Abstract
To understand the fundamental physical properties of calcium maltobionate (MBCa), its water sorption isotherm, glass transition temperature (T g), and viscosity (η) were investigated and compared with those of maltobionic acid (MBH) and maltose. Although amorphous maltose crystalized at water activity (a w) higher than 0.43, MBCa and MBH maintained an amorphous state over the whole a w range. In addition, MBCa had a higher T g and greater resistance to water plasticizing than MBH and maltose. These properties of MBCa likely originate from the strong interaction between MBCa and water induced by electrostatic interactions. Moreover, the effects of temperature and water content on η of an aqueous MBCa solution were evaluated, and its behavior was described using a semi-empirical approach based on a combination of T g extrapolated by the Gordon-Taylor equation and a non-Arrhenius formula known as the Vogel–Fulcher–Tammann equation. This result will be useful for understating the effect of MBCa addition on the solution’s properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maltobionic acid (MBH) is a type of aldonic acid in which glucose and gluconic acid are linked via an α-1,4-glycosidic bond; it is obtained from the chemical or enzymatic oxidation of maltose [1, 2]. MBH has antioxidant/chelation, barrier strengthening, and moisturizing effects, and it is commonly used in cosmetics products [3]. Moreover, maltobionate can form various types of salts depending on the cation used. Among those, calcium maltobionate (MBCa) is expected to be an effective calcium soluble material for use in pharmaceutical and food products because of its high water solubility and mild bitter taste.
MBCa powder is amorphous and thus readily adsorbs moisture and aggregates during processing and preservation. It is known that aggregation of an amorphous powder can be explained via glass transition effects [4, 5]. At a temperature below the glass transition temperature (T g), the amorphous powder is in a glassy state and behaves as a physically stable powder. Above T g, the amorphous powder is in a rubbery state. As rubbery materials have viscoelastic properties, the fluidity of the powder is lost and it aggregates into a solid. T g of hydrophilic amorphous materials decreases with increasing water content because of water plasticizing [6–12]. Therefore, glass transition can occur in an amorphous powder held at a constant temperature when its water content is increased by water sorption.
When MBCa is used as an aqueous solution, viscosity (η) is treated as an important physical parameter for drying operation [13, 14] and quality control of liquid food [15, 16]. Similar to T g, η of an aqueous solution depends on temperature and water content [17]. The effect of temperature on η of a solution can be described by Arrhenius formula in a limited temperature range; however, it deviates from Arrhenius behavior at low temperatures near T g [18]. To understand the effect of temperature on η of solutions over the whole temperature range, a non-Arrhenius formulation that is applicable around T g has often been employed [17–21]. These results suggest that the effect of water content on η of solutions will also correspond to the T g curve.
As mentioned above, water sorption, glass transition, and viscosity are practically important parameters for amorphous hydrophilic materials. Although there have been many studies on the subject, MBCa has not yet been investigated to the best of our knowledge. The purpose of this study thus was to understand the water sorption isotherm (water content versus a w) and the T g-curve (T g versus water content) of the MBCa powder, and the effects of temperature and water content on η of MBCa aqueous solutions. For comparison, water sorption isotherm and the T g-curve of amorphous MBH and maltose were also investigated.
Materials and Methods
Sample Preparation
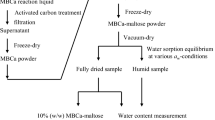
For the preparation of MBH and MBCa, reagents (analytical grade) of maltose monohydrate, calcium carbonate, catalase, and glucose oxidase were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan) and/or Amano Enzyme Inc. (Aichi, Japan). A 30 % (w/v) maltose solution (1000 mL) was reacted with 900 U of glucose oxidase, 60,000 U of catalase, and 55 g of calcium carbonate at 35 °C with aeration (1 L/min) and agitation (500 rpm). For MBCa purification, an activated carbon treatment at 80 °C for 30 min was carried out, and then the active carbon and enzymes were removed using vacuum filtration with Whatman glass microfiber filters, Grade GF/F, and membrane filters with a mixed cellulose ester pore size of 0.2 μm (Whatman Inc., USA). For MBH purification, the MBCa solution was desalted by cation exchange resin (Dowex88, Dow Chemical Company, USA) and decolorized mixed bed of anion (WA30, Mitsubishi Chemicals, Tokyo) and cation-exchange resins (Dowex88, Dow Chemical Company, USA). The eluate was concentrated in a rotary evaporator (RE1-D, AGC TECHNO GLASS CO., LTD. Japan). It was preliminarily confirmed that the purity of the MBH and MBCa solutions was more than 99 % by HPLC. In order to obtain freeze-dried maltose, MBH, and MBCa, a 10 % (w/w) solution (10 mL) was prepared in a glass vial and then placed in a freezer. The frozen preparation was set on the pre-cooled stage of the freeze-drier and vacuumed at a pressure below 70 Pa while increasing the temperature from −35 °C to 5 °C over a period of 2.5 days. The obtained samples were hermetically stored in the vial.
