Abstract
2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate ([BHEM][mesy]) displayed good ability for separating cellulose from biomass material in previous works. In order to have a better understanding of the structure–property relationship and facilitate further potential applications in industry, it is necessary to obtain more data for the physicochemical properties of [BHEM][mesy] and its mixtures. In this work, the densities (ρ) and viscosities (η) of [BHEM][mesy] + solvents (water, methanol, ethanol, n-propanol and isopropanol) mixtures were measured at six temperatures from (298.15 to 323.15) K over the full molar composition range and fitted by an empirical quadratic equation and Vogel–Fucher–Tammann (VFT) equation. Moreover, the excess molar volumes (VE) and viscosity deviation (Δη) of five binary mixtures were calculated and correlated by the Redlich–Kister equation. In addition, the apparent molar volumes (\({V}_{\varphi }\)) and partial molar volumes (\(\stackrel{-}{V}\)) of [BHEM][mesy] and solvents were also calculated and all these physicochemical property data (density, viscosity, excess molar properties as well as the apparent molar properties) are discussed in terms of the structure and interaction of binary mixtures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In order to make use of cellulose from various biomass sources more effectively, a number of techniques with different solvent systems have been used in the past few decades, including dilute acid treatment [1], alkaline treatment [2], the sulfite pulping process [3], organosolv isolation [4], etc. All these systems can promote cellulose separation from biomass materials to some extent, but they also generate severe consequences to the environment. Therefore, finding media that can extract cellulose effectively, in environmentally friendly manner, is extremely important for the utilization of biomass. In the last decades, ionic liquids (ILs) have attracted a lot of attention from both academia and industry as they are regarded as a kind of promising green solvent for the separation of cellulose owing to their unique characteristics, like high thermal stability, designability, recyclable as well as non-volatile properties [5,6,7,8,9,10].
Protic ILs, containing hydroxy groups, are drawing more and more attention because the formation of strong hydrogen bonds between the ILs and lignocelluloses facilitates the disruption of inter-/intra-molecular hydrogen bonds among biomass [11]. In addition, protic ILs are prepared by a one-step protonation reaction without any by-products, thus reducing the cost for multi-ton IL production [12]. Based on previous work [13], it was found that the polyhydric protic IL 2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate ([BHEM][mesy]) had good efficiency in the pretreatment of corn straw. The content of cellulose was up to 90 wt% in the cellulose material obtained at 413 K for 6 h in [BHEM][mesy]. Therefore, [BHEM][mesy] displayed a promising future in the biomass pretreatment industry.
In order to promote academic research and industrial applications of ILs, knowledge of binary systems containing ILs and solvents is of great significance. On the one hand, the relevant properties of IL mixtures can reveal useful information about the interaction forces between molecules and assist researchers in establishing theoretical models to predict the behaviors of pure components and their mixtures. On the other hand, as the properties directly affect the processes of mixing, stirring and pumping, they are important to the design of processing units and heat and mass transfer processes in industrial applications [14]. In recent years, a large amount of work has focused on the physical and chemical properties of hydroxyl-type protic ILs and their mixtures including density [15,16,17,18,19,20,21], viscosity [16,17,18,19,20,21,22], refraction index [15, 17, 21], vapor–liquid equilibria [15, 23], ionic conductivity [20, 22], acid–base properties [20], as well as acoustical properties [21, 24]. The anions mainly center on formate [16, 18, 19, 22], propionate [23], butyrate [24], hexanoate [15], various carboxylic acid anions [21], trifluoromethanesulfonate [20], bis(trifluoromethanesulfonyl)amide [20] and so on. However, what deserves our attention is that there is almost no relevant literatures in the field of physicochemical properties about the hydroxyl-type of protic ILs containing methanesulfonate, which can be applied to the field of pretreatment of biomass. Therefore, it is necessary to explore the physicochemical properties of binary system consisting of [BHEM][mesy] for potential applications in industry.
In this work, the density and viscosity of [BHEM][mesy] mixtures with water, methanol, ethanol, n-propanol, and isopropanol are first reported at temperatures from (298.15 to 323.15) K over the full molar composition and were fitted with an empirical quadratic equation and Vogel–Fucher–Tammann (VFT) equation, respectively. More than that, the excess molar volumes (VE) and viscosity deviation (Δη) of five binary mixtures were calculated and fitted with the Redlich–Kister equation. In addition, apparent molar volumes (\({V}_{\varphi }\)) and partial molar volumes (\(\stackrel{-}{V}\)) of the IL and solvents were also calculated. These physicochemical property data could provide useful information for the applications of [BHEM][mesy] in the biomass pretreatment industry.
