Abstract
There is an increasing interest in the food industry to use potato protein as an alternative plant protein. However, its bitter taste often limits the utilization in novel foods and beverages. Coacervation is a promising technique to mask bitterness of certain food compounds. In the present study, we aimed to reduce the degree of bitterness of potato protein by generating protein-pectin complexes based on coacervation. Potato protein isolate and pectins derived from various origins having different degree of esterification (DE) were initially mixed under acidic conditions to promote the formation of complexes. Single and complex biopolymers were then characterized in terms of surface charge, solubility, rheological and sensorial properties as a function of protein pectin ratio, pectin source, and the degree of esterification, respectively. The protein-pectin ratio and degree of esterification of pectins substantially influenced the interaction behaviour and phase separation of the protein-pectin mixtures. The bitterness score decreased with increasing surface charge and pectin concentration. Bitterness was strongly reduced for complexes formed with high DE citrus pectin at a protein pectin ratio 0.33. The complexes generated at this ratio were relatively stable based on visual observation and microscopic images. Our results might have significant implications for the utilization of potato proteins in beverage applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for protein-rich beverages increased over the past decade due to the fast growing trend of anti-aging and consumer preferences towards a healthier diet. Beverages with a high protein content (> 4%) currently available on the market mainly contain animal-derived proteins, such as whey, caseinate or gelatin. However, consumers’ demand in plant-based products is increasing due to moral, religious and cultural dietary prohibitions/decisions and concerns over the safety of animal-derived products become widespread. Because of these reasons, beverage industry has begun to utilize plants as an alternative to animal-based sources in this kind of beverages [1,2,3,4,5].
Plant proteins are known to have potential positive health effects such as decreasing the plasma cholesterol, decreasing coronary heart disease development and controlling calorie intake. They can provide an essential high quality protein in a concentrated form for specially designed low-calorie/high-nutrient-density meals [6]. In addition, mixtures of plant proteins serve as a well-balanced source of amino acids to meet human physiological needs [7]. However, organoleptic properties are as important as nutritional and health effects when introducing these proteins into foods and beverages. Mostly, plant proteins have been found to have a bitter taste and inferior flavor which limits their use in high protein beverages [8, 9]. The sensation of bitter taste was explained by a possible electrical response induced by bitter compounds in non-gustotary cells, such as neuroblastoma cells and olfactory cells. Moreover, it was suggested that, electrical response to bitterness compounds could prolong the bitterness sensation in an ionic medium which could also occur in the presence of proteins due to their cationic nature [10].
Currently, the food industry applies various methods such as the addition of sweet-tasting compounds, salts, odorants or texturants to reduce the bitterness [11]. However, these traditional techniques could negatively impact the healthiness of functional foods and beverages. In this context, various approaches including the removal, encapsulation and complexation are being investigated to reduce the sensation of bitterness [12, 13]. Several studies suggested that, polysaccharides such as alginate, chitosan or pectin could be utilized to form complexes with bitter molecules, thus preventing the contact with the receptor during in-mouth processing [14]. Accordingly, complex coacervation between proteins and polysaccharides which renders an electrostatic complexation between oppositely charged proteins and polysaccharides could lower the unpleasant bitter taste of plant proteins [13].
Potato proteins are by-products of starch production and its nutritional value has been shown to be greater than that of other vegetable or cereal proteins and casein while being comparable to that of egg protein [15,16,17,18]. Potato proteins are divided into three major fractions which are patatins, protease inhibitors and remainder proteins. Patatins (35–40%) are glycoproteins with a molecular weight around 40 kDa, having a pI at 4.8–5.2. The protease inhibitors (25–50%) are a diverse group of proteins with a molecular weight of 7–21 kDa and the pI varying between 5 and 8. The remainder proteins (~10%) are enzymes with different high molecular weight [19, 20]. In contrast, pectin is an anionic cell-wall polysaccharide which is composed of a backbone of (1 → 4) α-D-galacturonosyl residues interrupted with typically a 10% substitution of (1 → 2)-α-L-rhamnopyranosyl residues [21]. Generally, the main sources for commercial pectin production are apple pomace and citrus peel [22]. Apple pectins (AP) and citrus pectins (CP) have different functional properties due to their structural differences. Pectins are classified based on two parameters: the degree of methyl esterification of the carboxyl groups and the distribution of these methyl-esters along the pectin backbone. The ratio of methyl-esterified galacturonic acid groups to total galacturonic acid groups is termed as the degree of esterification (DE). Thus, high-methoxy pectins have ≥ 50% DE, whereas low methoxy pectins have < 50% DE [23]. The other parameter which is related to distribution of the methyl-esters is represented as the degree of blockiness (DB). A high DB value means that the methyl-esters are distributed in a blockwise manner while low DB value corresponds a random distribution [24].