Isothermal Water Sorption
The MBCa, MBH, and maltose samples were vacuum-dried at 80 °C for 8 h; the fully dried samples were then held under various water activity (a w) conditions at 25 °C. a w was adjusted with saturated salts: LiCl (a w = 0.11), CH3COOK (a w = 0.23), MgCl2 (a w = 0.33), K2CO3 (a w = 0.43), Mg(NO3)2 (a w = 0.53), NaBr (a w = 0.58), NaCl (a w = 0.75), KCl (a w = 0.84), and KNO3 (a w = 0.94). The equilibrium of water sorption was confirmed gravimetrically. A portion of each sample was used for DSC measurements. The remaining portions of each sample were used to measure the water content. Hydrated samples were dehydrated at 105 °C for 16 h, and their water content was evaluated gravimetrically. The measurements were performed in duplicate, and the results were averaged.
DSC Measurement
Glass transition of the MBCa, MBH, and maltose samples was investigated using DSC (DSC120; Seiko Instruments Inc., Tokyo, Japan). The water content of the samples was adjusted under various a w conditions as mentioned above. Alumina was used as a reference, and the temperature and heat flow were calibrated using indium and distilled water.
The sample (4–10 mg) was placed in an aluminum pan and then hermetically sealed. To evaluate anhydrous T g of MBCa, MBH and maltose, the samples were placed into the DSC pan and held at 105 °C for 1–6 h and then hermetically sealed [22]. DSC measurements were conducted at 5 °C/min in a temperature range between 0 °C and 180 °C. Glass transition is observed as an endothermic shift in a DSC thermogram. However, glass transition observed in the first scan shows an endothermic peak because of the enthalpy relaxation effect depending on the thermal history of glassy samples; the T g value may be affected by the enthalpy relaxation effect to various degrees. To cancel the thermal history of glassy samples on T g, DSC measurements were conducted again after the first scan and then T g was evaluated from the onset point of the endothermic shift observed in the second scan [22–24]. The T g measurements were performed in duplicate, and the results were averaged.
Viscosity
MBCa was directly mixed with distilled water in the glassy vial, and 10 %–60 % (w/w) MBCa aqueous solution samples were prepared. Viscosity was investigated using viscometer (Haake Mars III system; Thermo Fisher Scientific Inc., Kanagawa, Japan). A corn plate (φ = 60 mm, 1°) was employed, and the shear rate (1/s) was investigated by shear stress of up to 100 Pa. The temperature was adjusted between −5 °C and 45 °C. The measurements were performed in triplicate, and the results were averaged.
Results and Discussion
Water Sorption Isotherm
Water sorption isotherms (25 °C) for MBCa, MBH, and maltose are shown in Fig. 1. The water content of MBCa and MBH increased with a w, and the behavior showed a sigmoidal curve over the whole a w range. Amorphous maltose, on the other hand, showed an abrupt decrease of water content at a w > 0.43. This indicates that amorphous maltose crystallizes during water sorption in a similar fashion to amorphous lactose [25]. From the result, it is noted that MBCa and MBH are more stable amorphous material than maltose. The solid lines for MBCa, MBH, and maltose were obtained by fitting the Brunauer–Emmett–Teller (BET) equation (Eq. 1) and the Guggenheim-Anderson-de Boer (GAB) equation (Eq. 2) to the sorption data, respectively.
