Abstract
Correlation between lactic acid bacteria (LAB) survivability and physical properties of microcapsules is critical to revealing the protecting mechanism of microcapsules for LAB. In this paper, five formulae of microcapsules with increasing mechanical strength were chosen to encapsulate Lactobacillus acidophilus CGMCC1.2686 using the method of emulsification/internal gelation. Morphological and size characteristics showed that particle diameter of the five LAB microcapsules ranged from 263.4 to 404.6 μm, with a span factor from 0.87 to 1.12. The increased mechanical strength of the microcapsules was accompanied with increased viscoelasticity and structural compactness as observed by scanning electron microscopy. Most of the microspheres shrinked in simulated gastric juice (SGJ), whilst swelling in bile salts solution (BS). Regression analysis showed that cell viability in SGJ was positively correlated with the mechanical strength of microcapsules. However, increasing mechanical strength did not significantly improve cell survival in BS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics were defined as “live microorganisms (bacteria or yeasts), which when ingested or locally applied in sufficient numbers confer one or more specified demonstrated health benefits for the host” (FAO/WHO, 2001). As the most common probiotics, lactic acid bacteria (LAB) are typically associated with the human and animal gastrointestinal tract and play an import role in the gut microbiota and are frequently incorporated to food products for health benefits [1]. The viable bacterial or yeast numbers is usually estimated by colony-forming units (cfu). A minimum viable population of 107–109 cfu/mL was recommended for probiotic drinks in order to colonize the intestine [2]. However, LAB are susceptible to adverse conditions, such as acid, heat, stress and oxygen, resulting in a significant reduction in LAB viable number. Therefore, providing viable LAB with physical barriers to resist harmful conditions is the key technology for their application in foods. Microencapsulation has been recognized as an effective approach to enhance the LAB viability both in food products and in the gastro-intestinal environment [3, 4].
Alginate is one of the most widely used encapsulating materials, which is a linear heteropolysaccharide of β-D-mannuronic acid [M] and α-L-guluronic acid [G] and is extracted from various species of algae [5]. Emulsion is one of the most common techniques to prepare alginate beads. There are two basic methods to achieve this, namely emulsification/external gelation and emulsification/internal gelation. Compared with emulsification/external gelation, emulsification/internal gelation avoids the clumping of microspheres caused by directly adding CaCl2 solution and is more practicable in controlling particle size [6–8]. Emulsification/internal gelation has been widely used to encapsulate probiotics [9–11], islet cells [6, 12], Bacillus Calmette-Guerin [13], Clostridium sulfatireducens [14], microalgae [15], enzymes [16–18] and insulin [19, 20].
Several studies reported the analysis of mechanical strength of alginate microcapsules containing LAB [7, 21–23]. However, information on the correlation between the mechanical properties and the encapsulated LAB survivability is limited. Sandoval-Castilla et al. analyzed the correlation between encapsulated Lb. casei survival and physical properties of microspheres [22]. The results showed that the survivability of Lb. casei in yoghurt, and in simulated gastric juice, was positively correlated with the textural properties of the beads (hardness, springiness, cohesiveness, and resilience).
In a previous study, we compared the physical properties and probiotic survivals of alginate-CaCO3 and alginate-Ca-EDTA microcapsules, and found that CaCO3 was a more suitable calcium source than Ca-EDTA to solidify alginate for the encapsulation of Lactobacillus acidophilus CGMCC1.2686 using the emulsification/internal gelation method [24]. The main objective of this work is to systemically analyze the correlation between the capsule mechanical strength and the encapsulated L. acidophilus CGMCC1.2686 survivals in simulated gastric juice (SGJ) and in bile salts solution (BS).
Materials and Methods
Materials
Sodium alginate (Manucol DM, Mw 200 kDa) was obtained from FMC BioPolymer (Philadelphia, USA), which consists of 35.8 % G monomer and 64.2 % M monomer. Nano-sized CaCO3 was purchased from Zhenxin Reagent Factory (Shanghai, China), which is in powder form and of analytical reagent. Bile salt No. 3, pepsin (3000 U/mg) and other chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Preparation of Cell Culture
L. acidophilus CGMCC1.2686 from China General Microbiological Culture Collection Center was stationarily cultivated in the de-Man, Rogosa and Sharpe (MRS) medium [25] at 37 °C for 24 h. The cells were collected by centrifugation at 8000 g for 10 min, washed twice with sterile saline solution (9 g/L NaCl) and re-suspended, resulting in a cell concentrate of about 1010 cfu/mL.