Although its potential to use as an alternative food ingredient to animal protein, the bitter taste coming from potato protein isolate could limit its use in new food or beverage formulations [13]. Complex coacervation is one of the promising methods to mask bitterness by means of biopolymer utilization. Biopolymers could prevent bitter compounds coming in contact with the human taste buds and delay the dissolution progress in the mouth [25,26,27]. According to our knowledge, there are no studies investigating the complex formation of potato proteins, using different sources of pectins with varying DE, on the effect on bitterness. We hypothesized that the magnitude of bitterness would be reduced with an increase in protein-polysaccharide complex formation as it decreases the electrical charge of the cationic proteins. Thus, the degree of esterification could change the optimum protein-pectin ratio for complexation and accordingly the perception of bitterness. In addition, the pectin’s origin could also have an influence, as the degree of blockiness that affects protein-pectin interactions is highly depended on the pectin source. Therefore, in this study the effect of the following parameters on protein-pectin complex formation as well as on the perception of bitterness were investigated: The pectins’ degree of esterification (high and low), their origin (apple and citrus) and the ratio of protein to pectin. All biopolymers were individually characterized in terms of solubility and surface charge.
Materials and Methods
Materials
Food grade potato protein isolate was donated by Döhler GmbH (Darmstadt, Germany). Potato protein isolate contained (min.) 90% protein, (max.) 9% moisture, (max.) 5% ash and (max.) 0.5% sulphite according to specification provided by manufacturer. Apple pectin with 71% DE (AP DE71, Pectin Classic AU-L 051/15), apple pectin with 35% DE (AP DE35, Pectin Classic AU-L 051/15), citrus pectin with 70% DE (CP DE70, Pectin Classic CU-L 053/15) and citrus pectin with 35% DE (CP DE35, Pectin Classic CU-L 054/15) were supplied from Herbstreith & Fox KG (Neuenbürg, Germany) and used without further purification. Bradford reagent Roti®-Quant, analytical grade sodium hydroxide (NaOH), and hydrochloric acid (HCl) were obtained from Carl Roth GmbH+Co. KG (Karlsruhe, Germany). Lactic acid solution was supplied by Döhler GmbH (Darmstadt, Germany). All samples were prepared in double-distilled water and all concentrations are expressed in mass percentage (% w/w).
Biopolymer Solution Preparation
Powdered potato protein isolate (0.5% w/w) and pectins (0.5% w/w) were dissolved in double-distilled water and stirred at ambient temperature overnight to ensure complete hydration. After hydration, the biopolymer solutions were adjusted to pH 3.0–8.0 using HCl and/or NaOH (0.1 M, 1 M).
Biopolymer Complex Formation
The biopolymer complexes were generated based on the method described in our previous study [13]. The potato protein isolate (1% w/w) and pectin (1% w/w) solutions were initially adjusted to pH 3 using HCl and/or NaOH (0.1 M, 1 M). A series of complex dispersions were generated with different protein-pectin ratios (0.33–5.00). All biopolymer dispersions were mixed for at least 2 min at ambient temperature on a stirrer plate (mixing speed 100 rpm) to ensure evenly distributed dispersions. The final concentration of protein was 0.25% w/w and the pectin concentration was between 0.05–0.75% w/w in complexes.
ζ-Potential
The ζ-potential of individual biopolymer dispersions (0.5% w/w) at pH 3–8 and complex biopolymer dispersions (0.3–1% w/w) at pH 3 were measured by using a Laser-Doppler-micro-electrophoresis device (Zetasizer Nano-ZS, Malvern Instruments, Malvern, UK). Samples were diluted to a biopolymer concentration of 0.05% with double-distilled water and then loaded into an appropriate cuvette. The ζ-potential was determined by measuring the direction and velocity that the droplets moved in the electric field applied. All measurements were carried out at 25 °C. The Smoluchowski equation was utilized to calculate the ζ-potential according to Eq. (1).
where νE is the mobility, ζ is the ζ-potential, ε0 and εr are the relative dielectric constant and the electrical permittivity of a vacuum, respectively, μ is the solution viscosity, r is the particle radius and κ is the Debye–Hückel parameter. The ζ-potential measurements were conducted on two replicates of freshly prepared samples with four readings on each replicate.
Solubility
The solubility of the protein was determined using a method published previously [28]. In brief, protein solution (2%) in double-distilled water was prepared with stirring at ambient temperature for 2 h to ensure a complete hydration. pH values were adjusted from 3 to 8 using 0.1 M and 1 M HCl and/or NaOH and samples were centrifuged for 30 min at 36000 rpm (Biofuge 28 RS, Heraeus Sepatech, Germany). The protein concentration in the supernatant after centrifugation was measured with microplate procedure of Coomassie Brilliant Blue reagent which is a modificated procedure of Bradford assay [29]. 5 μL of the supernatant and 250 μL of Coomassie reagent mixed for 30 s and incubated for 10 min. The absorbance of mixtures was measured at 595 nm using a spectrophotometer (BioTek Synergy 2 Multi-Mode Reader, Winooski, USA). Quantification was carried out using a calibration curve (0–25 μg mL−1) obtained with albumin.