where W, W m, C, and K are the moisture content (g-H2O/100 g-DM), the moisture content of the monolayer (g-H2O/100 g-DM), a factor correcting the sorption properties of the first layer with respect to the bulk liquid, and a factor correcting the properties of the multilayer with respect to the bulk liquid, respectively [26, 27]. For K = 1, the GAB equation is equivalent to the BET equation. Eq. 1 and Eq. 2 have been used for the water sorption analysis up to a w = 0.45 and for the whole range (a w = 0–1), respectively [28–32]. Maltose was analyzed using Eq. 1 because crystallization occurred at a higher a w than 0.43. On the contrary, MBCa and MBH were analyzed using both of Eq.1 (a w ≤ 0.43) and Eq. 2 (a w ≤ 0.94) to understand the water sorption behavior. The obtained parameters (W m, C, and K) are listed in Table 1. The W m and C values of BET were slightly different from those of GAB, because their parameters were affected by K value of GAB [32]. GAB gave better fitting results (R 2 > 0.952) than BET did (R 2 > 0.662). In the comparison of GAB parameters between MBCa and MBH, there were minor differences of W m and K values. It is noted that MBCa has the highest C among them. This is likely related to the electrolytic dissociation of MBCa; ionized groups hydrate strongly [33] and form hydration shells with water molecules; further, calcium ions can interact with six water molecules [34].
T g-curve
Typical DSC thermograms (second scan) for the MBCa samples are shown in Fig. 2. The MBCa samples showed an endothermic shift owing to glass transition, and the T g value was evaluated from the onset point of the shift. The anhydrous sample (a w = 0) showed an exothermic peak at a higher temperature than T g. This originated from the thermal decomposition of MBCa.
The obtained T g was plotted against water content (g-H2O/100 g-DM) as shown in Fig. 3. As expected, T g decreased with an increase in water content. The solid line in Fig. 3 was obtained by fitting the Gordon-Taylor (GT) equation (Eq. 3) to the T g data,
where T gs (K) and T gw (K) are the T g values of the anhydrous solute (MBCa, MBH, and maltose) and water, W s and W w are the mass fractions of the solute and water, and k is a constant depending on the system. The k value indicates the sensitivity to water plasticizing [8]; the higher the k value, the greater the water content dependence of T g. Both T g and T gs were determined experimentally. T gw was set to 136 K as determined in previous publications [35, 36], and k was obtained as a fitted parameter. The GT parameters (T gs and k) are also listed in Table 1. From these results, glass transition induced by water sorption at a constant temperature could be evaluated. From Fig. 3, the water content at which MBCa, MBH, and maltose showed T g = 25 °C (critical water content) was determined to be 13.3 g-H2O/100 g-DM, 6.41 g-H2O/100 g-DM, and 5.86 g-H2O/100 g-DM, respectively. Their critical water contents were also matched to a w = 0.56, 0.37, and 0.35, respectively, using Eq. 1 or Eq. 2. At a higher critical water content or a w, the amorphous powder products became physically unstable; there was a risk of aggregation and re-crystallization during storage [4, 5, 13, 14]. Considering this, it is concluded that MBCa is a more stable glassy material than MBH and maltose.
The T gs of maltose was evaluated to be 95.5 °C. This value was almost equivalent to previous reports given by other researchers (90 ~ 97 °C [37–40]) and by another technique (90 °C by FT-IR [40]). The minor variation of the T gs appeared in the literature will be caused by the fact that glass transition is a relaxation phenomenon of amorphous materials; T g depends more or less on the preparing method [41] and measuring condition [42, 43].
The T gs of MBH (73.7 °C) was lower than maltose (95.5 °C). This indicates that oxidation reduces the T gs of maltose as similar to reduction; the T gs of maltitol is reported to be 47.3 °C [44]. Similar effect of reduction on the T gs of saccharide is also confirmed in the comparison between glucose (37 ~ 38 °C) and sorbitol (−1.9 ~ 0 °C), lactose (112 °C) and lactitol (54.9 °C), and matrotriose (134 °C) and maltotriitol (88.6 °C), respectively [37, 44]. On the other hand, MBCa (148.1 °C) had a much higher T gs than maltose, maltitol, and MBH. Similar effects of oxidation and Ca-addition on the glass transition properties are expected to other saccharides; for example, T gss of calcium gluconate (103 °C) and calcium lactobionate (137 °C) are much higher than that of glucose and lactose [45].