2 Experimental Section
2.1 Chemicals Used
N-methyldiethanolamine (> 99%) and methanesulfonic acid (> 99%), ethanol (> 99.9%) and propanol (> 99.9%) were purchased from Aladdin Industrial Corporation. Methanol (> 99.9%) and isopropanol (> 99.9%) were purchased from Fisher chemical. The water of the experiments was deionized by a reverse osmosis unit with an ion-exchange system (Milli-Q, French). [BHEM][mesy] was synthesized and purified based on the previous work, the synthetic procedure and structures of the [BHEM][mesy] are displayed in Scheme 1 [13].
2.2 Measurement of Density and Viscosity
[BHEM][mesy] was dried in a vacuum drying oven (DZG-6021, Shanghai Sumsung Laboratory Instrument Co., Ltd., China) under the condition of using the water absorbent P2O5 at temperature 323 K for 120 h. The P2O5 was replaced every 10 h in order to keep its best water absorption capability. All solvents were degassed in an ultrasonic bath (KQ-400KDE, Kunshan Ultrasonic Instruments Co., Ltd., China) at 100 W and the temperature about 320 K for 2 h. Binary mixtures were prepared by weight, using an electronic analytical balance (Mettler Toledo, Switzerland, ± 0.0001 g) in a drying shed. The uncertainty of mole fraction was estimated as being ± 0.0001.
The density and viscosity of pure [BHEM][mesy], solvents (water, methanol, ethanol, n-propanol and isopropanol) and binary mixtures ([BHEM][mesy] + water/alcohols) were measured by an Anton Paar DMA™ 4500 M density meter and an Anton Paar micro viscometer Lovis 2000 ME at temperatures from (298.15 to 323.15) K, respectively. The density meter’s uncertainty is ± 0.00005 g·cm−3 and the uncertainty of temperature in the density chamber is ± 0.02 K. The uncertainty of the viscosities measurements is 5 × 10−3 mPa·s and the temperature of glass capillaries can be precise to ± 0.02 K. There were three kinds of glass capillaries used with diameters 1.59 mm, 1.8 mm and 2.5 mm. The viscosity ranges for the capillary diameters of 1.59 mm, 1.8 mm, 2.5 mm are (0.3–15, 10–100 and 100–10,000) mPa·s, respectively. The viscosity over the full molar fraction at setting temperature was determined to confirm which capillary should be chosen. In the course of normal measurments, the glass capillary with diameter of 1.59 mm was selected to measure the samples with viscosity of 0.3–12.5 mPa·s, of 1.8 mm for 12.5–100 mPa·s and of 2.5 mm for 100–10,000 mPa·s. The capillaries were calibrated by the Anton Paar company before use.
About 5 mL samples of binary mixtures were added in 10 mL sample bottles, and then heated to temperature of 320 K in the ultrasonic instrument for 2 h to mix samples well. The density and viscosity tubes were repeatedly cleaned and dried, and then about 4 mL of samples were drawn into a 5 mL disposable syringe and slowly injected into the density and viscosity tubes, ensuring that no bubbles were generated. The temperature program run (from (298.15 to 323.15) K with one data point every 5 K) was used for measurements. When the temperature program ended and the temperature returned to 298.15 K, the injection syringe was slowly pushed to refresh the mixtures in the density chamber and viscosity glass capillary the temperature program was run again to make the second measurements, followed by a third measurement with the same procedures. The final data were obtained by calculation of the average of these three replicate measurements.
The measured densities and viscosities values of five pure solvents at different temperatures are compared with data provided in the literature in Table 1. In order to evaluate the accuracy and precision of collected data comparing with literature data, the relative deviations of density and viscosity were calculated and are displayed in the same table. The results indicate that all deviations of density data are less than 0.1% and of viscosity did not exceed 5% for most of data; thus the measuring systems used here are credible.
3 Results and Discussion
3.1 Characterizations of [BHEM][mesy]
The structure and purity of the [BHEM][mesy] were identified by 1H NMR (av-600 MHz, DMSO-d6, Bruker, Switzerland), FT-IR (10,000–370 cm−1, Nicolet 380, America) and Karl Fisher titration (C20 Coulometric KF Titrator, Mettler Toledo, Switzerland). All peaks and chemical shifts could be assigned to [BHEM][mesy] (Figs. S1, S2). No impurity peaks were found in the 1H NMR and FT-IR spectra, which indicated that the only impurity was water. The water content was measured by Karl Fisher titration and the result displayed that the water content was less than 1000 ppm. According to these analyses, the purity of [BHEM][mesy] was above 99%.
3.2 Densities
The density data of five pure components and binary mixtures are collected in Table 2. The following function is used to correlate the density of mixtures and temperatures at constant mole fraction [37, 38]:
where \(\rho\) is the density of the binary mixtures at a given molar fraction. T is the absolute temperature in Kelvin from (298.15 to 323.15) K. A (g·cm−3), B (g·cm−3·K−1) and C (g·cm−3·K−2) are the fitting coefficients.