Viscosity
The flow behavior of individual and complex dispersions was analyzed using a modular compact oscillatory rheometer (Anton Paar, Stuttgart, Germany) which was equipped with a coaxial cylinder (CC-27, cup diameter: 28.92 mm, bob diameter: 26.66 mm). Flow curves were recorded by increasing the shear rate of the coaxial cylinder system equilibrated to 25 °C logarithmically from 1.0 to 300 s−1. The viscosity η was calculated as the slope of the shear stress vs. strain curve and plotted as a function of protein-pectin ratio at a shear rate γ = 203 s−1.
Microstructure
Microstructure of samples were assessed by light microscopy. Micrographs were taken with an axial-mounted Canon Powershot G10 digital camera (Canon, Tokyo, Japan) mounted on an Axio Scope optical microscope (A1, Carl Zeiss Microimaging GmbH, Göttingen, Germany). The images presented are representative examples of the samples investigated during the study.
Visual Observation
The individual and complex biopolymer dispersions stored for 24 h at room temperature and phase separation behavior was observed using a digital camera (PowerShot SX200 IS, Canon, Tokyo, Japan).
Sensorial Evaluation
A series of complex dispersions was generated with different potato protein-pectin ratios (0.33–5.00) at pH 3 using lactic acid. In preliminary tests, various organic and inorganic acids (namely lactic acid, phosphoric acid, citric acid, malic acid) were tested on the formation and stability of the complexes formed, whereas no differences on the size, shape and charge was identified. As such, we decided to utilize an organic acid for the sensorial tests, since lactic acid is commonly used in many food applications. Moreover, it was reported that lactic acid is advantageous to use in food industry as it does not mask or overpower the other weak aromatic flavors and it has a milder taste than other organic acids have [30].
Initially, the panel including 28 panelists was trained for bitterness according to DIN 10959 with caffeine solutions of 0.05, 0.1, 0.15, 0.175, 0.2, 0.25, and 0.3 g L−1. The panelists selected were able to identify a critical threshold concentration of at least 0.25 g L−1 as bitter. A rank sum test was used to determine the bitterness of complex dispersions. Sensory evaluation was performed in a standardized sensory setup, according to DIN 10950:2012–10.27. The data were interpreted with nonparametric analysis which was specified as appropriate analysis for rank sum test by Meilgaard et al. [31]. The detailed information regarding the sensory data analysis was given in the section of statistical analysis.
Statistical Analysis
All measurements were repeated at least three times using duplicate samples. Means and standard deviations were calculated from these measurements using Excel (Microsoft, Redmond, WA, USA). A Fisher test with a confidence interval of 95 was used to evaluate the statistical differences (Minitab® 16 Statistical Software, Minitab Inc. State College, Pennsylvania, USA). Pearson correlation coefficient and p value was found by Excel – Data Analysis (Microsoft, Redmond, WA, USA). For microscopic imaging, representative images were chosen from amongst at least five similar images. Regarding the sensorial datas, Friedman analysis was used to determine if significant differences (p < 0.05) exist among samples and then an appropriate multiple comparison procedure was applied to find which samples were significantly different (p < 0.05). Friedman analysis was performed with IBM SPSS 19 (Chicago, IL, USA). Multiple comparison procedure which is appropriate for rank data was chosen as the method of nonparametric analog to Fisher’s LSD for rank sums which was proposed by Meilgaard et al. [32]. Two samples are declared to be significantly different (p < 0.05) if their rank sums differ by more than the value of LSDrank in Eq. (2)
where b is the number of panelists and t is the number of samples. The value of zα/2 was taken 1.96 which corresponds to confidence interval of 95.
Results and Discussion
Physicochemical Characterization of Individual Biopolymers
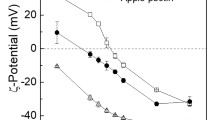
The impact of pH (3–8) on the ζ-potential of potato protein and pectin solutions (0.5% w/w) in distilled water was investigated (Fig. 1). The ζ-potential of potato protein solution changed from negative (−1.05 ± 0.09 mV) to positive (+23.7 ± 1.74 mV) as the pH was decreased from 8 to 3. As the pH decreases, carboxyl groups and amino groups of the protein undergo a gradual protonation which results a change in electrostatic repulsion pattern [33]. The measured pI of potato protein was between pH 7–8 which shows that the potato protein samples used in this study mostly consisted of protease inhibitors, that were reported to have a varying pI between 5 and 8 [19, 34].