It is known that T gs of carbohydrate materials increases with molar weight (MW) or formula weight (FW) because of the enhancement of intermolecular interactions [22]. One of the reasons why MBCa had a high T gs is likely that MBCa (FW = 754) has a much higher weight than MBH (MW = 358), maltitol (MW = 344), and maltose (MW = 342). In comparison with glucan, it should be noted that MBCa has an equivalent T gs to maltohexaose (MW = 991) [40]. In addition, it is known that Ca2+ can elevate the T g of hydrogen-bonging system because of thermally reversible self-polymerizing [46].
MBCa and MBH had a lower k (greater resistance to water plasticizing) than maltose. It is known that k of mono- and disaccharide increases linearly with increasing T gs (°C) and the relationship is empirically expressed as follows [8]:
In fact, it was confirmed that k = 7.4 for maltose and k = 4.7 for MBH are relatively in a good agreement with the value calculated by Eq. 4 (k = 6.4 for maltose and k = 5.8 for MBH). On the other hand, k = 5.7 for MBCa differed significantly from the calculated value (k = 7.9). As mentioned above, the lower the k value, the greater the resistance to water plasticizing. From these results, it was concluded that MBCa has a high T g and large resistance to water plasticizing. This property is similar to that of oligo- and polysaccharides. For example, it is known that for amorphous inulin T g increases from 111 °C to 141 °C and k decreases from 6.4 to 5.2 with an increase in the average molar mass between 1197 g/mol and 4395 g/mol [22]. The high T g and low k of MBCa likely originates from the strong interaction between MBCa and water induced by electrostatic interactions.
Effects of Temperature and Water Content on η of MBCa
Every MBCa aqueous solution exhibited Newtonian behavior; thus, η (Pa·s) was evaluated from the linear relationship between shear stress and shear rate (data not shown). The effect of temperature on η of each aqueous MBCa sample is shown in Fig. 4a. As mentioned above, the effect of temperature on η of an aqueous solution can be described by the Arrhenius formula in a limited temperature range, but it deviates for temperatures near T g. Over the whole temperature range, the dependence of η on temperature can be described instead by the Vogel–Fulcher–Tammann (VFT) equation (Eq. 5):
where η 0 (Pa·s), D (dimensionless), and T 0 (K) were constants [18]. It is known that η 0 is approximately 10−5 Pa·s [18]. On the other hand, T g is known to be the temperature at which η becomes approximately 1012 Pa·s [18]. When these values are input into Eq. 5, D can be empirically represented as a function of T g and T 0 (Eq. 6).
T g of the aqueous MBCa sample cannot be evaluated experimentally because water crystalizes before the system falls into the glassy state during the cooling process. Therefore, T g was extrapolated through Eq. 3 in this study. When the extrapolated T g was input into Eq. 6, D could be described as a function of T 0. Thus, the only unknown parameter in Eq. 5 was T 0 and then the solid lines in Fig. 4a were obtained by fitting Eq. 5 to the η data. In addition, effect of temperature on the η for MBCa aqueous solution (Fig. 4a) was described as a T g-scaled Arrhenius plot (i.e. Angell plot) in Fig. 4b. From the result, it was confirmed that effect of temperature of the η for MBCa aqueous solution was roughly described by a master curve (Fig. 4b). Similar results were also reported for maltose, trehalose, and trehallose-borax aqueous solutions [17, 21, 47].
From the fitting results, it was noted that T 0 increased with decreasing water content of MBCa, which was similar to the trend in T g. This observation agreed with trehalose aqueous solution [17]. To understand the relation between T g and T 0, T 0 was plotted against T g in this study, and then a linear relationship was obtained (Fig. 5). As mentioned above, T g is the temperature at which η becomes 1012 Pa·s. On the other hand, T 0 is the temperature of viscosity divergence (i.e., η = ∞) [18]. Since both of T g and T 0 have a similar physical meaning of which viscosity becomes the certain value, it is reasonable that T g has a liner proportion to T 0. The T g of water was 136 K [35, 36], and thus the linear relation shown in Fig. 5 gave T 0 = 115 K. When the η data of water in the temperature range between 0 °C and 100 °C (11 points) were taken from a general chemical handbook, it was found that the parameters well described the dependence of η on temperature (R 2 = 0.9962). This fact supports the validity of the empirical linear relation for the prediction of T 0 from T g.
