC, et al. Midterm Clinical and Radiologic Outcomes after Percutaneous Interspinous Spacer Treatment for Neurogenic Intermittent Claudication. J Vasc Interv Radiol. 2015; 26(11): 1687-93.e1-2.
Fabrizi AP, Maina R, Schiabello L. Interspinous spacers in the treatment of degenerative lumbar spinal disease: our experience with DIAM and Aperius devices. Eur Spine J. 2011;20(Suppl 1):S20–6.
Menchetti PP, Postacchini F, Bini W, Canero G. Percutaneous surgical treatment in lumbar spinal stenosis with Aperius-PercLID: indications, surgical technique and results. Acta Neurochir Suppl. 2011;108:183–6.
Galarza M, Fabrizi AP, Maina R, Gazzeri R, Martinez-Lage JF. Degenerative lumbar spinal stenosis with neurogenic intermittent claudication and treatment with the Aperius PercLID System: a preliminary report. Neurosurg Focus. 2010;28(6):E3.
Masala S, Marcia S, Taglieri A, Chiaravalloti A, Calabria E, Raguso M, et al. Degenerative lumbar spinal stenosis treatment with Aperius™ PerCLID™ system and Falena® interspinous spacers: 1-year follow-up of clinical outcome and quality of life. Interv Neuroradiol. 2016;22(2):217–26.
Moojen WA, Arts MP, Jacobs WC, van Zwet EW, van den Akker-van Marle ME, Koes BW, Leggeert-Lankamp CL, Peul WC, Leiden The Hague Spine Intervention Prognostic Study Group (SIPS). IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2-year results of a double-blind randomized controlled trial. Eur Spine J. 2015;24(10):2295–305.
van den Akker-van Marle ME, Moojen WA, Arts MP, Vleggeert-Lankamp CL, Peul WC, Leiden-The Hague Spine Intervention Prognostic Study Group (SIPS). Interspinous process devices versus standard conventional surgical decompression for lumbar spinal stenosis: cost-utility analysis. Spine J. 2016;16(6):702–10.
Hong P, Liu Y, Li H. Comparison of the efficacy and safety between interspinous process distraction device and open decompression surgery in treating lumbar spinal stenosis: a meta analysis. J. Invest. Surg. 2014;28:1–10.
Machado GC, Ferreira PH, Harris IA, Pinheiro MB, Koes BW, van Tulder M, Rzewuska M, Maher CG, Ferreira ML. Effectiveness of surgery for lumbar spinal stenosis: a systematic review and meta-analysis. PLoS ONE. 2015;10(3):e0122800.
Machado GC, Ferreira PH, Yoo RI, Harris IA, Pinheiro MB, Koes BW, van Tulder MW, Rzewuska M, Maher CG, Ferreira ML. Surgical options for lumbar spinal stenosis. Cochrane Database Syst Rev. 2016;11:CD012421.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest to declare
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Filippiadis, D.K., Marcia, S., Ryan, A. et al. New Implant-Based Technologies in the Spine. Cardiovasc Intervent Radiol 41, 1463–1473 (2018). https://doi.org/10.1007/s00270-018-1987-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-1987-z