In addition, the lowest solubility (53.52 ± 1.00%) for potato protein solution was measured under alkaline conditions (pH 8) whereas a gradual increase was observed with decreasing pH reaching a maximum value (92.99 ± 2.03%) at pH 3 (Table 1). A statistical evaluation confirmed that solubility was directly dependent on net ζ-potential values (r = 0.922; p < 0.01) proving the effect of alteration in electrostatic repulsion on the solubility with a change in degree of surface charge. It was mentioned that increased net surface charge can overcome various attractive forces (e.g., van der Waals, hydrophobic and depletion) which contributes to the solubility at acidic pH [35, 36]. Near the pI, the repulsive electrostatic interactions between molecules are of very low magnitude so that protein molecules form aggregates [37].
In contrast, all pectins tested had negative surface charge over the entire pH range (Fig. 1) – a typical pH-depended behavior for that type of hydrocolloid. It can be clearly seen that all pectin types had higher negative surface charge at higher pH values and there is a sharp decrease in net negative surface charge due to pKa values around 3.5 of carboxylic acid groups. The carboxylic acid groups would be uncharged (–COOH) at the pH values lower than pKa while they can be fully charged (–COO−) at high pH values [38]. However, significant differences between pectins with various DE was observed. As such, pectins with a low DE had a higher net negative surface charge over the whole pH range regardless of origin due to a larger number of dissociated carboxylic acid groups [39]. The differences in ζ-potential plots of pectins with the same DE and from different sources could be due to blockwise distribution of free carboxylic acid groups in citrus pectin [40, 41]. It was previously reported that the most ordered pectin in terms of non-esterified carboxylic acid groups has the net highest negative ζ-potential among pectins with the same DE [41].
Based on the characterized properties of individual biopolymers, we chose pH 3 for the formation of complexes. At this pH both biopolymers (protein and polysaccharides) showed a high solubility and nearly equal net surface charge values which potentially promotes electrostatic attraction [42, 43].
Physicochemical Characterization of Complexes
It was previously observed in studies conducted in our lab [13, 44,45,46] that proteins could be utilized as potential ingredients for the formation of protein-pectin complexes. In general, it was demonstrated that the complex formation was promoted under conditions where the biopolymers carry opposite charges, whereas the pH-value, protein-pectin ratio, and the pectin type could be used to modulate the size and morphology of the complexes formed. ζ-potential measurements have been used as a rough measure in the study as it is the commonly used method to evaluate the complex formation between oppositely charged biopolymers [47]. Viscosity measurements, microstructure images and visual observations were used to characterize the complexes formed.
Impact of Biopolymer Ratio
The purpose of this set of experiments was to determine an optimum protein-pectin ratio to generate complexes which are stable to gravitational separation and small enough to avoid light scattering. As such, potato protein and various pectin types were mixed under acidic conditions (pH 3) at different protein-pectin ratios ranging between 0.33–5, whereas the overall protein concentration (0.25%) was kept constant. The impact of protein-pectin ratio on the surface charge of complexes is shown in Fig. 2. The net negative surface charge of the complexes decreased with protein proportion until achieving electroneutrality (ζ-potential = 0 mV), whereas higher protein-pectin ratios produced complexes with higher positive surface charge. At the point of electroneutrality, all negative sites of pectin are saturated by positive sites of potato protein. Neutral surface charge was obtained at near to a protein-pectin ratio of 2.5 for the complexes formed with high DE pectins. Protein-pectin ratios of 1 and 1.67 were required for charge neutralization for the complexes formed by AP DE35 and CP DE35, respectively. The ζ-potential values of the mixtures turned to positive at higher protein-pectin ratios, which can be explained by low amount of carboxylic groups of pectins in solution to interact with amino groups of protein. It was indicated that, protein-pectin ratio controls the equilibrium of macromolecule charges and thus the degree of electrostatic interactions and the extent of self-aggregation during complex formation [48]. Sanchez et al. [49] also stated that the structure of coacervates is affected by biopolymer mixing ratio.
ζ-potential results were supported by visual observations of the mixtures as a function of protein-pectin ratio (0.33–5) (Fig. 3). For samples with lower net ζ-potential values (< 20 mV), phase separation and precipitation observed, while at higher values (> 20 mV) stable coacervates with a turbid appearance formed. Kim and Wicker [50] also stated that insufficient pectin concentration results in bridging flocculation while too high pectin concentration cause a strong increase in viscosity preventing the precipitation. In addition, microscopic images showed that intensity of interaction between biopolymers was enhanced by the protein-pectin ratio (Fig. 4). The complexes at the ratio of 0.33 showed dispersed small aggregates while larger clusters with a firmer appearance were observed for those complexes generated at ratio of 1, 2.5 and 5. There were also dispersed small aggregates which probably resulted from protein-protein interactions at high protein-pectin ratio. Wang et al. [40] explained complex formation at high protein-pectin ratio with binding of protein separately on the pectin chain network and aggregation of some protein dimers with each other to form small protein domains.