From the relationship between T 0 and T g, it is noted that the temperature difference between T g and T 0 decreased with increase water content (with decrease T g). In addition, the D calculated by Eq. 6 decreased from 8.1 (60 % MBCa) to 7.5 (10 % MBCa). Similar effect of water content on the D is reported for sucrose [18]. It is known that D can be related to strong-fragile classification of liquid. Effect of temperature on the η of strong liquid shows Arrhenius-like behavior. In contrast, the lower D, the greater non-Arrhenius behavior is observed; such a liquid is described as “fragile liquid”. It is commonly known that hydrogen-bonding system is classified into fragile liquid. For example, D values for sucrose and trehalose aqueous solutions are reported to be approximately 6 [18] and 3.32 ~ 4.85 [17], respectively. MBCa aqueous solution is slightly stronger liquid than these saccharides. Water, on the other hand, shows irregular strong/fragile behavior; fragile behavior at a higher temperature than 236 K (limitation of supercoiling), but strong behavior at a temperature near T g (in the temperature range between 136 K and 150 K) are observed [18, 36]. Although further discussion on the molecular dynamics of water is avoided in this paper, it is emphasized that water showed fragile behavior in the temperature range between 273 K and 373 K. Consequently, the reason why the D of MBCa aqueous solutions decreased with increase in water content will be that strong liquid-structure formed by MBCa was destructed by fragile liquid (i.e., water).
When T 0 is treated as a function of T g according to Fig. 5, effects of temperature and water content on η of MBCa can be predicted from the combination of Eqs. 3, 5, and 6; T 0 and D of Eq. 5 can be replaced by T g, and T g can be described as a function of water content by Eq. 3. The combined equation gives a three-dimensional diagram as shown in Fig. 6. As mentioned above, η is an important physical parameter for drying operation [13, 14] and quality control of liquid food [15, 16]. The diagram as shown in Fig. 6 will be useful for understating the effect of MBCa addition on the solution’s properties. Taking the background of this predictive approach into account, the temperature range should be limited between 0 °C and 100 °C; water molecules exist as fragile liquid in the temperature range. In addition, MBCa content should be limited between 0 and 60 % (w/w); non-Newtonian behavior may be appeared at a higher MBCa content.
Conclusion
Water sorption, glass transition, and viscosity of MBCa were measured. These data are practically useful not only for the production of MBCa powder (drying operation and storage stability) but also for the quality prediction of food modified by adding MBCa. Because MBCa has the advantages of having a high T g and great resistance to water plasticizing, MBCa will be a useful food modifier. In addition, a semi-empirical η-prediction approach based on T g was proposed. To confirm the validity of this approach in more detail, it is necessary to apply it to many types of aqueous materials, which will be the subject of future studies.
References
A. J. Kluyver, J. de Ley, A. Rijven, The formation and consumption of lactobionic and maltobionic acids by pseudomonas species. Anton. Leeuw. 17, 1–14 (1951)
A. Mirescu, U. Prüße, A new environmental friendly method for the preparation of sugar acids via catalytic oxidation on gold catalysts. Appl. Catal. B 70, 644–652 (2007)
B. A. Green, R. J. Yu, E. J. Van Scott, Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 27, 495–501 (2009)
J. M. Aguilera, J. M. del Valle, M. Karel, Caking phenomena in amorphous food powders. Trends Food Sci. Technol. 6, 149–155 (1995)
S. Palzer, The effect of glass transition on the desired and undesired agglomeration of amorphous food powders. Chem. Eng. Sci. 60, 3959–3968 (2005)