The data of fitting coefficients are collected in Table 3 and the fitting curves are displayed in Fig. S3. The coefficients of determination (R2) of all five binary mixtures all approach 1, which indicates that Eq. 1 represents the experimental data very well. It is observed that the pure [BHEM][mesy] has the largest density in all binary mixtures and the densities of mixtures at given molar fraction decrease with the increase of temperature as displayed in Fig. S3. In addition, from Fig. S4, the densities of mixtures gradually decrease with the decrease of molar fraction of [BHEM][mesy] at each given temperature and the slopes become smaller with the increase of the mole composition of [BHEM][mesy]. These results indicate that the magnitude of density change becomes smaller as the molar fraction of [BHEM][mesy] increases and also illustrates that a small amount of ionic liquid can have a large effect on the density of the solvent.
3.3 Apparent Molar Volumes
The apparent molar volume is considered as the molar volume of the solute in the solvent. It is generally different from the molar volume of pure material and can provide useful information to unveil the interactions between different molecules in mixtures, such as interactions between ion–ion, ion–solvent and solvent–solvent [39]. The apparent molar volumes of [BHEM][mesy] and solvents (water, methanol, ethanol, n-propanol and isopropanol) were calculated based on the following equations [39, 40]:
where \({V}_{\varphi ,1}\) and \({V}_{\varphi ,2}\), x1 and x2, M1 and M2 are the apparent molar volumes, the molar fractions and molar mass of the [BHEM][mesy] and solvents, respectively. ρ1, ρ2 and ρm are the densities of [BHEM][mesy], solvents and their mixtures, respectively.
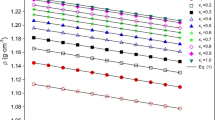
All these experimental results are presented in Table 4 and displayed in Fig. 1. In general, the apparent molar volumes increase with the increase of temperature and of molar fraction in binary mixtures [41]. The same trend can be seen for the five sets of mixtures. For all these mixtures, [BHEM][mesy] has the biggest apparent molar volumes compared with water, methanol, ethanol, n-propanol and isopropanol at all compositions. In addition, with the increase of molar fraction of [BHEM][mesy], a clear trend of increasing apparent molar volumes of [BHEM][mesy] and decreasing apparent molar volumes of solvents can be observed from Fig. 1. What’s more, the apparent molar volumes of both [BHEM][mesy] and solvents increase with the increase of temperature just as displaying in Fig. 1.
Apparent molar volumes of [BHEM][mesy] and solvent molecules over the full molar fraction range at temperatures from (298.15–323.15) K. a1–e1 are the apparent molar volumes of [BHEM][mesy] in water, methanol, ethanol, n-propanol and isopropanol, respectively. a2–e2 are the apparent molar volumes of water, methanol, ethanol, n-propanol and isopropanol, respectively
3.4 Viscosity
The dynamic viscosity values of all five binary mixtures at all molar compositions at temperatures from (298.15 to 323.15) K are collected in Table 5. The viscosities of each mixture were fitted with the Vogel–Fulcher–Tammann equation (VFT, Eq. 4), which can precisely describe the relationship between dynamic viscosity and temperature at constant molar composition [14, 37, 42]:
In this equation, the A (mPa·s), B (K) and T0 (K) are adjustable parameters. All these adjustable parameters and the correlation coefficients (R2) obtained and are listed in Table 6.
As can be seen from Fig. S5, the experimental data are well represented with the Vogel–Fulcher–Tammann equation and the viscosities of mixtures sharply decrease as the temperature increases at constant mole composition. Compared with Fig. S3, temperature has greater influence on viscosity than it has on density. This is because the viscosity of the liquid is generated by the cohesion of molecules; the higher of their temperature, the stronger of molecular vibration, the smaller is cohesion between them, and the viscosity goes down. In addition, from Fig. 2, the viscosities of all five binary mixtures rapidly increase with the increase of molar fraction of [BHEM][mesy] at constant temperature. As the molar concentration of [BHEM][mesy] goes up, the distance of cation and anion becomes smaller. Thus, the interaction forces, especially the electrostatic interaction, become stronger, which leads to the viscosity becoming larger.