Impact of Pectins’ DE
As it is seen in Fig. 2, the surface charge pattern of the complexes was clearly affected by DE of pectins. The slope of surface charge plot of complexes generated with low DE pectins was higher than complexes formed with high DE pectins. Higher protein-pectin ratios required for net ζ-potential values < 10 mV in the complexes generated with low DE pectins. This is probably due to higher amount of galacturonic acid content of low DE pectins. In addition, previous literature [51, 52] indicated that the difference in water affinity between protein and pectin decrease when the pectin is less hydrophilic which is possible when it has lower DE. It was reported that when both biopolymers have similar and low water affinity, they do not show a tendency to dissolve in water as an individual biopolymer, instead their compatibility to each other increase and stronger interactions are formed [51]. We assumed that plant proteins which contains relatively higher hydrophobic amino acids than animal proteins could make stronger interactions with pectins with low DE. It was also stated by Einhorn-Stoll et al. [51] that compatibility of sodium caseinate which is a relatively hydrophobic protein has increased when pectin with low DE has used for complexation.
Visual observations also demonstrated that DE of pectins clearly affects the complex formation especially at higher protein-pectin ratios (Fig. 3). All complexes at the protein-pectin ratio of 0.33 promoted a turbid appearance without visible sedimentation. However, high DE pectins caused a precipitation at the ratio of 0.5 in addition to a turbid supernatant. At higher protein-pectin ratios, the complexes generated with high DE pectins looked more like a precipitate than a coacervate while there was a cloudy phase separation formed with low DE pectins. This is probably due to viscous structure of low DE pectins interfering with the sedimentation of coacervate [53]. Microscopic images (Fig. 4) supported the visual observation that large number of small features in microscopic images gave a low degree of phase separation in visual observation while small number of large features lead to precipitation or coacervation. Protein-pectin mixtures obtained with low DE pectin had a different visual observation and microscopic images than the mixtures obtained with high DE. This could be due to the presence of hydrogen bonding between pectin chains and minor contribution of hydrophobic interactions to electrostatic interactions in complexes formed with low DE. These interactions could be neglected for the complexes generated with high DE due to their high hydrophilicity [54].
Impact of Pectin Source
The pectin source also had an effect on complexation and the viscosity of the formed complexes. The degree of blockiness, molecular weight and neutral sugar side chains could be the influencing factors depending on the source of pectins.
As can be seen from Fig. 2, surface charge patterns were similar for complexes formed with high DE pectins, irrespective of their origin. For low DE pectins, however, clear differences could be seen. Even though the DE of CP DE35 and AP DE35 was the same, the complexes formed with the latter reached to neutralization at a lower protein-pectin ratio. This could be explained by differences in degree of blockiness values in these two pectins.
The distribution of carboxylic acid groups was largely studied in relation to the formation of gels in the presence of Ca2+. It was reported in several studies that pectins with low DE which are able to form gel in the presence of Ca2+ have higher gelling properties when those have higher amount of blockwise distributed carboxylic acid groups. Blockwise distribution of reactive sites generates a local charge concentration and hold Ca2+ ions in place in gel structure [55,56,57,58,59,60,61]. Lutz et al.[41] explained that, the blockwise arrangement allows stronger and more effective intermolecular interactions, probably because the sequence of carboxylic acids permits better contact between the pectin chains and better utilization of the carboxylic acid groups is provided.
As such, it was indicated that pectins having a higher local charge density might form complexes with proteins which could withstand harsher environmental conditions [62]. Warnakulasuriya et al. [63] also found that dispersions including pectins with high DB gave higher OD values meaning the formation of more complexes with pea protein isolate. In addition, we recently demonstrated that pectin-protein complexes grew in size with decreasing temperatures, increasing degree of charge density and decreasing pectin concentration [64].
It was indicated that citrus pectin shows a blockwise esterification due to its partial de-methylation by native pectin esterase prior to processing [41, 62, 65]. However, for complexes formed with low DE pectins, higher differences in ζ-potential were observed. A reason for this could be the higher sensitivity of low DE pectins than that of high DE pectins to cationic charge groups when they possess a blockwise distribution [66]. Besides the degree of blockiness, Warnakulasuriya et al. [63] mentioned that neutral sugar side chains could also have an important role on complex coacervation by means of hydrogen bonding or hydrophobic interactions.