Preparation of Empty and Encapsulated Microspheres
Microspheres were prepared by emulsification/internal gelation method as described in a previous work [24]. A basal encapsulation protocol (2.0 % w⁄w sodium alginate, calcium⁄alginate monomer molar ratio ([Ca]/[M+G]) = 0.25, acidification pH 3.5) was described in the following. The monomer (guluronic or manuronic acid residue, C6H10O7, Mw = 194) concentration of 2.0 % sodium alginate was about 100 mM, and thus a final calcium concentration of 25 mM was added to achieve a [Ca]/[M+G] ratio of 0.25. At first, an alginate mixture of 3.0 % w⁄w sodium alginate and 37.5 mM CaCO3 was prepared and homogenized. Then, 20 mL of the alginate mixture was well blended with 10 mL cell concentrate to prepare cell mixture. The cell mixture was dispersed into 70 mL soybean oil containing 1 % v⁄v Span-80. After emulsification for 15 min at 300 rpm, 20 mL of soybean oil containing 0.8 g glacial acetic acid was added to reach an acidification pH value of 3.5. The system was continuously stirred for 30 min to allow the reaction of alginate with calcium ions and consequently the solidification of capsules. Phosphate buffer (0.1 M, pH 7.0) was added to separate the microspheres from oil phase. Microspheres were collected and washed with buffer to remove oil. Microspheres were harvested by centrifugation, stored at 4 °C and used for the following measurements.
To design microcapsules with different mechanical strengths, alginate concentration or [Ca]/[M+G] was changed with all other parameters being fixed. Alginate concentration varied as 1, 2, 3, 4 and 5 % (w/w) and [Ca]/[M+G] as 0.25, 0.33, 0.5, 0.67 and 1.
Morphological and Particle Size Analysis
Microsphere morphology was investigated using scanning electron microscope (JEOL JSM-6390lv, Tokyo, Japan). Size distribution was measured in distilled water using Malvern Mastersizer 2000 (Malvern Instruments Ltd, Malvern, UK). Particle size is expressed as volume mean diameter (D[4, 3], μm). Particle size distribution is calculated using span factor as an index: span = [D(v, 90) − D(v, 10)]/D(v, 50), where D(v, 90), D(v, 10) and D(v, 50) are the diameters at 90, 10 and 50 % of the cumulative volume, respectively [26]. It provides a direct indication of the range of droplet sizes relative to D[4, 3]. A higher span value indicates a wider distribution in size and a higher polydispersity [26].
Analysis of Mechanical Strength and Viscoelasticity
Haake Rheostress 6000 rheometer (Thermo Scientific, USA) with parallel plate geometry (35 mm in diameter) at 25 °C was used to evaluate the mechanical strength and viscoelasticity of microspheres. Mechanical strength was determined by compression using a normal force mode. Three grams microsphere slurry was loaded onto the measuring plate and was pressed by the upper plate at a constant speed of 0.005 mm/s, from an initial gap of 1 mm to a final gap of 0.14 mm. The change of normal force (Fn) during the compression was recorded. Fn at 0.15 mm was chosen to characterize the mechanical strength of microsphere. Viscoelastic properties were evaluated using a dynamic oscillatory mode. Storage (G′) and loss (G″) moduli were recorded in the frequency range of 0.1 to 100 rad/s and within the linear viscoelastic region of the microspheres.
Encapsulation Efficiency
The encapsulation yield (EY) of microcapsules was calculated according to previous reports [11, 24]. One gram microspheres were added to 9 g tempered (37 °C) peptone saline (1 g/L peptone, 8.5 g/L NaCl). The suspension was homogenized at 10 000 rpm for 30 s (PT-MR 2100, Kinematica, Switzerland) and was then gently shaked in a rotary shaker for 30 min. EY of microcapsules was calculated as:
where N is the number of viable cells released from the microspheres and N0 is the number of viable cells in the cell concentrate for microencapsulation. The enumeration of viable cells in microsphere or cell concentrate was carried out through 10-fold serial dilution, spread plating on MRS agar, and counting the number of colonies formed on the plates after anaerobic incubation at 37 °C for 48 h.