Viscosity for mixtures of ax1 [BHEM][mesy] + x2 water, bx1 [BHEM][mesy] + x2 methanol, cx1 [BHEM][mesy] + x2 ethanol, dx1 [BHEM][mesy] + x2n-propanol, ex1 [BHEM][mesy] + x2 isopropanol as a function of mole fractions of x1 [BHEM][mesy] at six different temperatures. Filled square, 298.15 K; open square, 303.15 K; filled triangle, 308.15 K; open triangle, 313.15 K; filled inverted triangle, 318.15 K; open inverted triangle, 323.15 K. The solid curves are trendlines and the symbols denote experimental values
3.5 Excess Molar Volume and Viscosity Deviation
The excess molar volume is an important thermodynamic property, which indicates the difference of molar volumes between the experimental binary mixture and the corresponding ideal mixture fluid as well as providing useful information to the molecular interactions [43]. Thus, it is necessary to evaluate the excess molar volume (\({V}^{\mathrm{E}}\)) and viscosity deviation (\(\Delta \eta\)) of binary mixtures to obtain the difference between the real solution and the corresponding ideal one. Among them, the ideal viscosity of binary mixtures is obtained by the Arrhenius expression [44]; thus \({V}^{\mathrm{E}}\) and \(\Delta \eta\) can be calculated by the following equations [45,46,47,48]:
where \({x}_{1}\) and \({x}_{2}\), \({\rho }_{1}\) and \({\rho }_{2}\), \({\eta }_{1}\) and \({\eta }_{2}\), \({M}_{1}\) and \({M}_{2}\) are the molar fraction, density and viscosity of a pure component at a certain temperature and the molar masses of [BHEM][mesy] and solvent, respectively. \({\rho }_{m}\) and \({\eta }_{m}\) are the experimental data at a certain composition and temperature. The results of the calculations are displayed in Table 7.
The experimental values of VE and Δη were fitted with Redlich–Kister type polynomials [47, 48]:
where the adjustable parameters (Ai) were obtained by fitting the equation with the experimental values with a least-squares type algorithm. The standard deviation (σ) is defined by the following equation [47, 48]:
where n is the number of experimental values and p is the number of parameters. The subscripts, exptl and calc, stand for the experimental and the calculated values, respectively. VE and Δη were fitted perfectly with the Redlich–Kister equation just as displayed in Figs. 3 and 4. The values of the parameters Ai and standard deviations σ are listed in Table 8.
Excess molar volumes for mixtures of ax1 [BHEM][mesy] + x2 water, bx1 [BHEM][mesy] + x2 methanol, cx1 [BHEM][mesy] + x2 ethanol, dx1 [BHEM][mesy] + x2n-propanol, ex1 [BHEM][mesy] + x2 isopropanol at different temperatures. Open inverted triangle, 298.15 K; filled inverted triangle, 303.15 K; open triangle, 308.15 K; filled triangle, 313.15 K; open square, 318.15 K; filled square, 323.15 K; the solid curves were calculated with the Redlich − Kister equation, and the symbols represent experimental values
Viscosity deviations for mixtures of ax1 [BHEM][mesy] + x2 water, bx1 [BHEM][mesy] + x2 methanol, cx1 [BHEM][mesy] + x2 ethanol, dx1 [BHEM][mesy] + x2n-propanol, ex1 [BHEM][mesy] + x2 isopropanol at different temperatures. Open inverted triangle, 323.15 K; filled inverted triangle, 318.15 K; open triangle, 313.15 K; filled triangle, 308.15 K; open square, 303.15 K; filled square, 298.15 K; the solid curves were calculated with the Redlich − Kister equation, and the symbols represent experimental values
From Fig. 3, it is clear that all binary mixtures at all temperatures have negative excess molar volumes. The values decrease first and then begin to increase at a particular molar fraction. Especially, the binary mixtures, [BHEM][mesy] + water and [BHEM][mesy] + methanol have a minimum VE at a molar fraction of [BHEM][mesy] between 0.2 and 0.3, the mixture of [BHEM][mesy] + ethanol has a minimum VE at molar fraction about 0.3–0.4, and the mixtures containing propanol and isopropanol have a minimum VE at 0.4–0.5. The negative VE values indicate the interactions between [BHEM][mesy] and solvents are strong, thus making the deviations between real mixtures and ideal ones large [49]. These interaction forces include van der Waals’ forces, strong dipole–dipole, charge transfer complexes and ion–dipole interactions. But the most important factor in these binary mixtures may be hydrogen bonds because each component has one or more hydroxyl group, which can generate strong hydrogen bonds between each other. At the same time, the difference of size between ionic liquid and solvents is also significant in the course of determining VE because the large volume of [BHEM][mesy] provide enough space for small molecules of solvents to embed in it, thus giving rise to negative VE values. Furthermore, the deviations of all five binary mixtures become smaller with increasing temperature from (298.15 to 323.15) K. This trend can be attributed to the reason that the movement of molecules become more intense with increasing temperature, which leads to weaker interaction among molecules. Moreover, the binary mixtures containing water have smaller VE values than the mixtures containing alcohols. This phenomenon may be attributed to the fact that the molecules of alcohols have alkyl (methyl, ethyl, propyl) groups, which generate stronger dispersion interactions in the mixtures due to their alkyl chains [50].