Based on the viscosity measurements, complexes formed with apple pectin had a higher viscosity than citrus pectin with the same DE at lower protein-pectin ratios (Fig. 5). Wang et al. [40] indicated that, viscosity of pectin solutions increase with molecular weight and neutral sugar side chains at similar galacturonic acid contents. However, neutral sugar side chains appeared to be predominant factor affecting the viscosity in our case as apple pectin has lower molecular weight but higher neutral sugar side chains than citrus pectin. This finding was supported by Wang et al. [40] and Renard et al. [67] who observed a higher viscous shares for apple pectin than citrus pectin.
Impact of Complexation with Different Pectins on Bitterness
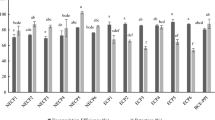
Bitterness perception was evaluated for complexes formed with AP DE71 and CP DE 70 at different protein-pectin ratios. As the low DE pectins had a high viscosity, sensorial evaluation could not be performed due to being not drinkable at low protein-pectin ratios. Despite all complexes having the same protein concentration (1% w/w) bitterness score increased with increasing protein-pectin ratio (Fig. 6). A very similar pattern obtained for bitterness score and surface charge properties. The complexes with a positive surface charge were found to be more bitter than the ones with a negative surface charge, which is in agreement with our previous paper [13]. The relationship between bitter compounds and surface charge was supported by several authors [68,69,70] who indicated that most of the bitter compounds are cationic in nature. Moreover, previously published literature [70, 71] showed that bitter off-notes were increasingly reduced with higher pectin concentrations with a constant protein amount.
Sensorial ranking sums (bitterness) of potato protein-pectin complexes as a function of biopolymer ratio (cprotein = 1.0%). Please note: Results of bitterness of apple pectin potato protein mixtures with the same uppercase letter were not significantly different (p < 0.05). Results of bitterness of citrus pectin potato protein mixtures with the same lowercase letter were not significantly different (p < 0.05)
According to sensorial results, complexes formed at the ratios of 0.33 and 1 had significantly lower bitterness rank sum than that of protein. Bitterness rank sum was lower in complexes generated with citrus pectin than in complexes generated with apple pectin at the protein-pectin ratio of 0.33. The reason for this could be the blockwise distribution of citrus pectin on pectin backbone. Blockwise distribution could lead to more stable complexes, affecting the perception of bitterness. However, the bitterness rank sum of complexes formed at the ratio of 2.5 were not statistically different from the protein and the complexes formed at the protein-pectin ratio of 1. This could be explained by the increasing viscosity of complexes with pectin concentration as it affects the oral perception of bitter taste. This was also indicated by panelists’ comments as undesirable texture and mouthfeel especially at higher pectin concentrations. This effect was also described by other researchers [72, 73] who observed an impact on texture, flavor release and other sensorial qualities when polysaccharides were utilized above a critical polymer concentration.
Conclusion
The results of this study showed that the pectin type and degree of esterification had a clear impact on the association of potato protein-pectin complexes. Based on the measured surface charge, microscopic images and visual observation, it can be concluded that the ratio between protein and polysaccharide had a substantial effect on charge balance and therefore on the intensity of interactions. Bitterness, as evaluated by a sensory panel, was highly depended on surface charge of complexes. This resulted in the lowest bitter taste for samples with the highest net negative surface charge on complexes generated at the protein-pectin ratio 0.33 which also gave a relatively stable mixture. Citrus pectin proved to be better to prepare soluble complexes at low protein-pectin ratio as complex solutions had a lower viscosity. Moreover, high DE pectins provided less viscous complexes than low DE pectins which could make their utilization easier in high protein beverage applications. It was inferred from the study that complex coacervation of potato protein with citrus pectin with a high DE could be an efficient method for reduction of bitter taste in potato protein products. A further study could be conducted using pectins with a known degree of blockiness at the same esterification degree to clarify the effect on complex formation and sensorial acceptance of proteins. Moreover, a sensory analysis could also be performed at incremental viscosity values of pectins with a same amount of bitter compound to observe the impact of viscosity on bitterness quantitatively.