Cell Survival in SGJ
Simulated gastric juice (SGJ) consisted of 0.2 % (w/v) NaCl and 0.32 % (w/v) pepsin at pH 2.0. One gram of microsphere slurry was added to 9 g pre-warmed (37 °C) SGJ, and then incubated in a 37 °C water bath. After 120 min of incubation, pH of the microsphere suspension was adjusted to 6.0–8.0 with 1 M NaOH. The viable cells in microcapsules after exposure to SGJ were enumerated by breaking the microcapsules (homogenization at 10 000 rpm for 30 s), shaking, dilution, spread plating, anaerobic incubation and counting, as described in Section Encapsulation efficiency. Microsphere slurry in saline solution, instead of SGJ, namely blank control, was analyzed to measure the initial counts. The same approach was taken for free cells to determine the survival in SGJ. Cell survival rate in SGJ (RSGJ) was calculated according to the following formula:
where NSGJ is the number of viable cells in microcapsules after exposure to SGJ, and N0-SGJ is the initial counts from the blank control test, using saline solution instead of SGJ.
Cell Survival in BS
Bile salts solution (BS) consisted of 0.68 % (w/w) KH2PO4 and 1.0 % (w/w) bile salts at pH 6.8. One gram of microcapsules was added into 9 g pre-warmed (37 °C) BS, and incubated at 100 rpm in a 37 °C water bath. Microsphere suspension was sampled after 30 min of incubation. The viable cells in microcapsules after exposure to BS were counted following the procedure of enumerating viable cells exposed to SGJ. Blank control (microsphere slurry in saline solution, instead of BS) was analyzed to measure the initial counts. The same approach was taken for free cells to determine the survival in BS. Cell survival rate in BS (RBS) was calculated from the following equation:
where NBS is the number of viable cells in microcapsules after exposure to BS, and N0-BS is the initial counts from the blank control test, using saline solution instead of BS.
Swelling Behavior of Microspheres in SGJ and BS
Similar to cell survival test, 3 g microspheres was added to 27 g pre-warmed SGJ or BS, and incubated at 37 °C for 2 h and 30 min, respectively. Swollen microspheres were harvested by centrifugation. Size of the particles before and after swelling process was determined using Malvern Mastersizer 2000. The swelling index was calculated from the ratio of the size of swollen particles in SGJ or BS to that of initial spheres.
Statistical Analysis
All the analysis experiments for each microsphere sample were performed in triplicate, and the results were expressed as mean ± SD (standard deviation). Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The significance of differences in mechanical strengths of selected microspheres, encapsulation yields and cell survivals was determined by ANOVA and the correlation between bacterial survivals and mechanical strengths was analyzed using the simple linear regression. Values of p < 0.05 were considered significant [27].
Results and Discussion
Preparation of Empty Alginate Beads with Different Mechanical Strength
Microcapsules with different mechanical strength were obtained before analyzing the correlation between encapsulated LAB survivability and capsule mechanical properties. Mechanical strength was evaluated in compression test using a normal force (Fn) mode. In a previous research, rupture behaviour was clearly observed during the compression of alginate capsules and hence Fn at rupture point was used to characterize capsule mechanical strength [24]. In the present work, we failed to observe the rupture phenomena due to the hardness of prepared capsules well beyond the Fn limit of the rheometer used. Instead, the Fn at a compression gap of 0.15 mm was employed to characterize the mechanical strength of alginate capsules.
As indicated in Table 1, capsule mechanical strength could be varied by using different alginate concentration and calcium⁄alginate monomer molar ratio ([Ca]/[M+G]). Both the two factors greatly affected the mechanical strength of alginate microcapsules. Mechanical strength increased with increasing alginate concentration and [Ca]/[M+G] ratio, and was in the range of 1.23 to 53.47 N. Five formulae for microparticles with gradually increasing mechanical strength were chosen to encapsulate LAB and were used for analysis of the correlation between LAB survivability and capsule mechanical properties. The five samples were named A, B, C, D and E, respectively. Among them, A, B and C had alginate concentrations of 2, 3 and 4 % (w/w), respectively, and owned a fixed [Ca]/[M+G] ratio of 0.33, while C, D and E had a fixed alginate concentration of 4 % with increasing [Ca]/[M+G] ratio from 0.33, 0.67 to 1.
Morphological and Size Characteristics of LAB Microcapsules
As shown in Table 2, the mean diameters of capsule A, B and C increased from 268.2 to 404.6 μm when alginate concentration was increased from 2 to 4 % (w/w), and this increase in size was accompanied with an increase in span factor from 0.87 to 0.93. Larger beads with a wider distribution as a result of higher alginate concentration have been reported in previous researches [12, 20, 28, 29]. This phenomenon is due to the effect of increased viscosity, which is unfavorable for emulsification during stirring [20].