P. D. Orford, R. Parker, S. G. Ring, A. C. Smith, Effect of water as a dilutent on the glass transition behavior of malto-oligosaccharides, amylose and amylopectin. Int. J. Biol. Macromol. 11, 91–96 (1989)
L. H. Mosquera, G. Moraga, P. F. de Córdoba, N. Martínez-Navarrete, Water content–water activity–glass transition temperature relationships of spray-dried borojó as related to changes in color and mechanical properties. Food Biophys. 6, 397–406 (2011)
Y. Roos, Melting and glass transitions of low molecular weight carbohydrates. Carbohydr. Res. 238, 39–48 (1993)
V. R. N. Telis, N. Martínez-Navarrete, Collapse and color changes in grapefruit juice powder as affected by water activity, glass transition, and addition of carbohydrate polymers. Food Biophys. 4, 83–93 (2009)
F. Avaltroni, P. E. Bouquerand, V. Normand, Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohydr. Polym. 58, 323–334 (2004)
M. Le Meste, D. Champion, G. Roudaut, G. Blond, D. Simatos, Glass transition and food technology: a critical appraisal. J. Food Sci. 67, 2444–2458 (2002)
D. S. Chaudhary, B. P. Adhikari, S. Kasapis, Glass-transition behavior of plasticized starch biopolymer system – a modified Gordon-Taylor approach. Food Hydrocoll. 25, 114–121 (2011)
P. Zhu, S. Méjean, E. Blanchard, R. Jeantet, P. Schuck, Prediction of dry mass glass transition temperature and the spray drying behaviour of a concentrate using a desorption method. J. Food Eng. 105, 460–467 (2011)
P. Stähle, V. Gaukel, H. P. Schuchmann, Influence of feed viscosity on the two-phase flow inside the exit orifice of an effervescent atomizer and on resulting spray characteristics. Food Res. Int. 77, 55–62 (2015)
E. Maltini, D. Torreggiani, E. Venir, G. Bertolo, Water activity and the preservation of plant foods. Food Chem. 82, 79–86 (2003)
I. Aguiló-Aguayo, R. Soliva-Fortuny, O. Martín-Belloso, Color and viscosity of watermelon juice treated by high-intensity pulsed electric fields or heat. Innov. Food Sci. Emerg. Technol. 11, 299–305 (2010)
M. E. Elias, A. M. Elias, Trehalose + water fragile system: properties and glass transition. J. Mol. Liq. 83, 303–310 (1999)
C. A. Angell, R. D. Bressel, J. L. Green, H. Kanno, M. Oguni, E. J. Sare, Liquid fragility and the glass transition in water and aqueous solutions. J. Food Eng. 22, 115–142 (1994)
C. A. Angell, R. C. Stell, W. Sichina, Viscosity-temperature function for sorbitol from combined viscosity and differential scanning calorimetry studies. J. Phys. Chem. 86, 1540–1542 (1982)
T. R. Noel, S. G. Ring, M. A. Whittam, Kinetic aspects of the glass transition behabior of maltose-water mixtures. Carbohydr. Res. 212, 109–117 (1991)
D. P. Miller, J. J. de Pablo, H. R. Corti, Viscosity and glass transition temperature of aqueous mixture of trehalose with borax and sodium chloride. J. Phys. Chem. B 103, 10243–10249 (1999)
K. Kawai, K. Fukami, P. Thanatuksorn, C. Viriyarattanasak, K. Kajiwara, Effects of moisture content, molecular weight, and crystallinity on the glass transition temperature of inulin. Carbohydr. Polym. 83, 934–939 (2011)
B. C. Hancock, S. L. Shamblin, G. Zografi, Molecular mobility of amorphous pharmaceutical solids below their glass transition temperature. Pharm. Res. 12, 799–806 (1995)
K. Kawai, Y. Hagura, Discontinuous and heterogeneous glass transition behavior of carbohydrate polymer-plasticizer systems. Carbohydr. Polym. 89, 836–841 (2012)
N. Potes, J. P. Kerry, Y. H. Roos, Additivity of water sorption, alpha-relaxations and crystallization inhibition in lactose–maltodextrin systems. Carbohydr. Polym. 89, 1050–1059 (2012)
J. E. Zimeri, J. L. Kokini, The effect of moisture content ion the crystallinity and glass transition temperature of inulin. Carbohydr. Polym. 48, 299–304 (2002)
J. E. Zimeri, J. L. Kokini, Phase transitions of inulin-waxy maize starch systems in limited moisture environments. Carbohydr. Polym. 51, 183–190 (2003)