References
A. Nesterenko, I. Alric, F. Silvestre, V. Durrieu, Ind. Crop. Prod. 42, 469–479 (2013)
S. Liu, C. Elmer, N.H. Low, M.T. Nickerson, Food Res. Int. 43(2), 489–495 (2010)
S. Sethi, S.K. Tyagi, R.K. Anurag, J. Food Sci. Technol. 53(9), 3408–3423 (2016)
M. Yavuz and B. Özçelik, Academic Food Journal/Akademik GIDA 14 (4) (2016)
A. Görgüç, P. Özer, F.M. Yılmaz, J. Food Compos. Anal. 87, 103424 (2020)
E. Sipos and L. Foster, in Dietary Proteins (AOCS Publishing, 1992), pp. 243–282
V.R. Young, P.L. Pellett, The American Journal of Clinical Nutrition 59(5), 1203S–1212S (1994)
A. Tarrega, M.O. Ramírez-Sucre, J.F. Vélez-Ruiz, E. Costell, J. Food Eng. 109(3), 467–474 (2012)
I. Siro, E. Kapolna, B. Kápolna, A. Lugasi, Appetite 51(3), 456–467 (2008)
D. Asker, J. Weiss, D.J. McClements, J. Agric. Food Chem. 59(3), 1041–1049 (2011)
N.J. Gaudette, G.J. Pickering, Crit. Rev. Food Sci. Nutr. 53(5), 464–481 (2013)
D. Sun-Waterhouse, S.S. Wadhwa, Food Bioprocess Technol. 6(3), 607–627 (2013)
B. Zeeb, M. Yavuz-Düzgun, J. Dreher, et al., Food & Function 9(4), 2261–2269 (2018)
A.S. Sivam, D. Sun-Waterhouse, C.O. Perera, G.I.N. Waterhouse, Food Res. Int. 50(2), 574–585 (2013)
M. Friedman, J. Agric. Food Chem. 44(1), 6–29 (1996)
R.L. Jackman, R.Y. Yada, J. Food Sci. 53(5), 1427–1432 (1988)
M.S. Kaldy, Econ. Bot. 26(2), 142–144 (1972)
A.C. Kapoor, S.L. Desborough, P.H. Li, Potato Res. 18(3), 469–478 (1975)
L. Stounbjerg, C. Vestergaard, B. Andreasen, R. Ipsen, Colloids Surf. A Physicochem. Eng. Asp. 566, 104–112 (2019)
S. Løkra, K.O. Strætkvern, Food 3(1), 88–95 (2009)
H.A. Schols, A.G.J. Voragen, Carbohydr. Res. 256(1), 83–95 (1994)
W.G.T. Willats, P. Knox, J.D. Mikkelsen, Trends Food Sci. Technol. 17(3), 97–104 (2006)
H. Salminen, J. Weiss, Food Biophysics 9(1), 29–38 (2014)
P.J.H. Daas, K. Meyer-Hansen, H.A. Schols, G.A. De Ruiter, A.G.J. Voragen, Carbohydr. Res. 318(1–4), 135–145 (1999)
M. Maniruzzaman, J.S. Boateng, M. Bonnefille, A. Aranyos, J.C. Mitchell, D. Douroumis, Eur. J. Pharm. Biopharm. 80(2), 433–442 (2012)
D. Douroumis, Expert Opinion on Drug Delivery 4(4), 417–426 (2007)
M. Pein, M. Preis, C. Eckert, F.E. Kiene, Int. J. Pharm. 465(1–2), 239–254 (2014)
C. Morr, B. German, J. Kinsella, et al., J. Food Sci. 50(6), 1715–1718 (1985)
M.M. Bradford, Anal. Biochem. 72(1), 248–254 (1976)
W. H. Gardner, Handbook of Food Additives, 225–270 (1972)
M. Meilgaard, G. Civille, B. Carr, Sensory Evaluation Techniques. 4th ed. (CRC Press, New York, 2007), pp. 105–128
M. Meilgaard, G. Civille, B. Carr, Sensory Evaluation Techniques, 4th edn. (CRC Press, New York, 2007)
C.-H. Tang, X. Sun, Food Hydrocoll. 25(3), 315–324 (2011)
Y. Du, F. Chen, Y. Zhang, C. Rempel, M.R. Thompson, Q. Liu, J. Appl. Polym. Sci. 132(44) (2015)
S.-W. Yin, J.-C. Chen, S.-D. Sun, C.H. Tang, X.Q. Yang, Q.B. Wen, J.R. Qi, Food Chem. 128(2), 420–426 (2011)
D.J. McClements, Curr. Opin. Colloid Interface Sci. 9(5), 305–313 (2004)
R. Townend, R.J. Winterbottom, S.N. Timasheff, J. Am. Chem. Soc. 82(12), 3161–3168 (1960)
Y. Yang, M.E. Leser, A.A. Sher, D.J. McClements, Food Hydrocoll. 30(2), 589–596 (2013)
A. Krzeminski, K.A. Prell, J. Weiss, J. Hinrichs, Food Hydrocoll. 35, 332–340 (2014)
X. Wang, Q. Chen, X. Lü, Food Hydrocoll. 38, 129–137 (2014)
R. Lutz, A. Aserin, L. Wicker, N. Garti, Food Hydrocoll. 23(3), 786–794 (2009)
S. Turgeon, C. Schmitt, C. Sanchez, Curr. Opin. Colloid Interface Sci. 12(4–5), 166–178 (2007)