As for viscosity deviations of binary mixtures, they are displayed in Fig. 4 and a similar overall change trend can be observed. From this figure, all five mixtures have positive Δη values that increase first and then decrease at any particular molar fraction. What is different from the VE is that the turning point appearing in the molar fraction is at about x1 = 0.9, which is larger than the corresponding point in VE. Particularly, the binary mixtures containing water have larger Δη values in general and its maximum is about 500 mPa·s compared to the maximum Δη about 250 mPa·s for the binary mixtures containing alcohols. In addition, at low molar fraction of [BHEM][mesy] (about x1 = 0–0.5), the intensity of change of Δη is small. However, when x1 exceeds 0.5, the intensity of change of Δη becomes very large. At the same time, the viscosity deviations of the binary mixtures change very rapidly from the turning point, and they decrease to zero from the maximum in a very narrow range (about 0.9–1.0); this phenomenon indicates that very small amounts of impurities can have a great effect on the viscosity of pure ionic liquids. Also, the regularity of Δη is similar with the VE with regards to the influence of temperature. With the increase of temperature, the Δη become smaller in all binary mixtures.
3.6 Partial Molar Volumes
In general, the partial molar volume represents the change in volume caused by the addition of one mole of matter to a mixed solution under the condition of constant pressure and temperature. The partial molar volume of [BHEM][mesy] and solvent compounds of binary mixtures can be calculated basing on the following equations [51, 52]:
where \({V}^{\mathrm{E}}\) represents the excess molar volume of the binary mixture, \({V}_{1}^{0}\) and \({V}_{2}^{0}\) denote for molar volume of pure [BHEM][mesy] and solvents at constant temperature, respectively.
The calculated partial molar volumes are listed in Table 9 and all of them are positive over the full mole composition range at temperatures from (298.15 to 323.15) K. With the increase of temperature, the partial molar volumes of both [BHEM][mesy] and solvent compounds (water and alcohols) increase at constant molar fraction. However, at constant temperature, the partial molar volumes of [BHEM][mesy] decrease and the partial molar volumes of solvent compounds increase with the increase of the molar fraction of [BHEM][mesy]. In addition, the partial molar volumes of [BHEM][mesy] are commonly much larger than the solvent compounds, as [BHEM][mesy] has larger molecular weight and volume. What’s more, [BHEM][mesy] has smaller partial molar volumes in water compared to its values in alcohols at the corresponding mole fraction. For binary mixtures containing alcohols, [BHEM][mesy] has similar partial molar volumes, which indicates that there are no significant difference in the influences of methanol, ethanol, propanol and isopropanol in terms of partial molar volumes of [BHEM][mesy]. For partial molar volumes of solvents, their order is water < methanol < ethanol < n-propanol ≈ isopropanol, which corresponds with their molecular weight and volume.
4 Conclusions
The density and viscosity of five binary mixtures including [BHEM][mesy] and water or alcohols (methanol, ethanol, n-propanol and isopropanol) were systematically measured. The data of density and viscosity were correlated by an empirical polynomial and the Vogel–Fulcher–Tammann equations, respectively. The calculated standard deviations indicate that all fitting equations accurately reproduce the experimental data. It is clearly shown that density and viscosity of all binary mixtures decrease with increasing temperature and decrease with the [BHEM][mesy] molar fraction. It was also observed that the influences of temperature and molar composition are larger for viscosity than for density in all mixtures. Then, excess molar volumes and viscosity deviations were calculated and fitted with Redlich–Kister type polynomials. The excess molar volumes vary similarly in all five binary mixtures; all values are negative and decrease first but then have an increasing trend at a particular mole fraction. For the viscosity deviations, they increased slowly at first but then increased rapidly above a particular molar fraction. At the same time, the deviations of mixtures become smaller with increasing temperature for both density and viscosity. In addition, apparent molar volumes of [BHEM][mesy] and the organic solvents were also calculated; the results indicate that [BHEM][mesy] has larger apparent molar volumes than the solvents under the experimental conditions. With the increase of molar fraction of [BHEM][mesy], the apparent molar volumes increased for the ionic liquid but decreased for the solvents. Moreover, partial molar volumes of [BHEM][mesy] and solvents were calculated and the results indicate that all values are positive and they all increase with increase of temperature. However, the partial molar volumes of [BHEM][mesy] decrease and the partial molar volumes of solvents increase with the increase of molar fraction of [BHEM][mesy] at constant temperature.