Increasing calcium concentration also affected particle diameter and size distribution. As [Ca]/[M+G] ratio increased from 0.33 to 1, the mean diameter of capsules C, D and E decreased from 404.6 to 263.4 μm but the span factor increased significantly from 0.93 to 1.12. This might be explained that higher degree of crosslinking in the presence of more calcium ions leads to more compact structure of alginate microspheres. Similar to our results, a slight decrease in mean size of insulin microcapsules with increasing Ca/alginate ratio was observed by Silva et al. [20].
Scanning electron micrographs of freeze dried microcapsules are shown in Fig. 1. All the microcapsules were sphere-like and it seemed that the structure of alginate-CaCO3 microcapsules became more and more compact as the mechanical strength increased (from capsules A to E). The surface of all the microspheres was quite rough. It has been reported that the wrinkled surface was due to the loss of water during freeze-drying process [30].
Mechanical Properties of LAB Microcapsules
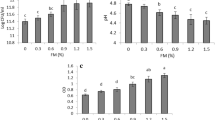
As shown in Fig. 2a, mechanical strength of capsules A, B, C, D and E was 4.27 ± 0.17, 13.10 ± 0.54, 26.90 ± 1.13, 31.80 ± 0.73 and 35.10 ± 0.23 N, respectively, which increased in sequence and conformed to our design. However, compared to the corresponding mechanical strength (8.02 ± 0.54, 20.18 ± 1.35, 32.10 ± 0.64, 42.15 ± 0.39, and 53.47 ± 2.43 N) of empty capsules, mechanical strength of LAB-loaded capsules was lower, indicating that cell loading reduced the mechanical strength of alginate capsules. The result is in agreement with the previous reports [31, 32], where incorporation of cells into capsules was believed to weaken the physical properties and the structure of polymeric matrix of the capsules. Junter & Vinet explained that cell particles could bind Ca2+ ions and screen part of the cross-linking sites (i.e., carboxyl groups of guluronate residues) on alginate chains [31].
Figure 2b shows the viscoelastic properties of capsules A, B, C, D and E. G′ and G″ represent the storage and loss moduli of the measured materials, respectively. For all the microcapsules, G′ > G″ and was almost independent of frequency, which is the characteristic of elastic bodies and is expected for the gelled microcapsules. As shown in Fig. 2b and Table 3, increasing alginate concentration or [Ca]/[M+G] led to increase in elasticity (G′) and viscosity (G″).
Both the compression test and dynamic oscillatory rheology pointed to higher mechanical strength and larger elasticity for capsules with higher alginate concentration. The reason might be that increasing alginate concentration resulted in an increased solid volume fraction and an increased density of cross-linking, which impart greater hardness to the gel [33, 34]. Similar tendency was obtained for capsules with higher [Ca]/[M+G]. This might be due to that higher CaCO3 concentration provided more free calcium and more cross-linking for alginate.
Swelling Behavior of Microcapsules in SGJ and BS
Swelling behavior of microcapsules in aqueous phase indicates the influence of external solution on the internal encapsulants. In SGJ, most of the capsules shrinked, except capsule E (Table 4). Underlying mechanisms for capsule swelling in SGJ are complicated and possible explanations could be: 1) capsule swelled in SGJ due to osmotic pressure; 2) acidic SGJ (pH 2) caused the protonation of carboxylic groups of alginate chains, leading to shrinking of alginate network due to reduced electrostatic repulsion and/or dissociation of alginate network. These mechanisms could work jointly, leading to an overall shrinking of alginate capsules in SGJ.
In BS, all the capsules swelled and expanded (Table 4). Capsules with higher mechanical strength could resist higher osmotic pressure and tended to swell less, as is reflected by a decreased swelling index in capsules A, B and C. On the other hand, the phosphate anions present in BS can effectively sequester calcium ions, leading to the formation of calcium phosphate precipitate (white precipitates observed) and the dissociation of alginate network [35]. Increasing [Ca]/[M+G] ratio in capsules C, D and E showed an increased swelling index, possibly due to more crosslinking sites being dissociated by phosphate anions leading to a higher capacity of swelling. Detailed studies on the swelling of alginate capsules in SGJ and BS should be the topic of a future investigation.