F. Weinbreck, R. De Vries, P. Schrooyen, C. De Kruif, Biomacromolecules 4(2), 293–303 (2003)
B. Zeeb, C. Stenger, J. Hinrichs and J. Weiss, Food Structure 10, 10–20 (2016)
C. Stenger, B. Zeeb, J. Hinrichs, J. Weiss, J. Dispers. Sci. Technol. 38(9), 1258–1265 (2017)
K. Protte, C. Bollow, A. Sonne, O. Menéndez-Aguirre, J. Weiss, J. Hinrichs, Food Biophysics 11(3), 226–234 (2016)
Y.P. Timilsena, T.O. Akanbi, N. Khalid, B. Adhikari, C.J. Barrow, International Journal of Biological Macromolecules 121, 1276–1286 (2019)
A. Ye, International Journal of Food Science & Technology 43(3), 406–415 (2008)
C. Sanchez, G. Mekhloufi, C. Schmitt, et al., Langmuir 18(26), 10323–10333 (2002)
Y. Kim, L. Wicker, Food Hydrocolloids 25(3), 419–425 (2011)
U. Einhorn-Stoll, T. Salazar, B. Jaafar, H. Kunzek, Nahrung-Food 45(5), 332–337 (2001)
T. Nordmark and G. R. Ziegler, Food Hydrocolloids 14(6), 579–590 (2000)
E. Hans-Ulrich, M. Frank and N. Karl, in Handbook of Food Science, Technology, and Engineering - 4 Volume Set (CRC Press, 2005)
Y.A. Antonov, M. Celus, C. Kyomugasho, M. Hendrickx, P. Moldenaers, R. Cardinaels, Food Hydrocoll. 94, 268–278 (2019)
D. Powell, E. Morris, M. Gidley, D. Rees, Journal of Molecular Biology 155(4), 517–531 (1982)
R. Kohn, O. Markovič, E. Machová, Collect. Czechoslov. Chem. Commun. 48(3), 790–797 (1983)
J. Thibault, M. Rinaudo, Biopolymers: Original Research on Biomolecules 24(11), 2131–2143 (1985)
M.-C. Ralet, V. Dronnet, H.C. Buchholt, J.-F. Thibault, Carbohydrate Research 336(2), 117–125 (2001)
C. Löfgren, S. Guillotin, H. Evenbratt, H. Schols, A.-M. Hermansson, Biomacromolecules 6(2), 646–652 (2005)
G. Limberg, R. Körner, H.C. Buchholt, T.M. Christensen, P. Roepstorff, J.D. Mikkelsen, Carbohydr. Res. 327(3), 293–307 (2000)
C. Rolin, Pectins and their Manipulation, 222–241 (2002)
B.L. Sperber, H.A. Schols, M.A.C. Stuart, W. Norde, A.G. Voragen, Food Hydrocolloids 23(3), 765–772 (2009)
S. Warnakulasuriya, P.K. Pillai, A.K. Stone, M.T. Nickerson, Food Chemistry 264, 180–188 (2018)
B. Zeeb, L. Mi-Yeon, M. Gibis, J. Weiss, Food Hydrocolloids 74, 53–61 (2018)
M. Buchweitz, M. Speth, D. Kammerer, R. Carle, Food Chemistry 139(1–4), 1168–1178 (2013)
A.T. Nasseri, J.-F. Thibault, M.-C. Ralet, Tree Sci. Biotechnol 2, 60–70 (2008)
C.M. Renard, M.-J. Crépeau, J.-F. Thibault, Carbohydr. Res. 275(1), 155–165 (1995)
N. Funasaki, I. Uratsuji, T. Okuno, S. Hirota, S. Neya, Chemical and Pharmaceutical Bulletin 54(8), 1155–1161 (2006)
Y. Miyanaga, A. Tanigake, T. Nakamura, et al., International Journal of Pharmaceutics 248(1–2), 207–218 (2002)
K. Kurihara, Y. Katsuragi, I. Matsuoka, M. Kashiwayanagi, T. Kumazawa, T. Shoji, Physiology & Behavior 56(6), 1125–1132 (1994)
T. Kumazawa, M. Kashiwayanagi, K. Kurihara, Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 888(1), 62–69 (1986)
E. Guichard, S. Issanchou, A. Descourvieres, P. Etievant, J. Food Sci. 56(6), 1621–1627 (1991)
J.P. Ley, Chemosens. Percept. 1(1), 58–77 (2008)
Acknowledgements
We thank Döhler GmbH (Darmstadt, Germany) and Herbstreith & Fox KG (Neuenbürg, Germany) for generously providing us with biopolymer samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yavuz-Düzgün, M., Zeeb, B., Dreher, J. et al. The Impact of Esterification Degree and Source of Pectins on Complex Coacervation as a Tool to Mask the Bitterness of Potato Protein Isolates. Food Biophysics 15, 376–385 (2020). https://doi.org/10.1007/s11483-020-09631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-020-09631-1