G. Balasubrahmanyam, A. K. Datta, Prevention of moisture migration in coated biscuit. J. Food Eng. 21, 235–244 (1994)
E. Palou, A. López-Malo, A. Argaiz, Effect of temperature on the moisture sorption isotherms of some cookies and corn snacks. J. Food Eng. 31, 85–93 (1997)
S. S. Arogba, Effect of temperature on the moisture sorption isotherm of a biscuit containing processed mango (Mangifera indica) kernel flour. J. Food Eng. 48, 121–125 (2001)
W. A. M. McMinn, D. J. McKee, T. R. A. Magee, Moisture adsorption behaviour of oatmeal biscuit and oat flakes. J. Food Eng. 79, 481–493 (2007)
M. Z. Islam, Y. Kitamura, Y. Yamano, M. Kitamura, Effect of vacuum spray drying on the physicochemical properties, water sorption and glass transition phenomenon of orange juice powder. J. Food Eng. 169, 131–140 (2016)
B. A. Bolton, J. R. Scherer, Raman spectra and water absorption of bovine serum albumin. J. Phys. Chem. 93, 7635–7640 (1989)
P. P. Lewicki, Water as the determinant of food engineering properties. A review. J. Food Eng. 61, 483–495 (2004)
G. P. Johari, A. Hallbrucker, E. Mayer, The glass–liquid transition of hyperquenched water. Nature 330, 552–553 (1987)
S. Sastry, Going strong or falling apart? Nature 398, 467–470 (1999)
P. D. Orford, R. Parker, S. G. Ring, Aspects of the glass transition behaviour of mixtures of carbohydrates of low molecular weight. Carbohydr. Res. 196, 11–18 (1990)
T. R. Noel, R. Parker, S. G. Ring, Effect of molecular structure and water content on the dielectric relaxation behaviour of amorphous low molecular weight carbohydrates above and below their glass transition. Carbohydr. Res. 329, 839–845 (2000)
T. R. Noel, R. Parker, S. G. Ring, A comparative study of the dielectric relaxation behaviour of glucose, maltose, and their mixtures with water in the liquid and glassy states. Carbohydr. Res. 282, 193–206 (1996)
K. Imamura, K. Sakaura, K. Ohyama, A. Fukushima, H. Imanaka, T. Sakiyama, K. Nakanishi, Temperature scanning FTIR analysis of hydrogen bonding states of various saccharides in amorphous matrixes below and above their glass transition temperatures. J. Phys. Chem. B 110, 15094–15099 (2006)
R. Surana, A. Pyne, R. Suryanarayanan, Effect of preparation method on physical properties of amorphous trehalose. Pharm. Res. 21, 1161–1176 (2004)
K. J. Crowley, G. Zografi, The use of thermal methods for predicting glass-former fragility. Thermochim. Acta 380, 79–93 (2001)
L. Wang, V. Velikov, C. A. Angell, Direct determination of kinetic fragility indices of glassforming liquids by differential scanning calorimetry: kinetic versus thermodynamic fragilities. J. Chem. Phys. 117, 10184–10192 (2002)
S. Kadoya, K. Fujii, K. Izutsu, E. Yonemochi, K. Terada, C. Yomota, T. Kawanishi, Freeze-drying of proteins with glass-forming oligosaccharide-derived sugar alcohols. Int. J. Pharm. 389, 107–113 (2010)
M. Erber, G. Lee, Cryopellets based on amorphous organic calcium salts: production, characterization and their usage in coagulation diagnostics. Powder Technol. 280, 10–17 (2015)
D. R. MacFarlane, J. Pringle, G. Annat, Reversible self-polymerizing high Tg lyoprotectants. Cryobiol. 45, 188–192 (2002)
T. Matsuoka, T. Okada, K. Murai, S. Koda, H. Nomura, Dynamics and hydration of trehalose and maltose in concentrated solutions. J. Mol. Liq. 98–99, 319–329 (2002)
Acknowledgments
A part of this work was financially supported by JSPS KAKENHI (Grant-in-Aid for Scientific Research C: 15 K07453).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukami, K., Kawai, K., Takeuchi, S. et al. Effect of Water Content on the Glass Transition Temperature of Calcium Maltobionate and its Application to the Characterization of Non-Arrhenius Viscosity Behavior. Food Biophysics 11, 410–416 (2016). https://doi.org/10.1007/s11483-016-9455-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-016-9455-2