Encapsulation Yields
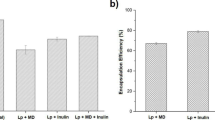
Encapsulation yield is an important factor to determine the production cost of microspheres. As shown in Table 5, the encapsulation yield of capsules A, B and C increased significantly from 11.6 to 42.3 % as the alginate concentration increased from 2 to 4 %. Using similar encapsulation method, higher drug encapsulation yield was obtained with increasing alginate concentration as described by Silva et al. and Sultana et al. [20, 29] When [Ca]/[M+G] ratio increased from 0.33 to 1, the encapsulation yield of capsules C, D and E increased slightly from 42.3 to 55.5 %. Higher cross-linking agent concentration was favorable for cell encapsulation. The results are also in accordance with the reports by Silva et al. and Sultana et al. [20, 29]
Encapsulation yields in the work are mostly lower than those reported for Bifidobacterium bifidum F-35 (48.1 %) [11], L. fermentum CECT5716 (74.41 %) [10] and yeast cells (Y235) (77 %) [36] in alginate microcapsules, which were prepared by a similar method of emulsification/internal gelation. One reason could be that our acidification pH is lower than those used in the literature and causes more biological damage. A control study was done by incubating L. acidophilus cell (1.07 ± 0.25 * 1010 cfu) in MRS medium (pH 3.5) at 37 °C for 1 h and it was found that only 51.7 ± 2.6 % cells survived. This indicated that acidification pH had a great influence on cell survival during microencapsulation. Other alginate encapsulation methods, such as extrusion and emulsification/external gelation, avoided acid stress of emulsification/internal gelation. However, extrusion is deficient in diameter control and emulsification/external gelation showed a significant degree of microsphere clumping after CaCl2 solution was added. Internal gelation approach overcomes the above problems and the acidification parameters, eg. acid/calcium molar ratio and acidification time, should be optimized to achieve a high encapsulation yield.
Cell Survivals in SGJ
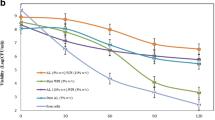
Acid resistance of free and encapsulated L. acidophilus CGMCC1.2686 in capsules with different mechanical strength was studied. After exposure to SGJ for 120 min, survivals of free cells and encapsulated cells of capsules A, B, C, D and E were 1.00*10−4, 5.4, 19.4, 23.5, 25.4 and 26.3 %, respectively (Table 5). Much higher cell survivals of encapsulated cells than free cells suggested that microencapsulation using the technique of emulsification/internal gelation greatly improved probiotic resistance to acidic condition, which is in agreement with previous reports [11, 24]. Results from the present study indicate that cell survivals in SGJ increased with increasing alginate concentration and [Ca]/[M+G] ratio. No previous studies dealt with the effects of alginate concentration and [Ca]/[M+G] ratio on cell survival for capsules prepared using emulsification/internal gelation method. In other encapsulation approaches, increasing alginate concentration was found to increase the viability of probiotics in SGJ [37–39], while excessive calcium ions had limited effect [40].
It seemed that mechanical strength, rather than swelling index, played an important role in cell viability in SGJ. A simple linear regression analysis was used to analyze the correlation between cell survivals in SGJ and mechanical strength of the microcapsules. The results exhibited a positive linear correlation (Fig. 3). The regression equation Y = 0.608 X + 6.470 was statistically significant (F = 18.560 > F0.05(1, 3) = 10.1, p = 0.023 < 0.05), and the coefficient of determination R2 = 0.860, demonstrating that viability of L. acidophilus CGMCC1.2686 in SGJ was highly positively correlated with mechanical strength of the capsules, which agrees with the conclusion of Sandoval-Castilla et al. [22] This might be explained by more integrated structure and smaller mesh size of the network associated with higher mechanical strength. Acid in SGJ penetrated into beads through gel mesh, forming an acid gradient in the microspheres. This process could also be reflected by the swelling index (Table 4). Microspheres with low mechanical strength shrinked a lot in SGJ, indicating more alginate carboxylic groups in the outside layer were protonated by acid and more acid entered to damage cells.