References
Lamaming, J., Hashim, R., Leh, C.P., Sulaiman, O., Sugimoto, T., Nasir, M.: Isolation and characterization of cellulose nanocrystals from parenchyma and vascular bundle of oil palm trunk (Claeis guineensis). Carbohydr. Polym. 134, 534–540 (2015)
Chieng, B., Lee, S., Ibrahim, N., Then, Y., Loo, Y.: Isolation and characterization of cellulose nanocrystals from oil palm mesocarp fiber. Polymers 9, 355 (2017)
Prozil, S.O., Evtuguin, D.V., Lopes, L.P.C.: Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 35, 178–184 (2012)
Sindhu, R., Binod, P., Janu, K.U., Sukumaran, R.K., Pandey, A.: Organosolvent pretreatment and enzymatic hydrolysis of rice straw for the production of bioethanol. World J. Microbiol. Biotechnol. 28, 473–483 (2012)
Xie, W., Zhou, D., Ren, Y., Tang, S., Kuang, M., Du, S.: 1-Butyl-3-methylimidazolium chloride pretreatment of cotton stalk and structure characterization. Renew. Energy 125, 668–674 (2018)
Xiao, W., Yin, W., Xia, S., Ma, P.: The study of factors affecting the enzymatic hydrolysis of cellulose after ionic liquid pretreatment. Carbohydr. Polym. 87, 2019–2023 (2012)
Vancov, T., Alston, A.-S., Brown, T., McIntosh, S.: Use of ionic liquids in converting lignocellulosic material to biofuels. Renew. Energy 45, 1–6 (2012)
Elgharbawy, A.A., Alam, M.Z., Moniruzzaman, M., Goto, M.: I onic liquid pretreatment as emerging approaches for enhanced enzymatic hydrolysis of lignocellulosic biomass. Biochem. Eng. J. 109, 252–267 (2016)
Wang, J., Petit, C., Zhang, X., Park, A.-H.A.: Simultaneous measurement of CO2 sorption and swelling of phosphate-based ionic liquid. Green Energy Environ. 1, 258–265 (2016)
Ren, S., Hou, Y., Zhang, K., Wu, W.: Ionic liquids: functionalization and absorption of SO2. Green Energy Environ. 3, 179–190 (2018)
Mora-Pale, M., Meli, L., Doherty, T.V., Linhardt, R.J., Dordick, J.S.: Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 108, 1229–1245 (2011)
Clough, M.T., Geyer, K., Hunt, P.A., Mertes, J., Welton, T.: Thermal decomposition of carboxylate ionic liquids: trends and mechanisms. Phys. Chem. Chem. Phys. 15, 20480–20495 (2013)
Yang, S., Lu, X., Zhang, Y., Xu, J., Xin, J., Zhang, S.: Separation and characterization of cellulose I material from corn straw by low-cost polyhydric protic ionic liquids. Cellulose 25, 3241–3254 (2018)
González, E.J., González, B., Macedo, E.A.: Effect of the relative humidity and isomeric structure on the physical properties of pyridinium based-ionic liquids. J. Chem. Thermodyn. 86, 96–105 (2015)
Alvarez, V.H., Mattedi, S., Aznar, M.: Density, refraction index, and vapor–liquid equilibria of n-methyl-2-hydroxyethylammonium hexanoate plus (methyl acetate, ethyl acetate, or propyl acetate) at several temperatures. Ind. Eng. Chem. Rese. 51, 14543–14554 (2012)
Ghatee, M.H., Bahrami, M., Khanjari, N., Firouzabadi, H., Ahmadi, Y.: A functionalized high-surface-energy ammonium-based ionic liquid: experimental measurement of viscosity, density, and surface tension of (2-hydroxyethyl)ammonium formate. J. Chem. Eng. Data 57, 2095–2101 (2012)
Kurnia, K.A., Wilfred, C.D., Murugesan, T.: Thermophysical properties of hydroxyl ammonium ionic liquids. J. Chem. Thermodyn. 41, 517–521 (2009)
Iglesias, M., Torres, A., Gonzalez-Olmos, R., Salvatierra, D.: Effect of temperature on mixing thermodynamics of a new ionic liquid: {2-hydroxy ethylammonium formate (2-HEAF) + short hydroxylic solvents}. J. Chem. Thermodyn. 40, 119–133 (2008)
Hosseini, S.M., Alavianmehr, M.M., Gutiérrez, A., Khalifeh, R., Moghadasi, J., Aparicio, S.: On the properties and structure of 2-hydroxyethylammonium formate ionic liquid. J. Mol. Liq. 249, 233–244 (2018)
Song, X., Kanzaki, R., Ishiguro, S., Umebayashi, Y.: Physicochemical and acid-base properties of a series of 2-hydroxyethylammonium-based protic ionic liquids. Analy. Sci. 28, 469–474 (2012)
Álvarez, V.H., Dosil, H., Gonzalez-Cabaleiro, R., Mattedi, S., Martin-Pastor, M., Iglesias, M., Navaza, J.M.: Brønsted ionic liquids for sustainable processes: synthesis and physical properties. J. Chem. Eng. Data 55, 625–642 (2010)