Cell Survivals in BS
In order to exert health benefit, LAB should survive the harsh conditions of the stomach and upper intestine that contain bile [41]. Therefore, survivals of free and encapsulated L. acidophilus CGMCC1.2686 in BS were investigated. After exposure to BS for 30 min, survival rates of free and encapsulated L. acidophilus CGMCC1.2686 of A, B, C, D and E microcapsules were 6.9*10−5, 6.3*10−4, 1.4*10−4, 7.0*10−4, 4.3*10−4 and 3.6*10−4 %, respectively (Table 5). Microencapsulation enhanced cell viability in BS by 2–10 folds, whereas in SGJ by more than 10 000 folds. The big difference might be due to different capsule swelling behaviors in SGJ and BS. Capsules in SGJ tended to shrink, and protected LAB cells from acidic injury effectively. In contrary, capsules in BS swelled significantly and lost integrity due to phosphate anions, and could not provide effective protection against bile salts injury. Therefore, cell survival in BS-swollen capsules was only marginally better than that of free LAB. Furthermore, from the free cell survivals in SGJ and BS (Table 5), it is implied that L. acidophilus CGMCC1.2686 is more susceptible to bile salts than acid. Bile salts behave as biological surfactants and could penetrate into LAB cell membrane and damage membrane integrity [42, 43], resulting in low survivals of encapsulated cells. Because of the loss of capsule integrity cell viability in BS did not show positive correlation with capsule mechanical strength. This differs from the case in SGJ.
There have been reports that encapsulation of probiotics in alginate could significantly increase cell viability in bile salts [37–39]. The contradiction could be attributed to the use of phosphate in BS in the present study. The difference could also be caused by different encapsulation methods used or different strains of LAB having different resistance to bile salts.
Conclusions
Mechanical strength is one of the important physical properties of microcapsules regarding the survivability of probiotics. In this paper, five microcapsules with different mechanical strengths were used to encapsulate L. acidophilus CGMCC1.2686. Mechanical measurements demonstrated that capsules with higher mechanical strengths are always more elastic, and images observed under electron scanning microscopy confirmed their denser structure. Interestingly, bacteria survivals in SGJ of the five microcapsules were positively correlated with mechanical strength, while increasing mechanical strengths did not effectively enhance cell survival in BS. This might be explained by their difference of swelling behavior in SGJ and BS.
References
J. Walter, Appl. Environ. Microbiol. 74(16), 4985–4996 (2008)
T. Mattila-Sandholm, P. Myllärinen, R. Crittenden, G. Mogensen, R. Fondén, M. Saarela, Int. Dairy J. 12(2–3), 173–182 (2002)
S. Sarkar, Br. Food J. 112(4), 329–349 (2010)
J. Burgain, C. Gaiani, M. Linder, J. Scher, J. Food Eng. 104(4), 467–483 (2011)
P. Gacesa, Carbohydr. Polym. 8(3), 161–182 (1988)
C.A. Hoesli, K. Raghuram, R.L.J. Kiang et al., Biotechnol. Bioeng. 108(2), 424–434 (2011)
W. Krasaekoopt, B. Bhandari, H. Deeth, Int. Dairy J. 13(1), 3–13 (2003)
D. Poncelet, B. Poncelet De Smet, C. Beaulieu, M.L. Huguet, A. Fournier, R.J. Neufeld, Appl. Microbiol. Biotechnol. 43(4), 644–650 (1995)
B.C. Larisch, D. Poncelet, C.P. Champagne, R.J. Neufeld, J. Microencapsul. 11(2), 189–195 (1994)
M.J. Martin, F. Lara-Villoslada, M.A. Ruiz, M.E. Morales, LWT Food Sci. Technol. 53(2), 480–486 (2013)
Q. Zou, J. Zhao, X. Liu et al., Int. J. Food Sci. Technol. 46(8), 1672–1678 (2011)