Bicak, N.: A new ionic liquid: 2-hydroxy ethylammonium formate. J. Mol. Liq. 116, 15–18 (2005)
Oliveira, L.M.C., Ribeiro, F.R.G., Alcantara, M.L., Pisoni, G.O., Cabral, V.F., Cardozo-Filho, L., Mattedi, S.: High pressure vapor–liquid equilibria for binary methane and protic ionic liquid based on propionate anions. Fluid Phase Equilib. 426, 65–74 (2016)
Li, Y., Figueiredo, E.J.P., Santos, M.J., Santos, J.B., Talavera-Prieto, N.M.C., Carvalho, P.J., Ferreira, A.G.M., Mattedi, S.: Volumetric and acoustical properties of aqueous mixtures of N-methyl-2-hydroxyethylammonium butyrate and N-methyl-2-hydroxyethylammonium pentanoate at T = (298.15 to 333.15) K. J. Chem. Thermodyn. 97, 191–205 (2016)
Brennecke, H., Brennecke, J.F.: Temperature and composition dependence of the density and viscosity of binary mixtures of water + ionic liquid. J. Chem. Eng. Data 51, 2145–2155 (2006)
Zhang, L., Lu, X., Ye, D., Guo, Y., Fang, W.: Density and viscosity for binary mixtures of the ionic liquid 2,2-diethyl-1,1,3,3-tetramethylguanidinium ethyl sulfate with water, methanol, or ethanol. J. Chem. Eng. Data 61, 1023–1031 (2016)
Gong, Y.H., Shen, C., Lu, Y.Z., Meng, H., Li, C.X.: Viscosity and density measurements for six binary mixtures of water (methanol or ethanol) with an ionic liquid ([Bmim][Dmp] or [Emim][Dmp]) at atmospheric pressure in the temperature range of (293.15 to 333.15) K. J. Chem. Eng. Data 57, 33–39 (2011)
Blanco, A., García-Abuín, A., Gómez-Díaz, D., Navaza, J.M.: Density, speed of sound, viscosity and surface tension of 3-dimethylamino-1-propylamine + water, 3-amino-1-propanol + 3-dimethylamino-1-propanol, and (3-amino-1-propanol + 3-dimethylamino-1-propanol) + water from T = (293.15 to 323.15) K. J. Chem. Eng. Data 62, 2272–2279 (2017)
Domańska, U., Laskowska, M.: Temperature and composition dependence of the density and viscosity of binary mixtures of {1-butyl-3-methylimidazolium thiocyanate + 1-alcohols}. J. Chem. Eng. Data 54, 2113–2119 (2009)
Zhang, Z., Zhou, Q., Lu, X., Qiao, C., Zhang, S.: Densities and viscosities of binary mixtures containing 1,3-dimethylimidazolium dimethylphosphate and alcohols. J. Chem. Eng. Data 59, 2377–2388 (2014)
Martín, C.S.: Densities and viscosities of binary mixtures of 1,4-dioxane with 1-propanol and 2-propanol at (25, 30, 35, and 40) °C. J. Chem. Eng. Data 46, 1149–1152 (2001)
Kermanpour, F., Sharifi, T.: Measurement and correlation of the excess properties of ternary mixture of x1[Hmim][BF4] + x21-propanol + x32-propanol at different temperatures. J. Chem. Eng. Data 59, 1922–1929 (2014)
Dománska, U., Królikowska, M.: Density and viscosity of binary mixtures of thiocyanate ionic liquids + water as a function of temperature. J. Solution Chem. 41, 1422–1445 (2012)
Yu, Z., Gao, H., Wang, H., Chen, L.: Densities, viscosities, and refractive properties of the binary mixtures of the amino acid ionic liquid [Bmim][Ala] with methanol or benzylalcohol at T = (298.15 to 313.15) K. J. Chem. Eng. Data 56, 2877–2883 (2011)
Marigliano, A.C.G., Sólimo, H.N.: Density, viscosity, excess molar volume, viscosity deviation, and their correlations for formamide + three alkan-1-ols binary systems. J. Chem. Eng. Data 47, 796–800 (2002)
Castro, M.C., Arce, A., Soto, A., Rodríguez, H.: Thermophysical characterization of the mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate with 1-propanol or 2-propanol. J. Chem. Eng. Data 61, 2299–2310 (2016)
Yang, F., Ma, Q., Wang, X., Liu, Z.: Influence of aprotic cosolvents on the thermophysical properties of imidazolium-based ionic liquid. J. Chem. Eng. Data 62, 1628–1638 (2017)
Hiraga, Y., Koyama, K., Sato, Y., Smith, R.L.: High pressure densities for mixed ionic liquids having different functionalities: 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Chem. Thermodyn. 108, 7–17 (2017)
Abdulagatov, I.M., Tekin, A., Safarov, J., Shahverdiyev, A., Hassel, E.: High-pressure densities and derived volumetric properties (excess, apparent and partial molar volumes) of binary mixtures of methanol + [Bmim][PF6]. J. Solution Chem. 37, 801–833 (2008)
Ayranci, E., Sahin, E.M.: Interactions of imidazolium dased ionic liquids with water studied by density and speed of sound measurements: effect of the chain length of an alkyl substituent on the imidazolium ion. J. Solution Chem. 47, 246–261 (2018)
Bahadur, I., Deenadayalu, N.: Apparent molar volume and isentropic compressibility for the binary systems {methyltrioctylammonium bis(trifluoromethylsulfonyl)imide + methyl acetate or methanol} and (methanol + methyl acetate) at T = 298.15, 303.15, 308.15 and 313.15 K and atmospheric pressure. J. Solution Chem. 40, 1528–1543 (2011)
Verdía, P., Hernaiz, M., González, E.J., Macedo, E.A., Salgado, J., Tojo, E.: Effect of the number, position and length of alkyl chains on the physical properties of polysubstituted pyridinium ionic liquids. J. Chem. Thermodyn. 69, 19–26 (2014)
Hou, H., Jiao, B., Li, Q., Lin, X., Liu, S.: Physicochemical properties, 1H-NMR, ab initio calculations and molecular interaction in binary mixtures of N-methylimidazole with methanol. J. Solution Chem. 47, 1875–1901 (2018)
Katti, P.K., Chaudhri, M.M.: Viscosities of binary mixtures of benzyl acetate with dioxane, aniline and m-cresol. J. Chem. Eng. Data 9, 442–443 (1964)
Prasad, G., Muralidhar Reddy, K., Padamasuvarna, R., Madhu Mohan, T., Vijaya Krishna, T., Ramesh Kumar, V.: Thermophysical properties of 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide with 2-ethoxyethanol from T = (298.15 to 323.15) K at atmospheric pressure. J. Mol. Liquids 251, 335–344 (2018)
González, E.J., González, B., Macedo, E.A.: Thermophysical properties of the pure ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide and its binary mixtures with alcohols. J. Chem. Eng. Data 58, 1440–1448 (2013)
Shafaati, S., Almasi, M.: Influence of temperature and carbon chain on thermophysical properties of benzaldehyde/alkan-2-ol binary mixtures. J. Chem. Eng. Data 62, 2406–2412 (2017)
Chen, Y., Sun, Y., Li, Z., Wang, R., Hou, A., Yang, F.: Volumetric properties of binary mixtures of ionic liquid with tributyl phosphate and dimethyl carbonate. J. Chem. Thermody. 123, 165–173 (2018)
Prasad, G., Reddy, K.M., Padamasuvarna, R., Mohan, T.M., Krishna, T.V., Rao, S.G.: Investigations of molecular interactions in the binary mixtures of 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl) amide and 2-propoxyethanol from T = (298.15 to 323.15) K at atmospheric pressure. J. Solution Chem. 47, 1980–2006 (2018)
Kermanpour, F., Rabie, M.M., Broumand, S.: A thermodynamic and physical study on (1-hexyl-3-methylimidazolium chloride + 1-pentanol and ethylene glycol) binary mixtures at temperatures (293.15–333.15) K. J. Solution Chem. 46, 2091–2108 (2017)
Carmen Grande, M.d., Juliá, J.A., García, M., Marschoff, C.M.: On the density and viscosity of (water+dimethylsulphoxide) binary mixtures. J. Chem. Thermodyn. 39, 1049–1056 (2007)
Santos, Â.F.S., Moita, M.-L.C.J., Nobre, L.C.S., Lampreia, I.M.S.: A volumetric and acoustic study of pseudo-binary mixtures of (water + 1,3-propanediol + 3-butoxypropan-1-amine) from T = (283.15 to 303.15) K. J. Chem. Thermodyn. 128, 344–351 (2019)
Acknowledgements
This research was supported financially by the National Natural Scientific Fund of China (No. 21978291, 21776289, 21878292, 21606240) and K. C. Wong Education Foundation. Sincerely appreciate Prof. Suojiang Zhang (IPE, CAS) for his careful academic guidance and great support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, G., Yang, S., Wang, X. et al. Densities and Viscosities of Binary Mixtures Containing the Polyhydric Protic Ionic Liquid(2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate) and Water or Alcohols. J Solution Chem 49, 423–457 (2020). https://doi.org/10.1007/s10953-020-00968-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00968-2