C.A. Hoesli, R.L.J. Kiang, D. Mocinecová et al., J. Biomed. Mater. Res. B 100B(4), 1017–1028 (2012)
A. Esquisabelr, M. Hernáandez, M. Igartuaa, R. Gascóan, B. Calvo, J.L. Pedraz, J. Microencapsul. 14(5), 627–638 (1997)
R. Börner, M. Aliaga, B. Mattiasson, Biotechnol. Lett. 35(3), 397–405 (2013)
E. Pales Espinosa, L. Barillé, B. Allam, J. Exp. Mar. Biol. Ecol. 343(1), 118–126 (2007)
X. Wang, K.-X. Zhu, H.-M. Zhou, Int. J. Mol. Sci. 12(5), 3042–3054 (2011)
Q. Liu, A.M. Rauth, X.Y. Wu, Int. J. Pharm 339(1–2), 148–156 (2007)
A.W.J. Chan, I. Mazeaud, T. Becker, R.J. Neufeld, Enzym Microb. Technol. 38(1–2), 265–272 (2006)
C.M. Silva, A.J. Ribeiro, D. Ferreira, F. Veiga, Eur. J. Pharm. Sci. 29(2), 148–159 (2006)
C.M. Silva, A.J. Ribeiro, I.V. Figueiredo, A.R. Gonçalves, F. Veiga, Int. J. Pharma 311(1–2), 1–10 (2006)
L. Baruch, M. Machluf, Biopolymers 82(6), 570–579 (2006)
O. Sandoval-Castilla, C. Lobato-Calleros, H.S. García-Galindo, J. Alvarez-Ramírez, E.J. Vernon-Carter, Food Res. Int. 43(1), 111–117 (2010)
U. Schuldt, D. Hunkeler, J. Microencapsul. 24(1), 1–10 (2007)
S. Cai, M. Zhao, Y. Fang, K. Nishinari, G.O. Phillips, F. Jiang, Food Hydrocoll. 39, 295–300 (2014)
J.C. De Man, M. Rogosa, M.E. Sharpe, J. Appl. Bacteriol. 23(1), 130–135 (1960)
A. Lefebvre, G.A. Matthews, E.C. Hislop, Application technology for crop protection (CAB, Wallingford, 1993), pp. 85–100
A.A. Guerra-Ordaz, G. González-Ortiz, R.M. La Ragione et al., Appl. Environ. Microbiol. 80(16), 4879–4886 (2014)
X.D. Liu, D.C. Bao, W.M. Xue et al., J. Appl. Polym. Sci. 87(5), 848–852 (2003)
Y. Sultana, S. Mall, D.P. Maurya, D. Kumar, M. Das, Pharm. Dev. Technol. 14(3), 321–331 (2009)
X. Li, X. Chen, J. Ocean Univ. China 8(1), 39–44 (2009)
G.A. Junter, F. Vinet, Chem. Eng. J. 145(3), 514–521 (2009)
J.M. Van Raamsdonk, P.L. Chang, J. Biomed. Mater. Res. 54(2), 264–271 (2001)
C.K. Kuo, P.X. Ma, Biomaterials 22(6), 511–521 (2001)
M.A. LeRoux, F. Guilak, L.A. Setton, J. Biomed. Mater. Res. 47(1), 46–53 (1999)
Y. Murata, K. Nakada, E. Miyamoto, S. Kawashima, S.-H. Seo, J. Control. Release 23(1), 21–26 (1993)
H. Song, W. Yu, M. Gao, X. Liu, X. Ma, Carbohydr. Polym. 96(1), 181–189 (2013)
V. Chandramouli, K. Kailasapathy, P. Peiris, M. Jones, J. Microbiol. Meth. 56(1), 27–35 (2004)
K.-Y. Lee, T.-R. Heo, Appl. Environ. Microbiol. 66(2), 869–873 (2000)
S. Mandal, A.K. Puniya, K. Singh, Int. Dairy J. 16(10), 1190–1195 (2006)
I. Trabelsi, W. Bejar, D. Ayadi et al., Int. J. Biol. Macromol. 61, 36–42 (2013)
L.-S. Chou, B. Weimer, J. Dairy Sci. 82(1), 23–31 (1999)
D. Provenzano, C.M. Lauriano, K.E. Klose, J. Bacteriol. 183(12), 3652–3662 (2001)
J.Y. Sung, E.A. Shaffer, J.W. Costerton, Dig. Dis. Sci. 38(11), 2104–2112 (1993)
Acknowledgments
The research was supported by National Natural Science Foundation of China (31322043, 31171751), Natural Science Foundation of Hubei Province (2012FFB00705, 2012FFA004), Projects from Hubei Provincial Department of Education (Q20141401, T201307), Scientific Research Foundation of Hubei University of Technology (BSQD12051), Program for New Century Excellent Talents in University (NCET-12-0710), and Key Project of Chinese Ministry of Education (212117).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, M., Qu, F., Cai, S. et al. Microencapsulation of Lactobacillus acidophilus CGMCC1.2686: Correlation Between Bacteria Survivability and Physical Properties of Microcapsules. Food Biophysics 10, 292–299 (2015). https://doi.org/10.1007/s11483-014-9389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-014-9389-5